Abstract
Lithium-sulfur batteries have promise as next-generation energy storage device due to their high capacity and energy density. However, their wide application is faced with great challenges, in particular the polysulfide migration and poor electronic conductivity during the electrochemical cycles. Herein, we report the employment of metal sulfide MoS2 as catalysts to promote the polysulfide conversion. When used as cathode materials in Li–S batteries, the electrochemical results indicate that the MoS2/S cathode delivers high specific capacity and stable cycling performance. The excellent electrochemical performance is attributed to the presence of the metal sulfide, which could accelerate the polysulfide conversion and improve the whole electronic conductivity. This work provides new insights to enhance the electrochemical performance by using metal sulfide as sulfur matrix for high-performance lithium-sulfur batteries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The new energy storage devices with high energy and power density must be developed for satisfying the increasing energy demands for electric vehicles and other electric devices [1,2,3]. Lithium-sulfur batteries could meet the increasing high energy demand due to their high specific capacity (1675 mAh/g) and energy density (2600 Wh/Kg) [4,5,6]. In addition, the sulfur active cathode materials have following advantages, such as natural abundance and no pollution. Therefore, during the past decades, many researchers have been devoted themselves to study the lithium-sulfur batteries [7,8,9,10,11,12]. Although great improvements have been obtained, there are still problems to inhibit the wide application of the lithium-sulfur batteries [13]. On the one hand, the sulfur active materials suffer from poor electronic conductivity. On the other hand, it has poor polysulfide conversion during the electrochemical cycles due to polysulfide dissolution [14,15,16,17].
To deal with these problems, numerous efforts have been paid to improve the electronic conductivity and the polysulfide conversion. It can be concluded as following aspects: constructing perfect sulfur host structures as sulfur matrix [18], adding polar materials or doping elements [19], protecting lithium anode [20], and other functional interlayers and separators [21]. Overall, these methods make some achievements for improving the electrochemical performance of the lithium-sulfur batteries. However, there are still issues. Therefore, it is urgent to develop new functional host materials to improve the electronic conductivity and accelerating the polysulfide conversion during the electrochemical cycles. Metal sulfides are one of the most promising materials due to their unique structure and properties. And it has been applied in many areas, such as energy storage, catalysts, and other related fields [22]. Therefore, it is necessary to further construct MoS2 structure to increase the active site, which could accelerate the polysulfide conversion.
In this work, we report the employment of metal sulfide MoS2 nanosheets with abundant active sites as catalysts to promote the polysulfide conversion. When used as cathode materials in Li–S batteries, the electrochemical results indicate that the MoS2/S cathode delivers high specific capacity and stable cycling performance. It shows high initial specific capacity of 1536 mAh/g at the current density of 0.1 C, which is much higher than the reported cathode materials. The excellent electrochemical performance is attributed to the presence of the metal sulfide, which could accelerate the polysulfide conversion and improve the whole electronic conductivity. This work provides new insights to enhance the electrochemical performance by using metal sulfide as sulfur matrix for high-performance lithium-sulfur batteries.
Experimental
Preparation of MoS2/S composites
Typically, 0.8 g Na2MoO4·2H2O (99.9%, Aldrich) and 0.6 g thioacetamide (99.9%, Sigma) were dissolved in the ethanol. And then the mixture was transferred into high pressure reactor and then maintained at 180 °C for 12 h. After cooling to the room temperature, the samples were washed by using deionized water for three times and dried at 60 ℃ for 12 h. After that, the samples were heated with sublimed sulfur (Alfa Aesar) with ratio of 1:3 at 155 ℃ for 12 h to prepare MoS2/S composites.
Materials characterization
The morphology and microstructure of the samples are carried out by a scanning electron microscope (SEM, Tescan Mira 3). The crystal and phase compositions are performed on powder X-ray diffraction (XRD, Rigaku-mini Flex600) and Raman spectra (YT-LM). Brunauer Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) methods are applied to calculate the specific surface and the pore size distribution of samples.
