Abstract
Wind-induced soil erosion is a major global misfortune, which obliterates nearly one-third of worldwide soil. The windswept sand particles cover large areas including highways, and make the visibility vague. This results in accidents, damaged infrastructure, delayed flights, and various health issues. The erosive impact of the wind can be minimized by enhancing the intactness of the soil surface. There is a prerequisite to adopt viable measures to strengthen soil against wind erosion. There are certain nature-based solutions that can fortify soil against wind erosion and the application of biopolymers is one of them. The objective of this study is to examine the viability of non-toxic biopolymers for stabilizing desert sand by improving its erosion resistance property and strength. In the present experiment, three biopolymers, sodium alginate (SA), pectin (P), and acacia gum (AG), were used with 1, 2, and 3% concentrations for 1 and 0.75 PV as stabilizing agents. The treatment with biopolymers was performed either by surficial treatment (spraying or pouring of solution) or by mixing and compact method based on the viscosity of prepared biopolymer solutions. The biotreated sand samples were tested in a wind tunnel at varying wind speeds of 10, 20, and 30 m/s to assess sand erosion. Surface strengths were assessed by measuring compressive strength using a pocket penetrometer. Crust thickness measurement was performed to check the penetration depth of biopolymer solution and binding of sand particles. All three biopolymers with 1% concentration gave a feasible solution for erosion against wind and binding of particles through SEM analysis. SA and P could not be sprayed for 2 and 3% concentrations due to high viscosity. This solution is also not feasible for the field application. Simultaneously, AG with 2 and 3% concentration was highly soluble, less viscous, and gave more surface strength due to higher percentage of biopolymer concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The desert is an unproductive land area with scanty rainfall. Environment of desert is antagonistic for animal and plant survival. The Indian Desert (Thar) is a rolling sandy plain enclosed with sand dunes. It experiences an arid climate. The major portion of the Thar Desert lies in the north-western part of India. The temperature varies from 50 °C in summers to 0 °C in winters along with a high range of temperature differences between day and night. Strong wind carries fine sand and dust particles, resulting in sandstorms in many parts of the desert. The deleterious effects of sandstorms are manifold and lead to major environmental and agricultural issues including crop loss, plant damage and burial, and general soil infertility. Consequently, sandstorms give rise to desertification, environmental pollution, and ecological imbalance (Lemboye et al. 2021). Although there are several ground improvement techniques in trend, still they have various limitations in terms of field application, environment friendliness and cost factor. Thus there is a strong need for renewable, soil strengthening material with a pleasing environmental impact. The recent trend has witnessed an emerging use of sustainable and environment-friendly materials, for soil retrieval process, that reduces negative effects on humans and the environment. Biobased soil quality reclamation methods can be categorized based on the microbial activities and biomaterials to be utilized. These microbial activities include biofilm generation, bio-mineralization, biopolymer and biogas formation, etc. (Choi et al. 2020).
One of the techniques people have used is Microbial-induced calcite precipitation (MICP) for mitigation of wind-induced soil erosion. General ground-improvement practices comprise industrial waste, ashes, agricultural waste, and fibres (Sharma and Satyam 2021). The MICP method has advancement over other ground improvement practices due to its better prospective to reinforce soil particles (Dagliya et al. 2022a; Sharma et al. 2021, 2022). Despite the lead, MICP has various limitations in terms of transportation, cultivation, fixation performance of bacteria and ammonium chloride as a by-product (Chang et al. 2020). Also, MICP is time-consuming method in comparison with biopolymers.
Biopolymers are renewable, carbon-neutral, sustainable, green, and environment friendly materials in every way since they are prepared from ever-available crops (Ayeldeen et al. 2016). There are varieties of biopolymers, either produced by microorganisms, plants, animals, or laboratory made. Biopolymers allies components like sugar, amino acid, and oil displayed unique properties. Industries, namely oil extraction, textile mills, construction companies, cosmetics, pharmaceuticals, and food preservation, consume different types of biopolymers due to its trussing characteristic. The unification of biopolymers with the soil particles blocks the voids resulting in elements binding. Thus biopolymers can be used to enhance strength (Fatehi et al. 2018; Reddy et al. 2021) and erosion resistance (Almajed et al. 2020; Fatehi et al. 2019; Mahamaya et al. 2021; Refaei et al. 2020). It also reduces the compressibility (Burra et al. 2019) and permeability of soil (Chang et al. 2016). Most of the studies have been performed using sodium alginate, xanthan gum, gellan gum, and guar gum to improve geotechnical engineering properties of the soil (Choi et al. 2020). Furthermore, studies have shown that wind erosion can be controlled by stabilizing soil using biopolymers (Alsanad and Kavazanjian 2011; Fatehi et al. 2019). Ayeldeen et al. (2018), Lemboye et al. (2021) conducted a study on resistance to wind-induced sand erosion using biopolymers. The result of study indicated that wind-induced sand erosion can be controlled using biopolymers. However, the effect of biopolymer on surface strength of sand samples was rarely discussed.
