Abstract
In this manuscript, carbon quantum dots-reduced graphene oxide (CQDs-rGO) nanocomposite was modified on the surface of glass carbon electrode (GCE) via an one-step co-deposition method to fabricate an effective electrochemical senor for the simultaneous detection of ascorbic acid (AA), dopamine (DA), and uric acid (UA). Due to the strong synergetic effect between CQDs and rGO, the sensor exhibited good selectivity and high catalytic activity toward AA, DA, and UA. The peak potential differences of simultaneous detection for AA-DA, DA-UA, and AA-UA were calculated as 0.147 V, 0.135 V, and 0.282 V, respectively. And the linear responses of AA, DA, and UA were found in the ranges of 0.2–0.9 mM (R2 = 0.99) and 1.4–4.2 mM (R2 = 0.99), 5–9 μM (R2 = 0.98) and 11–81 μM (R2 = 0.99), and 20–90 μM (R2 = 0.99) and 140–300 μM (R2 = 0.99), respectively. The as-prepared sensor contributed a fresh idea to the simultaneous detection of AA, DA, and UA, and has a broad prospect in practical application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ascorbic acid (AA), dopamine (DA), and uric acid (UA) are three vital compounds coexisting in human physical fluid, which play an irreplaceable role in maintaining the normal life function of human body.
Ascorbic acid (AA), also known as vitamin C, is a kind of water-soluble antioxidant with a daily demand of 100 mg [1], which can only be obtained from food and drugs. Under normal conditions, AA remains a level of about 100 μM [2] in the extracellular fluid of the central nervous system. AA has significant influences on the life processes of cell division, tissue growth, collagen synthesis, amino acid metabolism, and gene expression [3,4,5]. The long-term deficiency of AA in human body can be related to the occurrence of scurvy, cold, atherosclerosis, infertility, liver disease, and cancer [6,7,8].
As an essential information transmitting substance, dopamine (DA) is tightly linked to the brain behaviors of motivation, cognition, emotion, memory, and reward [9,10,11]. The concentration of DA in human brain fluid is reported to be 0.01–1 μM [12], and the abnormal levels of DA will cause great damage to the nervous system of human body and then lead to a variety of related diseases, such as Parkinson’s disease, depression, and Schizophrenia [13, 14].
Uric acid is the end product of purine metabolism with a concentration range of 200–430 μM [15] in physical fluid, and its disordered metabolism may result in gout and pneumonia [16, 17].
Since AA, DA, and UA coexist in biological matrixes and have important impacts on human health, the simultaneous detection of them is highly desired for the prevention and diagnosis of diseases. In recent decades, electrochemical detection methods have drawn extensive attention of researchers owing to their advantages of good sensitivity, high selectivity, low cost, and simultaneous detection ability toward multiple analytes.
A large number of studies have shown that there exists a synergetic effect between nanomaterials, and the combination of nanomaterials through appropriate methods can effectively elevate the electrochemical performance of the modified electrode. Graphene is a kind of hexagonal honeycomb carbon nanomaterial with a thickness about 0.335 nm, which is composed of a single layer of sp2-bonded carbon atoms. The unique structure of graphene enables it to have strong stability, huge specific surface area, and high conductivity. Unfortunately, the poor water solubility of graphene makes it easy to agglomerate between layers [18], which greatly limits its application in the field of electrochemical sensing. In order to solve this problem, researchers mainly use electrodeposition method to reduce the graphene oxide (GO) to prepare graphene (also known as reduced graphene oxide (rGO)), while electrodeposition method can effectively control the amount of oxygen-containing groups on the surface of GO during reduction that makes the prepared rGO possesses good water solubility and conductivity. Up to now, rGO prepared by electrochemical reduction has been used in the electrochemical detection of paroxetine, hydrogen peroxide, mercury ion, glucose, levofloxacin, and so on [19,20,21,22,23,24].
Carbon quantum dots (CQDs) are a new type of carbon nanomaterials with a spherical-like structure and a particle size less than 10 nm that have attracted much attention of researchers in recent years. Due to its easy preparation, non-toxic, and strong fluorescence characteristics, CQDs have been widely used in the fields of cell imaging, drug delivery, gene delivery, and fluorescence sensor [25,26,27]. In addition, CQDs also have good dispersion, excellent biosafety, and strong electrochemical properties, which make them have extensive applications in the field of electrochemical sensors. To date, CQDs have been used in the eletrochemical detection of patulin, metanilyellow, curcumin, ATP-related physiological phosphates, anti-HIV drug, heavy metal ion, and so on [28,29,30,31,32,33,34].
Based on the synergetic effect between CQDs and rGO, this paper provided a simple and effective method to prepare high-performance electrochemical sensor. Firstly, CQD solution was prepared by microwave method of glucose, polyethylene glycol-200, and ultrapure water, and then it was fully mixed with GO solution by ultrasound at a volume ratio of 1:1. After that, CQDs and GO were co-deposited on the surface of glassy carbon electrode (GCE) by cyclic voltammetry (CV) to construct CQDs-rGO/GCE electrochemical sensor for simultaneous detection of AA, DA, and UA. Since CQDs contain a large number of negative functional groups that can absorb positive DA and repel negative AA and UA, and rGO has strong conductivity that can greatly promote the oxidation of AA, DA, and UA, the sensor showed high selectivity and catalytic activity for AA, DA, and UA.