Electrochemical measurement
The as-obtained MoS2/S composites with poly(vinylidene fluoride) (PVDF) binder and Ketjen black (KB) with a weight ratio of 8:1:1 are dispersed in N-methyl pyrrolidone (NMP) to form a uniform slurry and coated on Al foils and then placed in a vacuum drying oven at 70 ℃ for 24 h. Similarly, the pure S cathode was prepared by mixing sublimed sulfur (Alfa Aesar), KB, and PVDF with weight ratio of 7:2:1 in NMP and coated on Al foils. The electrode was subsequently punched out a circular disk with a 15 mm diameter. The sulfur loading is ~ 1.6 mg cm−2. Celgard 2400 is used as separator and Li metal acts as negative electrode. The electrolyte consists of 1 M lithium bis(trifluoromethane sulfonyl) imide (LiTFSI) in DOL: DME (1:1 by volume) solution with 1 wt% LiNO3. The electrolyte/S mass ratio is about 5:1. The charge–discharge test with voltage from 1.5 to 3.0 V is performed on a Neware multichannel battery tester at room temperature. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) are conducted on CHI660E electrochemical workstation.
Results and discussion
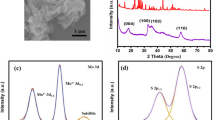
The crystal structure of all samples was characterized by using X-ray diffraction. As shown in Fig. 1, the pure MoS2 samples show typical diffraction peaks, which is corresponding to the crystal planes, which is according to the reported literatures [23]. Furthermore, it can be seen that the as-prepared MoS2/S composites exhibit diffraction peaks of the element sulfur, confirming the successful immersion of sulfur into the MoS2 samples. This also indicates the preparation of the MoS2/S composites via the heating method.
To investigate the pore structure of the samples, N2 adsorption/desorption was conducted. As shown in Fig. 2a, it can be seen that the MoS2 samples exhibit typical mesoporous structure. This can be judged by the shape of the N2 adsorption/desorption curve. It is a typical IV type hoop for the MoS2 samples, indicating the presence of the mesoporous structure [24]. The mesoporous structure is beneficial for the sulfur immersion during the heating process. In addition, the pore size of the samples is tested. As shown in Fig. 2b, it can be clearly observed that the pore size of the MoS2 samples is about 10 nm, further demonstrating the presence of the mesoporous structure. More importantly, the inset of Fig. 2b shows the SEM image of the MoS2 samples. It can be seen that it displays nanosheet structure with abundant surface active sites.
Furthermore, the electrochemical performance of the samples was tested by using corresponding electrochemical measurements. As shown in Fig. 3a, the CV curves of the MoS2/S cathode have two reduction peaks at 2.3 V and 2.1 V, respectively. These two peaks are corresponding to the multistep electrochemical reaction from sulfur to Li2S. During the discharging process, there are polysulfides in the electrolyte, which could lead to severe shuttle effect. In addition, there is one oxidation peak at 2.5 V, which is attributed to the reversible electrochemical reaction from Li2S to element sulfur. On the other hand, it can be seen that the CV curves overlap well with the increase of the cycle numbers. This indicates the superior cycling stability of the MoS2/S cathode, proving the excellent electrochemical reaction kinetic as cathode in the lithium-sulfur batteries. Furthermore, the constant discharge/charge profiles of the MoS2/S cathode were tested at different rates from 0.1 C to 3 C. As shown in Fig. 3b, it can be seen that the MoS2/S cathode delivers high capacity of 1536 mAh/g at 0.1 C, which is much higher than the other reported cathode materials in the lithium-sulfur batteries. With the increase of the current densities, the MoS2/S cathode still shows high capacity. This confirms the superior electrochemical performance at high current densities, which is attributed to the improved electronic conductivity and enhanced polysulfide conversion.
To further explain the superior electrochemical performance of the MoS2/S cathode, EIS was conducted. As shown in Fig. 4a, the as-prepared MoS2/S cathode exhibits much smaller resistance than the pure S cathode. This can be observed from the diameter of the semicircle in the high-frequency region. The diameter of the semicircle represents the charge transfer resistance of the electrode. The much bigger diameter of the semicircle, the higher charge transfer resistance in the electrode. Encouraged by the high specific capacity, long-term cycling stability of the MoS2/S cathode was tested at 2 C for 300 cycles. As shown in Fig. 4b, there is a capacity value of 902 mAh/g after 300 cycles at high current density of 2 C, demonstrating excellent cycling performance. The excellent electrochemical performance is ascribed to the presence of the MoS2 matrix for the element sulfur. On the one hand, it can enhance the electronic conductivity of the whole cathode. On the other hand, the polysulfide conversion kinetic can be greatly accelerated. Therefore, the MoS2/S cathode shows excellent electrochemical performance.