The purpose of the present study is to investigate the efficacy of biopolymers in suppressing wind-induced erosion. Further, the influence of biopolymer on surface strength is also quantified. In the present study, sodium alginate, pectin, and acacia gum have been used as binding agents. The desert sand was mixed with 1, 2, and 3% biopolymers of 1 and 0.75 PV. Treated specimens were tested for wind tunnel, surface strength, and thickness formation. SEM analysis was performed for micro-characterization and interpretation of results. The novelty of the study was to examine effect of pore volume against the varying percentages of biopolymer on biotreated sand samples. Wind tunnel testing up to 30 m/s wind speed and surface strength measurement test were also conducted on biotreated sand samples.
Materials and methods
Sand properties
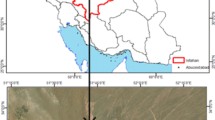
Figure 1 shows the site of sand dune from where the sand sample was collected for current study. The sand dune is located in the Tinwari village (26.584715 °N and 72.814687 °E) near Jodhpur city of Rajasthan, India. Figure 2 shows the grain size distribution curve of desert sand. As per the Indian standard classification system (IS 1498–1970, 2002), soil can be classified as poorly graded sand. Table 1 synopsizes various geotechnical engineering properties of the sand sample.
a Location of the sand dune (Dagliya et al. 2022a); b Sand dune site at Tinwari village Rajasthan, India
Properties of biopolymers used
Sodium alginate is a natural polysaccharide. It is a sodium salt form of alginic acid and gum, which is obtained from the cell walls of brown algae with chelation. It is usually white or yellow in colour and available in fibre or coarse powder form. It has been extensively used in the food preservation industry. Also due to its high water retention capacity, it is used in industrial and pharmaceutical fields (Almajed et al. 2020). Pectin is a regular polysaccharide having extreme galacturonic acid. It is a by-product obtained from vegetable and fruit wastes. For commercial purposes, it is made from apple pomace and citrus shells. It has been broadly used as a stabilizer, water binder, and thickener (Martau et al. 2019). Acacia gum is composed of D-galactose, L-arabinose, L-rhamnose, and D-glucuronic acid. It is highly soluble in water and contemplated to be a low glutinous polysaccharide. It has been extensively consumed in the food industry as a preservative, thickener, emulsifier, and gives flavour to food products (Lemboye et al. 2021).
Preparation of biopolymers solution
The biopolymers (Sodium alginate of Himedia, Pectin and Acacia Gum of Sisco research laboratories Pvt. Ltd.) were commercially procured. All biopolymers (in powdered form) were dissolved in water and a solution was prepared. The known weight (equal to the define percentage of pore volume) of biopolymer powder was added to water slowly for the desired concentration. Shaking was carried out to ensure homogeneous solution. The solutions were prepared for 1 and 0.75 PV with 1%, 2% and 3% biopolymer powder. Prepared solutions were either sprayed, poured, or mixed with sand samples depending on viscosity of the prepared solution.
Specimen preparation with different biopolymers
Sand sample of 790 gm was kept in a black coloured, rectangular shaped, microwave safe, plastic throwaway box, which weighed 13.8 gm and had measurements of 150 × 90 × 40 mm. Biopolymer solution was prepared and treatment of sand was performed as per viscosity of prepared solution as shown in Table 2. In case of lower viscosity biopolymer concentration, spraying was performed, whereas in case of average viscosity, pouring of solution was applied, while in case of higher viscosity, mixing and compact process was used to treat sand samples. The specimens were prepared for the wind tunnel laboratory tests and surface strength test including thickness of crust measurement. Three replicas of each combination were prepared to confirm the tests results. The specimens prepared for the surficial application (spraying and pouring of the biopolymer solutions) were prepared by placing the known weight of natural sand in a container. After tapping the sides of the box, top surface has been levelled. Then the prepared biopolymer solution was sprayed or poured directly on the surface. In mix and compact technique, the known weight of sand was placed on a plate for mixing it with biopolymer solution. After hand mixing, the specimen was filled in three layers with 25 times compaction using 850 gm weight hammer. After complete filling and compaction of sand, the top layer was smoothened and levelled. Biotreated samples by all methods were allowed to dry in the laboratory for 14 days (Fig. 3).
