Abstract
Purpose
To evaluate the efficacy and safety of a coaxial reservoir system with a non-braided spiral tip microcatheter and exclusive port for hepatic arterial infusion chemotherapy.
Materials and methods
In vitro evaluation included evaluation of pressure tolerance/flow rate of the coaxial reservoir system, and the strength of connection between the 2.7-F catheter and port. Due to the difficulty of implanting conventional reservoirs, coaxial reservoirs were implanted via the femoral artery of 80 patients. We implanted a non-braided 2.7-F microcatheter with a spiral shaped tip, 5-F catheter, and a port. Clinical assessment included evaluation of technical success and complications.
Results
In vitro evaluation of the coaxial reservoir at its maximum pressure load showed that flow rates for 300 mg I/mL iopamidol contrast medium were 0.25 ± 0.04 mL/s (undiluted), 1.03 ± 0.01 mL/s (50% dilution), and 2.91 ± 0.01 mL/s (30% dilution). Connection strength between the 2.7-F catheter and port was 13.4 ± 0.57 N. Percutaneous port catheter placement was successful in all patients (100%, n = 80). Complications included hepatic arterial occlusion (10%, n = 8), catheter tip dislocation (1.3%, n = 1), and catheter occlusion (1.3%, n = 1).
Conclusions
A coaxial reservoir system with a non-braided microcatheter and exclusive port is safe and effective for difficulty of implanting conventional reservoir.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatic arterial infusion chemotherapy (HAIC) is an important treatment option for unresectable advanced liver malignancies. This technique maintains a higher local concentration of the anticancer agents than systemic chemotherapy [1, 2]. In addition, the usefulness of HAIC refractory relative to systemic chemotherapy has recently been reported [3].
Tapered or non-tapered heparin-coated 5-F catheters have conventionally been used as indwelling catheters in the peripheral hepatic or gastroduodenal artery, whereas the fixed catheter tip method uses coils or the n-butyl cyanoacrylate–lipiodol mixture [4]. However, conventional reservoir implantation using these catheters can lead to occlusion, stenosis, and anatomical and arteriosclerotic issues, which can in turn pose problems for implantation. In these cases, a coaxial reservoir system using a microcatheter increases the technical success rate [5–7].
Breakage of the port silicone septum is one of complications of the coaxial reservoir system [5]. In addition, the connection between the microcatheter and the port connector may be weaker [8]. To the best of our knowledge, neither an in vitro evaluation of port performance nor a long-term evaluation regarding the use of non-braided microcatheters with anticoagulation coating in a coaxial reservoir system has been performed.
Materials and methods
Performance evaluation of the coaxial port system
Catheters and port
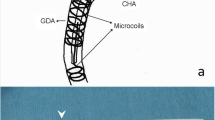
The non-braided 2.7-F catheter (W spiral catheter; Piolax Medical Devices, Yokohama, Japan) has a spiral tip onto which the nitinol (shape memory alloy) coil is mounted (Fig. 1). The shaft is coated with polyvinylpyrrolidone, which has anticoagulative, hydrophilic and lubricant properties. The catheter has a 0.022-in (0.56-mm) inner diameter, and can be inserted into a catheter with an inner lumen ≥0.038 in (0.97 mm). For the coaxial reservoir system, it can be implanted via a 5-F catheter. A side hole for anticancer agents is made at an appropriate site on the shaft with a special puncher included in the kit. Although a 0.018-in (0.46-mm) guide wire can pass through the catheter, a 0.016-in (0.41-mm) guide wire is recommended to avoid friction during insertion. The present study used the polyvinylpyrrolidone-coated non-braided 5-F catheter (Shepherd hook type, Piolax Medical Devices).
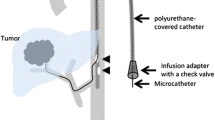
We also used an exclusive port (Access-port; Nipro, Osaka, Japan), which has two-stage structure connectors that can connect to both the 2.7-F catheter and the 5-F catheter (Fig. 2). Maximum pressure is 70 pounds per square inch (psi) (4.83 bar) for this port.
The catheters and port used in this study are approved and commercially available in Japan. The 2.7-F catheter and the exclusive port used in this study are specifically approved for coaxial reservoir use.