To further demonstrate the superior performance of the MoS2/S cathode, rate capabilities were tested. As shown in Fig. 5a, the as-prepared MoS2/S cathode delivers capacities of 1512, 1408, 1316, 1126, 965, and 816 mAh/g at 0.1, 0.2, 0.5, 1, 2, and 3 C, respectively, demonstrating excellent rate performance with high current densities. This is attributed to the improved electronic conductivity and enhanced polysulfide conversion. Figure 5b shows the schematic illustration of the MoS2 nanosheet for accelerating the polysulfide conversion. It can be seen that the MoS2 nanosheet could provide sufficient surface area as active sites for accelerating the polysulfide conversion. This is related to the catalysts effect of the MoS2 nanosheet, which could enhance the polysulfide conversion kinetic during the electrochemical cycles. Furthermore, the long-term cycling performance of MoS2/S cathode with high S loading of 3.6 mg cm−2 was tested at 1 C for 300 cycles. As shown in Fig. 5c, it can be seen that the MoS2/S cathode still has capacity of 716 mAh/g, indicating excellent cycling performance under high S loading.
Conclusion
In conclusion, we report the employment of metal sulfide MoS2 as catalysts to promote the polysulfide conversion. The MoS2 samples show perfect crystal structure and morphology, which is beneficial for the use in the lithium-sulfur batteries. When used as cathode materials in Li–S batteries, the electrochemical results indicate that the MoS2/S cathode delivers high specific capacity and stable cycling performance. It shows high initial specific capacity of 1536 mAh/g at the current density of 0.1 C, which is much higher than the reported cathode materials. The excellent electrochemical performance is attributed to the presence of the metal sulfide, which could accelerate the polysulfide conversion and improve the whole electronic conductivity. This work provides new insights to enhance the electrochemical performance by using metal sulfide as sulfur matrix for high-performance lithium-sulfur batteries.
References
Xiong ZS, Li JH, Sun YJ, Lin YX, Du L, Wei ZG, Wu M, Shi KX (2022) Fe3C@NCNT as a promoter for the sulfur cathode toward high-performance lithium-sulfur batteries. J Alloys Compd 899:163245
Hou LP, Yao LY, Bi CX, Xie J, Li BQ, Huang JQ, Zhang XQ (2022) High-valence sulfur-containing species in solid electrolyte interphase stabilizes lithium metal anodes in lithium–sulfur batteries. J Energy Chem 68:300–305
Wei ZZ, Zhang NX, Feng T, Wu F, Zhao T, Chen RJ (2022) A copolymer microspheres-coated separator to enhance thermal stability of lithium-sulfur batteries. Chem Eng J 430:132678
Qian M, Tang YK, Liu L, Gao Y, Li XH (2022) Well-dispersed Li2CoTi3O8 nanoparticles as a multifunctional material for lithium-ion batteries and lithium-sulfur batteries. J Alloys Compd 896:162926
Liu FR, Wang N, Shi CS, Sha JW, Ma LY, Liu EZ, Zhao NQ (2022) Phosphorus doping of 3D structural MoS2 to promote catalytic activity for lithium-sulfur batteries. Chem Eng J 431:133923
Ma BQ, Gao YC, Niu MX, Luo M, Li HJ, Bai Y, Sun KN (2021) ZIF-67/Super P modified separator as an efficient polysulfide barrier for high-performance lithium-sulfur batteries. Solid State Ionics 371:115750
Zhang X, Wan TT, Jia AZ, Li JD, Liu GH, Sun DL, Wang YJ (2021) Graphene oxide-wrapped cobalt-doped oxygen-deficient titanium dioxide hollow spheres clusters as efficient sulfur immobilizers for lithium-sulfur batteries. Electrochim Acta 397:139264
Li DS, Liu J, Wang WJ, Li SM, Yang GL, Wang P, Zhu KX, Li ZJ (2021) Synthesis of porous N deficient graphitic carbon nitride and utilization in lithium-sulfur battery. Appl Surf Sci 569:151058