Testing setup
Test setup for wind tunnel
Figure 4 shows the wind tunnel setup having total length of 1.5 m. out of which working section was 0.6 m in length and cross section dimension was 0.3 m × 0.3 m. The treated and natural sand sample were tested in the wind tunnel for a 1 min duration at increasing order wind velocities of 10, 20, and 30 m/s to calculate wind erosion resistance (Dagliya et al. 2022b; Miao et al. 2020). Anemometer was used to validate the wind speed of complete setup (Poulsen et al. 2020). Treated and natural sand samples were placed and fixed in the test section before starting the test (Wang et al. 2018). Sand loss was evaluated by mass variance and visual action. Mass loss was recorded by finding difference between pre- and post-weighing of the tested sample (Fattahi et al. 2020).
Wind tunnel laboratory setup (Dagliya et al. 2022b)
Surface strength test
Figure 5a shows the pocket penetrometer, used to assess surface strength of the biotreated sand samples. It can be used in the laboratory as well as in the field to measure penetration resistance of the surface of biotreated sand sample. The gadget is extensively consumed as it imparts immediate outcomes and is easy to operate (Cheng and Cord-Ruwisch 2012; Fick et al. 2020; Kou et al. 2020; Omoregie et al. 2018). However, constraint of the device is its range of gauging strength which is 0–4.5 kg/cm2. The least count of the device is 0.25 kg/cm2. To compute the surface strength (unconfined compressive strength) of the biotreated sample, device was punched at five different locations (see Fig. 5b) to check uniformity in treatment (Dagliya et al. 2022b).
a Pocket penetrometer used to measure surface strength; b Surface strength measurement at five positions (Dagliya et al. 2022b)
SEM images of biotreated sand particles
SEM tests were performed to examine the binding matrix of biotreated sand particles. Natural sand and biotreated sand with 1% biopolymer were chosen to study surface morphology. After wind tunnel testing, the sample was accumulated from surface and kept in an oven at 105 °C temperature for 1 day. Completely desiccated sample was crushed to fine powder and used for SEM analysis. For obtaining SEM images, the samples were coated with gold sputter and pictures were taken at different beam intensities.
Results and discussions
Observation through surface images
Biotreated sand samples were observed visually, pre- and post-wind tunnel testing. Figure 6 shows some selected images of natural and biotreated sand samples for 1 PV. Representative images were selected, that include all three treatment methods and biopolymers, to display comparison. Rest of the images were presented in the Appendix as supplementary figures. It was observed from images that untreated sand blows off from the surface, while no changes were perceived in biotreated sand samples for all proportions. Biotreated sand with all combinations and methods of treatment gave almost 100% result in terms of resistance to sand erosion, even at wind speed up to 30 m/s. This result was consistent with observation of Ayeldeen et al. (2018). Increase in biopolymer percentage enhanced trussing property of sand and reduced sand erosion. Also, none of the biopolymer treated soil samples showed any crack development on the surface. It was noted from images that in the pouring method black spots (fungus) were observed on the treated surface, which exhibited that biopolymer solution did not penetrate deeply and dried on the surface.
Measurement of soil erosion through wind tunnel testing
Table 3 summarizes the sand loss percentage, post-wind tunnel testing at varied wind speed for all treatment conditions. Sand specimens were weighed pre and post-wind tunnel testing with 0.01 gm accuracy. The mass loss percentage for natural sand were 9.2%, 36% and 59.5% for 10, 20, and 30 m/s wind speed, respectively. It was found that all three biopolymers (SA, P, and AG) minimized wind-induced erosion significantly. Maximum weight loss in percentage with biopolymers was 0.3%, 0. 5% and nil against the wind speed of 30, 20 and 10 m/s, respectively, which is negligible.