Pressure tolerance of the coaxial reservoir
To test pressure tolerance, we first created a side hole 8 cm from the distal end of the 2.7-F catheter. Next, the catheter and port were heated to 37°C and connected. The silicone septum of the port was punctured with a 22-gauge Huber point needle and filled with 300 mg I/mL iopamidol contrast medium (Iopamiron; Bayer Schering Pharma), which was also heated to 37°C and used undiluted or diluted with saline (50 or 30% dilution). Finally, 70 psi of pressure were applied as compressed air controlled by a regulator. Flow rate was calculated by dividing the volume of contrast medium entering from the side hole of the catheter by the infusion time. We measured this five times and calculated the median.
Connection strength of the port to the 2.7-F catheter
To test the connection strength between the port and the 2.7-F catheter, we first heated the catheter and port to 37°C and connected the two. Next, we set these up on the test machine (Tension Tester; Kotobuki Kikai Kougyou, Yokohama, Japan). After adjusting the catheter to an interval of 50 mm, the 2.7-F catheter was clamped by a vise. With the port still fixed in place, we withdrew only the catheter. The speed at which it was removed was set at 300 mm/min. Maximum strain at the point in time when the port and catheter were disconnected was measured. We tested this five times, and calculated the median.
Clinical evaluation of the coaxial port system
Patients
For this retrospective study, implantation of the coaxial reservoir was performed after approval by our institutional review board. Written informed consent was obtained from each patient or their family members before reservoir implantation.
From August 2003 to February 2009, we implanted a total of 315 reservoir systems, including conventional reservoir systems. Eighty of these (25.4%) were coaxial reservoirs implanted in 80 patients (men, n = 55; women, n = 25; mean age 62.3 years; age range 43–81 years) due to the difficulty of implanting conventional reservoirs. Patients underwent chemotherapy for hepatocellular carcinoma (n = 46) or liver metastases (n = 34). Hepatic metastases originated from the colon (n = 24), stomach (n = 5), esophagus (n = 2), gall bladder (n = 1), and bile duct (n = 2). The chemotherapeutic regimen included 5-fluorouracil (250 mg/day in 3 h, 5 days/week, for the first 3 weeks) and cisplatin (10 mg/day in 30 min, 5 days/week, for the first 3 weeks), then 5-fluorouracil (1,000 mg in 5 h) and cisplatin (10 mg/body) by weekly for patients with hepatocellular carcinoma; 5-fluorouracil (1,000 mg/m3 in 5 h, weekly) for patients with hepatic metastases from the colon; and 5-fluorouracil (1,000 mg/m3 in 5 h, weekly) and cisplatin (10 mg/body) by weekly for patients with hepatic metastasis from stomach, esophagus, gall bladder or bile duct.
Indication of coaxial reservoir implantation
Decisions regarding the necessity of the coaxial reservoir implantation for the different cases are described in Table 1. Cases were selected based on these criteria given that there is no major difference in cost between conventional and coaxial reservoirs. Two interventional radiologists who were experienced in reservoir implantation selected the cases for this study. When they disagreed, the conventional reservoir implantation using a 5-F catheter or a tapered 5-F catheter (2.7-F distal shaft and 5-F proximal shaft) was attempted. If conventional reservoir placement was difficult, coaxial reservoir implantation was performed.
Coaxial reservoir implantation technique
Insertion of the 2.7-F catheter
In all cases, coaxial reservoir systems were implanted via the common femoral artery approach. After regional local anesthesia around the common femoral artery, the artery was punctured using the Seldinger technique. To make the puncture as proximal as possible, the fluoroscopy was used to locate the superior border to center of the femoral head to puncture the common femoral artery in all cases. For diagnosis, a 4-F catheter (Shepherd Hook; Medikit, Tokyo, Japan) was inserted directly without a sheath to avoid leakage at the puncture site.
Location and anatomical variation of the celiac artery and superior mesenteric artery were then determined by digital subtraction angiography (DSA). To reduce infusion of anticancer agents to adjacent organs during HAIC, the right gastric artery and the gastroduodenal artery sometimes required embolization using a microcatheter (Turbotracker 18; Boston Scientific, Natick, MA, USA) and a fibered platinum microcoil (Vortex Diamond-18; Boston Scientific). When an aberrant hepatic artery was identified, hepatic arterial blood flow was redistributed using microcoils to convert multiple hepatic arteries into a single arterial blood supply. A 5-F catheter was then introduced in exchange for the 4-F catheter using an over-the-guide-wire technique, and the tip of the 5-F catheter was then placed into the celiac artery or superior mesenteric artery. Optimal positions of the side hole and tip of the 2.7-F catheter were defined using 0.016-in (0.41-mm) guide wire (GT wire; Terumo, Tokyo, Japan) to measure the length, which was advanced to the predetermined position on the 2.7-F catheter tip in the peripheral hepatic artery. Then, under fluoroscopic control, this guide wire was drawn out from the peripheral hepatic arterial branch to the proximal portion of the proper or common hepatic artery, and its length was measured. Before placement of the 2.7-F catheter, a side hole of 1.1 mm diameter was manually created. The spiral shaped tip of the 2.7-F catheter was placed through a 5-F catheter in a bend of the artery for stabilization, and we set the final position of the side hole to the position of the proper or common hepatic artery. The side hole was positioned toward the peripheral hepatic artery when tumors were unevenly distributed. Then, the 5-F catheter was withdrawn to the aortic bifurcation and stabilized. To check catheter fixation, the patient was asked to take several deep breaths.