Experimental
Reagents
Ascorbic acid (AA), dopamine hydrochloride (DA), and uric acid (UA) were provided by Lianxing Biotechnology (Tianjin), Sigma-Aldrich (Shanghai), and Tianjin Ron Chemical Reagent Company, respectively. Glucose, potassium chloride (KCl), potassium ferricyanidesodium, dihydrogen phosphate (NaH2PO4), and disodium hydrogen phosphate (Na2HPO4) were obtained from Tianjin Chemical Reagent Supply and Marketing company, and polyethylene glycol-200 was obtained from Beijing Solarbio Science & Technology, and graphene oxide (GO) was purchased from Xianfeng Nano Materials Technology (Nanjing). The water used in this paper is ultrapure water (18.20 MΩ).
Apparatus
AMETEK PARSTAT 4000 electrochemical workstation was provided by AMETEK Commercial Enterprise (Shanghai), Beijing Branch. All experiments were carried out based on a three-electrode system, which consist of CQDs-rGO/GCE (working electrode, WE), platinum electrode (counter electrode, CE), and saturated calomel electrode (reference electrode, RE). Field emission scanning electron microscopy (FESEM) images were obtained from Nova NanoSEM 430 (FEI, USA). High-resolution electron microscopy (HRTEM) and energy dispersive spectrometer (EDS) micrographs were acquired by using FEI Talos F200X equipped with an energy-dispersive spectrometer analyzer. The X-ray diffraction (XRD) patterns were recorded using a Rigaku D/max-rA with Cu Kαradiation (λ = 1.5418 Å) on a diffractometer (Rigaku, Japan). Microwave reactor, 100–500 D dialysis bags, LX-400 centrifuge, and Magnetic stirrer were obtained from LG Electronics Beijing Solarbio Science & Technology, Kylin-Bell Lab InstruMents, and Ronghua instrument manufacture (Jiangsu, China), respectively. Graphene oxide solution was prepared by ultrasonic cleaner (Kunshan Ultrasonic Instrument, Jiangsu). The parameters of DPV experiments in this paper were set as follows: peak 1 potential: − 1.5 V; peak 2 potential: + 1.5 V; pulse width: 0.2 s; amplitude: 50 mV.
Fabrication of electrochemical sensor based on CQDs-rGO composite
Preparation of CQD solution
CQDs were synthesized by one-step microwave method [35]. Briefly, the mixed solution containing 4 g of glucose, 20 mL of polyethylene glycol-200, and 6 mL of ultrapure water was stirred until colorless, and then the original CQD solution was obtained by microwave under the conditions of medium fire and 3 min. After the original CQD solution was cooled down, 2 mL of the solution was taken into a 100–500 D dialysis bag for 24 h, and then the obtained solution was centrifuged under the condition of 4000 r/min, and the supernatant was collected and stored at 4 °C, and marked as CQD solution.
Preparation of GO solution
GO solution was prepared by mixing 40 mg of GO powder and 40 mL of ultrapure water under ultrasonic condition for 60 min. In order to prevent precipitation, the solution was taken out and shaken for 1 min with every 10 min. The obtained solution was marked as GO solution.
Preparation of CQDs-rGO mixed solution
Twenty milliliters of CQD solution and 20 mL of GO solution were fully mixed under ultrasound condition and marked as CQDs-rGO mixed solution.
Construction of CQDs-rGO/GCE electrochemical sensor
Prior to modification, the bare glass carbon electrode (GCE) was polished with 1 μm, 0.3 μm, and 0.05 μm alumina slurry in turn. Then, the electrode was cleaned by ultrapure water, ethanol, and ultrapure water under ultrasonic condition for 3 min, respectively. After that, the clean GCE was put into the prepared CQDs-rGO mixed solution, and CQDs and GO were co-deposited on the surface of GCE by cyclic voltammetry (CV) to construct CQDs-rGO/GCE electrochemical sensor. Then the sensor was gently rinsed with ultrapure water and dried at room temperature. CV experimental parameters were set as follows: vertex 1 potential: − 1 V; vertex 2 potential: + 2 V; sweep speed: 50 mV s−1; cycle: 20 weeks.