A study was performed (Ayeldeen et al. 2018) using different biopolymers, i.e. xanthan gum, guar gum, carrageenan and modified starch at varying percentages to reduce wind-induced sand erosion. It was observed that with an increase in percentage of biopolymer, sand erosion can be reduced. However, biopolymer percentage has its own demarcation and can be increased to a certain limit due to its effect on viscosity. Almajed et al. (2020) demonstrated study on sand erosion control against wind speed up to 58.32 km/h and found that EICP (1:0.67) with SA 1% gave highest results in terms of surface strength and thickness formation.
Lemboye et al. (2021) performed study to control sand erosion up to 16.2 m/s wind speed using biopolymers and shown similar results. Experimental work was performed by (Mahamaya et al. 2021) to stabilize fly ash and coal mine overburden soil using xanthan gum, guar gum and carboxymethyl cellulose, and results shown that 1% of biopolymer could mitigate soil erosion and SEM results were also consistent with this study.
Crust thickness measurement
To examine the effectiveness of biopolymers treatment, crust formation test of the samples was performed. After wind tunnel testing, all samples of different proportions were removed from the container and were tested for crust thickness formation. Figure 7 displays crust thickness formation of treated samples with different percentage and pore volumes. Table 3 summarizes the thickness formation for all treatment conditions. To comprehend penetration and bonding of biopolymers with sand, study was performed to measure thicknesses formation as well as surface strength of the sand. It was observed from thickness measurement test that an increase in biopolymer percentage resulted in high viscosity due to which they were neither able to penetrate and nor mixed with the soil. Although surface strength was found good in mix and compact method with 100% thickness formation, but the process was not suitable for field application, and hence, spray method should be considered for surface treatment.
Figure 7 depicts that crust formation of biotreated samples SA11S, SA10.75S, SA21P, SA20.75P, P11S, P10.75S, P21P, P20.75P, AG10.75S, and AG20.75S was poor and the crust broke during reclamation and handling, while samples SA31M, SA30.75 M, P31M, P30.75 M AG11S, AG21S, AG31S, and AG30.75S were eventually harder and remained intact during reclamation and handling.
Surface strength test
Figure 8 displays the unconfined compressive strength (UCS) for all treatment condition. In present study, pocket penetrometer was used to quantify surface strength of biotreated sand samples. Surface strength was measured at five different positions to examine the variability in treatment (if any). The maximum strength was found for SA and P with 3% biopolymer solution using mix and compact method. Treatment with AG using 3% biopolymer with the spraying method also provided significant strength. Average surface strength value for pouring method was lower in comparison with other two methods due to lack of penetration in solution. Average surface strength for SA11S, SA21P, and SA31M was 102.96, 49.03, and 313.81 kPa, respectively. Compressive strength which was measured at five different positions was varied more with SA, compared to other two biopolymers. It was observed in case of AG bioploymer that surface strength enhanced with increase in the percentage of biopolymer and also variation in surface strength at different locations were less.
Figure 8 also shows the minimum and maximum value of MICP-treated sand samples in dotted line, which was adopted from previous study (Dagliya et al. 2022b). MICP treatment was conducted with minimum 5 days and maximum 20 days treatment cycle. It was observed that both minimum and maximum values were higher in case of biopolymer-treated specimen compared to MICP. Also MICP expended consistent 20 day’s treatment while biopolymer required single treatment only.
Micro-scale characterization analysis
SEM analysis was performed for natural and biotreated sand samples to observe pore structure. Figure 9a displays the image of natural desert sand. A clearly visible gap between particles can be observed. This suggests dearth of bonding between particles and also lack of shear resistance against wind-induced shear stress. Figure 9b shows SEM images of the biopolymer samples with 1% biopolymer concentration prepared using spraying method. It illustrated that biopolymer tends to fill the vacuums between sand particles, thereby enhanced bonding between them. This bonding helped to enhance resistance to wind-induced stresses. The spraying method can be used more easily in the field in comparison with the pouring method and mix and compact method.