Connection of the port to the 2.7-F catheter
At the puncture site, a subcutaneous tunnel was made in a loop from outside in the cranial direction. After connection of catheters to the port, the port was implanted into the subcutaneous space at the cranial side of the inguinal ligament. The 2.7-F catheter, which was inserted into the common femoral artery in a maximally proximal position, was connected first, and then the 5-F catheter was advanced over the 2.7-F catheter (Fig. 2). Catheter cutting was carried out as previously described by Koganemaru et al. [9].
Clinical evaluation
Clinical evaluation of the coaxial reservoir system assessed the technical success rate, hepatic arterial occlusion rate after catheter implantation, catheter tip dislocation, and other complications.
Follow-up and endpoints
Follow-up continued until withdrawal of the coaxial reservoir system due to termination of HAIC treatment, complications, or patient death. HAIC was initiated 5–7 days after the coaxial reservoir was implanted. After every HAIC treatment, the system was flushed with a sufficient volume of saline and 3,000 IU of heparin. DSA was conducted 1 week after implantation and every 2–3 months thereafter by injecting contrast medium via the port. If necessary (i.e., if changes occur in drug distribution via the indwelling catheter, often due to the development of collateral flow and/or parasitic blood supply), computed tomography (CT) angiography was conducted to evaluate drug distribution.
Results
In vitro evaluation of the coaxial reservoir system
Flow rates for coaxial reservoir system at 70 psi were 0.25 ± 0.04 mL/s for undiluted contrast medium, 1.03 ± 0.01 mL/s for contrast medium diluted to 50%, and 2.91 ± 0.01 mL/s for contrast medium diluted to 30%. The connection strength test showed that the mean force required for disconnection was 13.4 ± 0.57 N.
Performance and feasibility of reservoir implantation
Reservoir implantation using our coaxial system was successful in all 80 cases without immediate post-procedural complications. Of the total of 80 cases, reasons for choosing the coaxial reservoir system were ≥50% stenosis of the celiac artery (n = 23) (Fig. 3), including cases of median arcuate ligament compression (n = 3); celiac artery occlusion requiring the collateral artery approach (n = 12) (Fig. 4); a celiac artery angled steeply downward (≤30°) from the abdominal aorta (n = 35); and ≥50% stenosis or strong tortuosity of the peripheral hepatic artery (n = 10). The distal tip of the 2.7-F catheter was positioned at the peripheral hepatic artery (n = 80), and the side hole of the 2.7-F catheter was positioned at the right hepatic artery (n = 1), proper hepatic artery (n = 57) or common hepatic artery (n = 22).
A 65-year-old man with multiple metastatic liver tumors from colon cancer. a Superior mesenteric arteriogram obtained from a 5-F catheter inserted via the right common femoral artery shows hepatopetal blood flow through the pancreaticoduodenal arteries. The splenic artery is visualized through retrograde flow running in the common hepatic and celiac arteries. There may be severe stenosis at the celiac artery. The right gastric artery was embolized with a microcoil before reservoir implantation (black curved arrow). b Oblique view of celiac arteriogram. The black arrow indicates severe stenosis of the celiac artery. c The coaxial reservoir was implanted via the celiac artery approach (black arrow). The spiral shape tip of the 2.7-F catheter was inserted into a left hepatic artery and stabilized at a bend in the artery. Two platinum markers show the position of the 2.7-F catheter tip (white arrows). The side hole was located at the proper hepatic artery (white arrowhead). The 5-F catheter was withdrawn to the abdominal aortic bifurcation (tip position) and fixed there (black arrowhead). d The port was placed in the right region of the hypogastric area (white curved arrow). The catheter was placed in the subcutaneous tunnel (thin white arrows), making a loop from just under the inguinal ligament in the cranial direction at the superior border to center of the femoral head (thin black arrow)
A 70-year-old man with multiple hepatocellular carcinomas and a concomitant finding of celiac artery occlusion due to arteriosclerosis. a Superior mesenteric arteriogram revealed hepatopetal blood flow. b The coaxial reservoir was implanted via the superior mesenteric artery approach. The spiral shape tip of the 2.7-F catheter was inserted into the right hepatic artery (arrow). The side hole was located at the proper hepatic artery (arrowhead)
Complications
The mean follow-up period after coaxial reservoir implantation was 16.5 months (range 1–58 months). Of the total 80 patients, 36 died of cancer (45%) after a mean duration of 14 months (range 1–47 months). Of the 36 who died, 34 (94%) showed no evidence of complications immediately following reservoir implantation.