Cai DP, Zhuang YX, Fei B, Zhang CQ, Wang YG, Chen QD, Zhan HB (2022) Self-supported VN arrays coupled with N-doped carbon nanotubes embedded with Co nanoparticles as a multifunctional sulfur host for lithium-sulfur batteries. Chem Eng J 430:132931
Li JX, Dai LQ, Wang ZF, Wang H, Xie LJ, Chen JP, Yan C (2022) Cellulose nanofiber separator for suppressing shuttle effect and Li dendrite formation in lithium-sulfur batteries. J Energy Chem 67:736–744
Gu JH, Shi CY, Li ZY, Liu FY, Huang ZY, Hong B, Lai YQ (2022) Lithiated 3, 6-dioxa-1, 8-octane dithiol as redox mediator to manipulate polysulfides conversion for high-performance lithium-sulfur batteries. Chem Eng J 432:134379
Bertolini S, Jacob T (2022) Sulfurized-polyacrylonitrile in lithium-sulfur batteries: interactions between undercoordinated carbons and polymer structure under low lithiation. J Energy Chem 66:587–596
Gao XG, Huang Y, Sun XY, Batool S, Li TH (2022) Nanopolyhedron Co–C/Cores triggered carbon nanotube in-situ growth inside carbon aerogel shells for fast and long-lasting lithium–sulfur batteries. J Power Sources 520:230913
Zhang F, Niu TY, Wu FC, Wu LL, Wang GR, Li JD (2021) Highly oriented MIL-101(Cr) continuous films grown on carbon cloth as efficient polysulfide barrier for lithium-sulfur batteries. Electrochim Acta 392:139028
Li SL, Zhang WJ, Liu JD, Zhang YR, Zheng YH (2021) Maximizing catalytically active surface gallium for electrocatalysis of lithium polysulfides in lithium-sulfur batteries by silica@gallium core–shell particles. Appl Surf Sci 563:150381
Liu ZH, Lian RQ, Wu ZR, Li YJ, Lai XY, Yang S, Ma X (2022) Ordered Dual-Channel carbon embedded with molybdenum nitride catalytically induced High-Performance Lithium-Sulfur battery. Chem Eng J 431:134163
Yang LW, Li Y, Wang Y, Li Q, Chen YX, Zhong BH, Guo XD, Wu ZG (2021) Nitrogen-doped sheet VO2 modified separator to enhanced long-cycle performance lithium-sulfur battery. J Power Sources 501:230040
Zhang XM, Li GR, Zhang YG, Luo D, Yu AP, Wang X, Chen ZW (2021) Amorphizing metal-organic framework towards multifunctional polysulfide barrier for high-performance lithium-sulfur batteries. Nano Energy 86:106094
Song YW, Qin JL, Zhao CX, Zhao M, Hou LP, Peng YQ, Peng HJ, Li BQ (2022) The formation of crystalline lithium sulfide on electrocatalytic surfaces in lithium–sulfur batteries. J Energy Chem 64:568–573
Xue CH, Yue C, Yuan L (2021) A simple, robust and fast method for embedding sulfur nanoparticles in Ti3C2Tx MXene as stable lithium-sulfur batteries cathodes. J Alloys Compd 886:161152
Li J, He L, Qin FR, Fang J, Hong B, Lai YQ (2021) Dual-enhancement on electrochemical performance with thioacetamide as an electrolyte additive for lithium-sulfur batteries. Electrochim Acta 376:138041
Liu YY, Cui CJ, Liu Y, Liu W, Wei J (2020) Application of MoS2 in the cathode of lithium sulfur batteries. RSC Adv 10:7384–7395
Dong Q, Zhang FL, Ji S, Wang XY, Wang H, Linkov V, Wang RF (2021) Fe3C-inserted “tube plugging into porous network” nanohybrids as advanced sulfur hosts for lithium-sulfur batteries. J Alloys Compd 877:160286
Kang H, Park MJ (2021) Thirty-minute synthesis of hierarchically ordered sulfur particles enables high-energy, flexible lithium-sulfur batteries. Nano Energy 89:106459
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y. Metal sulfide as catalysts enabling fast polysulfide conversion for high electrochemical performance Li–S batteries. Ionics 28, 2227–2231 (2022). https://doi.org/10.1007/s11581-022-04493-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-022-04493-8