Conclusion
The current study explores the utilization of distinct types of biopolymers at various proportions of pore volumes for stabilizing desert sand. The biotreated samples were examined for visual observations, wind erosion resistance, crust thickness measurements, unconfined compressive strength, and SEM analysis. The subsequent conclusion can be depicted from the current experiment study:
1. The penetration depth of biopolymer solutions fluctuated due to the viscosity of the solutions. Solutions prepared using AG (ranging from 1 to 3% biopolymer concentration) were able to penetrate easily due to low viscosity. Conversely, solutions prepared by SA and P (ranging from 1 to 3% biopolymer concentration) depicted resistance to penetration due to high viscosity. These observations will help to narrow down the selection of appropriate biopolymer for stabilizing desert sand.
2. Results from wind tunnel testing revealed that untreated sand had loss of 9.2, 36 and 59.5% at the wind speed of 10, 20, and 30 m/s, respectively. In contrast, the percentage of sand loss in case of biotreated sand samples were almost negligible. This was mainly an outcome of binding between particles, due to the presence of biopolymer (as visible from SEM images).
3. Maximum surface strength was observed in 3% concentration for all biopolymers. It should be noted that biotreatment performed with AG biopolymer, using the spraying method, was more feasible for field application than the other method (mix and compact method).
Data availability
Data are available upon reasonable request.
References
Almajed A, Lemboye K, Arab MG, Alnuaim A (2020) Mitigating wind erosion of sand using biopolymer-assisted EICP technique. Soils Found 60:356–371. https://doi.org/10.1016/j.sandf.2020.02.011
Alsanad A, Kavazanjian E (2011) Novel biopolymer treatment for wind induced soil erosion. Diss Arizona State Univ. https://doi.org/10.1007/s13398-014-0173-7.2
Ayeldeen M, Negm A, El Sawwaf M, Gädda T (2018) Laboratory study of using biopolymer to reduce wind erosion. Int J Geotech Eng 12:228–240. https://doi.org/10.1080/19386362.2016.1264692
Ayeldeen MK, Negm AM, El Sawwaf MA (2016) Evaluating the physical characteristics of biopolymer/soil mixtures. Arab J Geosci 9:1–13. https://doi.org/10.1007/s12517-016-2366-1
Burra SG, Kolay PK, Kumar S, Puri VK (2019) Filler-stabilized xanthan gum for soil improvement justin Geo-Congress 2019 143–51
Chang I, Im J, Cho GC (2016) Geotechnical engineering behaviors of gellan gum biopolymer treated sand. Can Geotech J 53:1658–1670. https://doi.org/10.1139/cgj-2015-0475
Chang I, Lee M, Tran ATP, Lee S, Kwon YM, Im J et al (2020) Review on biopolymer-based soil treatment (BPST) technology in geotechnical engineering practices. Transp Geotech 24:100385. https://doi.org/10.1016/j.trgeo.2020.100385
Cheng L, Cord-Ruwisch R (2012) In situ soil cementation with ureolytic bacteria by surface percolation. Ecol Eng 42:64–72. https://doi.org/10.1016/j.ecoleng.2012.01.013
Choi SG, Chang I, Lee M, Lee JH, Han JT, Kwon TH (2020) Review on geotechnical engineering properties of sands treated by microbially induced calcium carbonate precipitation (MICP) and biopolymers Constr Build Mater https://doi.org/10.1016/j.conbuildmat.2020.118415.