Hepatic arterial occlusion occurred in 8 of 80 patients (10%). In 7 of the 8 cases (87.5%), hepatic arterial occlusion (proximal occlusion) was detected at 22.3 months (range 11–35 months) after reservoir implantation during anti-cancer drug infusion. In all 7 cases, the systems were removed and transcatheter arterial chemoembolization or systemic chemotherapy was conducted. In the remaining 1 case (1/8, 12.5%), occlusion was detected at follow-up DSA, the system was removed, and systemic chemotherapy was performed thereafter.
Catheter tip dislocation was observed 7 days after implantation in 1 case (1.3%). One case (1.3%) of catheter occlusion was also detected 280 days after reservoir implantation. Systems were replaced for these two patients by the same method.
Discussion
Use of a small diameter catheter increases pressure inside the coaxial reservoir system, and can cause breakage of the port silicone septum or disconnection of the catheter from the port [5, 8]. The following should be monitored after implanting a reservoir system: emerging stenosis or occlusion of a hepatic artery, recanalization of embolized vessels, catheter disconnection, catheter occlusion, and drug distribution [4]. Drug distribution can also be evaluated by CT or magnetic resonance imaging (MRI) by injecting contrast medium (30–50% dilution) at 0.5–1.5 mL/s for CT and 10 mL/h for MRI [4, 10]. Similarly, chemotherapy infusion, such as continuous infusion of 5-fluorouracil or 5-fluorodeoxyuridine, requires a flow rate ≤1 mL/min [1]. The coaxial reservoir system meets these conditions. However, DSA requires direct infusion of undiluted contrast medium from the port with a higher injection rate. Controlling flow rate by a hand injection makes it difficult to keep the maximum pressure under the pressure limit for the system. Using a small amount of contrast medium helped to obtain only the minimal required information.
Catheter disconnection from the port in reservoir systems has been reported, but causes of disconnection are not clear [8, 11]. Disconnection can cause leakage of anticancer agents into subcutaneous tissue and can make continuing HAIC impossible. Results of tensile tests between catheters and ports in the reservoir systems have not been reported. As for AFNOR (Association Française de Normalisation), the recommended connection strength between a catheter with an inside diameter of 2 mm and the port is more than 5 N [12]. The present study estimated the connection strength between the catheter and the port. The port we used has a two-stage node structure, which may have produced a stronger connection with the 2.7-F catheter.
The coaxial reservoir system has a high rate of successful placement, even for difficult conventional reservoir implantations [5, 6]. Because a 2.7-F catheter advances through a 5-F catheter in the system, placement is easy even through a collateral artery. Neither stenosis nor angle of bifurcation affected the technical success rate of the system.
Table 2 summarizes the various complications compared with that in the literature. Hepatic arterial occlusion is one of the most common complications after reservoir implantation, occurring in 3.3–16% of cases [5, 8, 11, 13–17]. The conventional reservoir using a fixed catheter tip method with a coil or n-butyl cyanoacrylate–lipiodol mixture is associated with a rate of 5–6.8% [11, 14, 16–18]. The present study found higher rates of hepatic arterial occlusion than observed for the conventional reservoir. One of the reasons that hepatic arterial occlusion was more common with the coaxial method was that these cases included placement through stenosed arteries or small collateral arteries. Previous reports have used braided diagnostic angiographic microcatheters without anticoagulation coating [5–7]; the polyvinylpyrrolidone coating on the catheter used in this study may have reduced the risk of hepatic arterial occlusion. Hydrogels like polyvinylpyrrolidone create a hydrophilic surface on medical devices that improves biocompatibility [19]. Therefore, anticoagulation actions and reduced risk of bacterial infection are expected [19]. Another way to reduce hepatic arterial occlusion is to select a fine, soft catheter with a smooth surface to prevent disruption of hepatic arterial blood flow and endothelial injury [20]. Less mechanical stimulation of the arterial inner walls by the catheter tip during respiration and polyvinylpyrrolidone coating may explain the lower hepatic artery occlusion rate.