Dagliya M, Satyam N, Garg A (2022a) Experimental Study on optimization of cementation solution for wind-erosion resistance using the MICP method 1–17
Dagliya M, Satyam N, Sharma M, Garg A (2022b) Experimental study on mitigating wind erosion of calcareous desert sand using spray method for MICP. J Rock Mech Geotech Eng. https://doi.org/10.1016/j.jrmge.2021.12.008
Fatehi H, Abtahi SM, Hashemolhosseini H, Hejazi SM (2018) A novel study on using protein based biopolymers in soil strengthening. Constr Build Mater 167:813–821. https://doi.org/10.1016/j.conbuildmat.2018.02.028
Fatehi H, Bahmani M, Noorzad A (2019) Strengthening of dune sand with sodium alginate biopolymer 157–66 https://doi.org/10.1061/9780784482117.015
Fattahi SM, Soroush A, Huang N (2020) Biocementation control of sand against wind erosion. J Geotech Geoenvironmental Eng 146:04020045. https://doi.org/10.1061/(asce)gt.1943-5606.0002268
Fick SE, Barger N, Tatarko J, Duniway MC (2020) Induced biological soil crust controls on wind erodibility and dust (PM10) emissions. Earth Surf Process Landforms 45:224–236. https://doi.org/10.1002/esp.4731
Kavazanjian E, Iglesias E, Karatas I (2009) Biopolymer soil stabilization for wind erosion control In Proceedings of the 17th International Conference on Soil Mechanics and Geotechnical Engineering (Vols 1, 2, 3 and 4) (pp 881–884) IOS press
Kou HL, Wu CZ, Ni PP, Jang BA (2020) Assessment of erosion resistance of biocemented sandy slope subjected to wave actions. Appl Ocean Res 105:102401. https://doi.org/10.1016/j.apor.2020.102401
Lemboye K, Almajed A, Alnuaim A, Arab M, Alshibli K (2021) Improving sand wind erosion resistance using renewable agriculturally derived biopolymers. Aeolian Res 49:100663. https://doi.org/10.1016/j.aeolia.2020.100663
Mahamaya M, Das SK, Reddy KR, Jain S (2021) Interaction of biopolymer with dispersive geomaterial and its characterization: an eco-friendly approach for erosion control. J Clean Prod 312:127778. https://doi.org/10.1016/j.jclepro.2021.127778
Martau GA, Mihai M, Vodnar DC (2019) The use of chitosan, alginate, and pectin in the biomedical and food sector-biocompatibility, bioadhesiveness, and biodegradability. Polymers (basel). https://doi.org/10.3390/polym11111837
Miao L, Wu L, Sun X, Li X, Zhang J (2020) Method for solidifying desert sands with enzyme-catalysed mineralization. Land Degrad Dev 31:1317–1324. https://doi.org/10.1002/ldr.3499
Omoregie AI, Siah J, Pei BCS, Yie SPJ, Weissmann LS, Enn TG et al (2018) Integrating biotechnology into geotechnical engineering: a laboratory exercise. Trans Sci Technol 5:76–87
Poulsen TG, Cai W, Garg A (2020) Water evaporation from cracked soil under moist conditions as related to crack properties and near-surface wind speed. Eur J Soil Sci 71:627–640. https://doi.org/10.1111/ejss.12926
Reddy NG, Nongmaithem RS, Basu D, Rao BH (2021) Application of biopolymers for improving the strength characteristics of red mud waste. Environ Geotech. https://doi.org/10.1680/jenge.19.00018
Refaei M, Arab MG, Omar M (2020) Sandy Soil Improvement through Biopolymer Assisted EICP 612–9. https://doi.org/10.1061/9780784482780.060
Sharma M, Satyam N (2021) Strength and durability of biocemented sands : wetting-drying cycles, ageing effects, and liquefaction resistance. Geoderma 402:115359. https://doi.org/10.1016/j.geoderma.2021.115359
Sharma M, Satyam N, Reddy KR (2022) Large-scale spatial characterization and liquefaction resistance of sand by hybrid bacteria induced biocementation. Eng Geol 302:106635. https://doi.org/10.1016/J.ENGGEO.2022.106635
Sharma M, Satyam N, Reddy KR (2021) Hybrid bacteria mediated cemented sand: microcharacterization, permeability, strength, shear wave velocity, stress-strain, and durability. Int J Damage Mech. https://doi.org/10.1177/1056789521991196
Wang Z, Zhang N, Ding J, Lu C, Jin Y (2018) Experimental study on wind erosion resistance and strength of sands treated with microbial-induced calcium carbonate precipitation. Adv Mater Sci Eng. https://doi.org/10.1155/2018/3463298
Acknowledgements
The first author is grateful to Prestige Institute of Engineering, Management, and Research, Indore (India), for their support throughout the research work.
Funding
The second and third authors are gratifying to the Department of Science and Technology, Guangdong for the “Overseas famous Teacher project” (Grant No. 2020A1414010268).
Author information
Authors and Affiliations
Contributions
First author executed the experiments and drafted initial manuscript. Second author was responsible for supervision of student and experimental facilities. Third author is responsible for co-supervision of first author, conceptualization and revising manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest with any third party.
Additional information
Edited by Dr. Michael Nones (CO-EDITOR-IN-CHIEF).
Appendix
Appendix






Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dagliya, M., Satyam, N. & Garg, A. Biopolymer based stabilization of Indian desert soil against wind-induced erosion. Acta Geophys. 71, 503–516 (2023). https://doi.org/10.1007/s11600-022-00905-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11600-022-00905-5