Catheter tip dislocation rate using the subclavian artery approach was reported to be 2.8–4.7% [15, 21], but was 3–12% using the femoral artery approach [5, 7, 8, 11, 13, 22, 23], possibly due to movement of the hip joint [24, 25]. Catheter tip dislocation was also high when the port was implanted anterior to the femoral area while the catheter was placed over the hip joint or below the inguinal ligament [7, 22, 23]. We used the femoral artery approach for all cases in our study, but the dislocation rate was lower than that previously reported. One possible reason for the low catheter dislocation rate is better catheter stabilization due to its unique spiral-shaped tip. Another reason may have been that the common femoral artery puncture point and location of the indwelling catheter and port in the subcutaneous space was the cranial portion.
Catheter occlusion was reported in 0–11.4% of cases [5, 7, 8, 11, 13–18, 21–23]. One study reported a high rate of occlusion using a 5-F catheter [23]; therefore, catheter size and occlusion rate do not appear to be related. We think that sufficient flushing of heparin into the system after chemotherapy infusion can prevent catheter occlusion.
There are several limitations for this retrospective, non-randomized study. Our study is limited by the small number of patients. Decisions regarding the necessity of implantation of this system were made by only two interventional radiologists; therefore, cases suitable for treatment by standard HAIC could have been included in this study, which may have affected the rate of complications. The types and dosages of anticancer agents used, difference of procedure time and cost between the two methods were not taken into account in our analysis.
Conclusion
In conclusion, transfemoral implantation of the coaxial reservoir system showed a high technical success rate that is not influenced by occlusion, stenosis, and tortuosity of arteries. Further studies will be needed to evaluate the indications, criteria and procedural time for the coaxial reservoir system, as well as for the conventional method with a long-tapered catheter. This technique could therefore be used as a good second line method in cases where conventional reservoir implantation is difficult.
References
Arai Y, Inaba Y, Takeuchi Y, Ariyoshi Y. Intermittent hepatic arterial infusion of high-dose 5-FU on a weekly schedule for metastases from colorectal cancer. Cancer Chemother Pharmacol. 1997;40:526–30.
Niederhuber JE, Ensminger W, Gyves J, Thrall J, Walker S, Cozzi E. Regional chemotherapy of colorectal cancer metastatic to the liver. Cancer. 1984;53:1336–43.
Martin RC, Joshi J, Robbins K, Tomalty D, Bonsnjakovik P, Derner M, et al. Hepatic intra-arterial injection of drug-eluting bead, irinotecan (DEBIRI) in unresectable colorectal liver metastases refractory to systemic chemotherapy: results of multi-institutional study. Ann Surg Oncol. 2011;18:192–8.
Arai Y, Takeuchi Y, Inaba Y, Yamaura H, Sato Y, Aramaki T, et al. Percutaneous catheter placement for hepatic arterial infusion chemotherapy. Tech Vasc Interv Radiol. 2007;10:30–7.
Hamada A, Yamakado K, Nakatsuka A, Takaki H, Takeda K. Clinical utility of coaxial reservoir system for hepatic arterial infusion chemotherapy. J Vasc Interv Radiol. 2007;18:1258–63.
Morita S, Hata Y, Morita Y, Horimi T. A new method of connection between a small caliber catheter and a reservoir system. Jpn J Interv Radiol. 1996;11:387–90.
Herrmann KA, Waggershauser T, Sittek H, Reiser MF. Liver intraarterial chemotherapy: use of the femoral artery for percutaneous implantation of catheter-port systems. Radiology. 2000;215:294–9.
Ricke J, Hildebrandt B, Miersch A, Nicolaou A, Warschewske G, Teichgräber U, et al. Hepatic arterial port systems for treatment of liver metastases: factors affecting patency and adverse events. J Vasc Interv Radiol. 2004;15:825–33.
Koganemaru M, Edamitsu O, Nonoshita M, Baba K, Hayabuchi N. Implantation of the intra-arterial port-catheter system via the femoral artery using a 2.7F double spiral coaxial catheter. Jpn J Interv Radiol. 2002;17:350–4.
Seki H, Ozaki T, Takaki S, Ooi H, Oda J, Shiina M. Using slow-infusion MR arteriography and an implantable port system to assess drug distribution at hepatic arterial infusion chemotherapy. Am J Roentgenol. 2003;180:681–6.
Irie T. Intraarterial chemotherapy of liver metastases: implantation of a microcatheter-port system with use of modified fixed catheter tip technique. J Vasc Interv Radiol. 2001;12:1215–8.
AFNOR (Association Française de Normalisation). Surgical implants. Implantable catheter chambers. Intravenous, intraarterial, intraperitoneal, intrathecal and epidural use. 1999;NFS94-370.
Tajima T, Yoshimitsu K, Kuroiwa T, Ishibashi T, Irie H, Aibe H, et al. Percutaneous femoral catheter placement for long-term chemotherapy infusions: preliminary technical results. Am J Roentgenol. 2005;184:906–14.
Yamagami T, Yoshimatsu R, Matsumoto T, Nishimura T. Evaluation of non-target arterial patency after implantation of hepatic arterial catheter using a modified implantation technique with the fixed catheter tip method. Clin Radiol. 2009;64:164–70.
Chen Y, He X, Chen W, Lu W, Mei Q, Zeng Q, et al. Percutaneous implantation of a Port-Catheter System using the left subclavian artery. Cardiovasc Interv Radiol. 2000;23:22–5.
Yamagami T, Kato T, Iida S, Tanaka O, Nishimura T. Value of transcatheter arterial embolization with coils and n-butyl cyanoacrylate for long-term hepatic arterial infusion chemotherapy. Radiology. 2004;230:792–802.
Yamagami T, Iida S, Kato T, Tanaka O, Hirota T, Nakamura T, et al. Using n-butyl cyanoacrylate and the fixed-catheter-tip technique in percutaneous implantation of a port-catheter system in patients undergoing repeated hepatic arterial chemotherapy. Am J Roentgenol. 2002;179:1611–7.
Yamagami T, Kato T, Hirota T, Yoshimatsu R, Matsumoto T, White RI Jr, et al. Value of Micronester coils in port-catheter implantation for continuous hepatic arterial infusion chemotherapy with fixed catheter tip method. Eur Radiol. 2008;18:152–7.
Francois P, Vaudaux P, Nurdin N, Mathieu HJ, Descouts P, Lew DP. Physical and biological effects of a surface coating procedure on polyurethane catheters. Biomaterials. 1996;17:667–78.
Watanabe M, Takita W, Tsuchiya M, Otsuka Y, Tamura A, Kaneko H, et al. Hepatic arterial cannulation using the side holed catheter. J Surg Oncol. 2005;91:145–9.
Tanaka T, Arai Y, Inaba Y, Matsueda K, Aramaki T, Takeuchi Y, et al. Radiologic placement of side-hole catheter with tip fixation for hepatic arterial infusion chemotherapy. J Vasc Interv Radiol. 2003;14:63–8.
Deschamps F, Rao P, Teriitehau C, Hakime A, Malka D, Boige V, et al. Percutaneous femoral implantation of an arterial port catheter for intraarterial chemotherapy: feasibility and predictive factors of long-term functionality. J Vasc Interv Radiol. 2010;21:1681–8.
Kuroiwa T, Honda H, Yoshimitsu K, Irie H, Aibe H, Tajima T, et al. Complications encountered with a transfemorally placed port-catheter system for hepatic artery chemotherapy infusion. Cardiovasc Interv Radiol. 2001;24:90–3.
Hirota T, Yamagami T, Tanaka O, Iida S, Kato T, Nakamura T, et al. Brain infarction after percutaneous implantation of port-catheter system via the left subclavian artery. Br J Radiol. 2002;75:799–804.
Ganeshan A, Upponi S, Hon LQ, Warakaulle D, Uberoi R. Hepatic arterial infusion of chemotherapy: the role of diagnostic and interventional radiology. Ann Oncol. 2008;19:847–51.
Conflict of interest
No conflict of interest for any author.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Koganemaru, M., Abe, T., Iwamoto, R. et al. Hepatic arterial infusion chemotherapy with a coaxial reservoir system using a non-braided spiral tip microcatheter. Jpn J Radiol 30, 10–17 (2012). https://doi.org/10.1007/s11604-011-0001-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-011-0001-3