Results and discussion
Characterization of CQDs-rGO nanocomposite
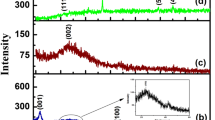
Morphology of CQDs-rGO was characterized by FESEM, HRTEM, XRD, and EDS in Fig. 1. As demonstrated in Fig. 1a, b, a typical thin-layer folded shape of rGO and a near spherical shape of CQDs were obtained, and CQDs were evenly distributed on the surface of rGO that can effectively increase the contact area between the surface of electrode and analytes; therefore, the detection signal of the electrode can be efficiently elevated. From Fig. 1c, the disappearance of a diffraction peak of GO at ~ 10° and the appearance of a diffraction peak at ~ 22° indicated the reductive reaction from GO to rGO. A broad amorphous peak appeared around ~ 25°, which was mainly due to the coexistence of CQDs. Moreover, from Fig. 1d of EDS, the prepared nanocomposite contains a dominant C peak and some weak Cu peak, where C peak should be came from CQDs and rGO, and Cu peaks should be originated from the substrate. The above results proved that CQDs-rGO nanocomposite has been successfully synthesized in this experiment.
Electrochemical characterization
Herein, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were used to analyze the interface properties of different electrodes in 20 mM potassium ferricyanide solution containing 0.1 M KCl. It can be seen from Fig. 2a, the modification of CQDs, rGO, and CQDs-rGO nanocomposite can all help to elevate the detection current of the electrode, and the current reaches the maximum at CQDs-rGO/GCE, which may be related to the strong synergistic effect between CQDs and rGO that can magnify the surface area of the electrode and accelerate the transfer rate of electrons. The parameters of CV experiment were set as follows: peak 1 potential: − 0.6 V; peak 2 potential: + 0.8 V; scanning speed: 50 mV s−1; scanning cycle: 3 weeks.
The EIS characterization results of different electrodes are shown in Fig. 2b. Compared with bare GCE, the semicircle diameter of CQDs/GCE, rGO/GCE, and CQDs-rGO/GCE was all significantly reduced, and the semicircle diameter reaches the minimum at CQDs-rGO/GCE, indicating that the impedance of CQDs-rGO/GCE was lowest and the CQDs-rGO nanocomposite has the most advantage in promoting the conductivity of the electrode. The results of EIS were consistent with the results of CV characterization. The experimental parameters of EIS were set as follows: start frequency: 10−2; end frequency: 105; bias voltage: + 0.24 V; amplitude: + 5 V.
Effect of pH
Since PBS was used as the electrolyte solution for the detection of AA, DA, and UA, its pH values have vital impacts on the oxidation of these three substances. Based on this, the effect of 0.1 M PBS solutions with different pH values (5.8–8.0) on the simultaneous detection of 0.5 mM AA, 1 μM DA, and 0.3 mM UA at CQDs-rGO/GCE was studied by DPV. And the obtained current values were plotted into a point-line diagram for visual comparison (Fig. 3). With pH value changing from 5.8 to 8.0, the oxidation peak current of AA, DA, and UA has all reached the maximum value at pH 7.0. Therefore, in order to obtain a better sensing system, 0.1 M PBS solution with pH value of 7.0 was chosen for subsequent experiments.
Effect of scan rate
To further study the reaction mechanism of AA, DA, and UA on the surface of CQDs-rGO/GCE electrochemical sensor, the effect of scan rate on the individual detection of 800 μM AA, 100 μM DA, and 500 μM UA were examined by CV method. As Fig. 4 shows, the oxidation peak current (Ipa) and reduction peak current (Ipc) of AA, DA, and UA increased linearly with increasing scan rates (from 50 to 250 mV s−1), and the linear equations were demonstrated as follows:
It can be inferred from the results that the oxidation and reduction of AA, DA, and UA on CQDs-rGO/GCE were adsorption controlled processes [36, 37]. The parameter settings of CV experiment were as follows: vertex 1 potential: − 0.6 V; vertex 2 potential: + 0.6 V; scanning speed: 50–250 mV s−1; scanning cycle: 3 weeks.
Electrochemical detection of AA, DA, and UA
Individual detection of AA, DA, and UA
Under the optimized experimental conditions, the individual detection of different concentrations of AA, DA, and UA on CQDs-rGO/GCE was carried out by DPV. It can be seen from Fig. 5a, c, and e that the initial peak oxidation potential of AA, DA, and UA appeared at about − 0.031 V, + 0.157 V, and + 0.286 V, respectively, which was then remain unchanged with rising levels of analytes. From Fig. 5b, d, and f, the linear relationships between concentrations of AA, DA, and UA and peak current were observed in the ranges of 70–1000 μM (R2 = 0.99), 1–10 μM (R2 = 0.99), and 4–100 μM (R2 = 0.99), with the detection limits of 3.33, 0.0167, and 1.33 μM (S/N = 3), and sensitivities of 18.50, 2.02, and 0.46 μA μM−1, respectively. The possible oxidation mechanism of AA, DA, and UA at CQDs-rGO/GCE can be described as Scheme 1. Under the experiment condition of pH 7.0, there exists an electrostatic attraction between cationic DA and negative functional groups on the surface of CQDs-rGO nanocomposite, which contributes to promote the oxidation of DA to dopamine o-quinone. In addition, the hydroxyl groups of AA, DA, and UA can interact with the oxygen-containing functional groups on the surface of CQDs-rGO nanocomposite to form hydrogen bonds, which then weakens the hydrogen bonding on the benzene ring of the three substances and promotes the oxidation of AA to dehydroascorbate, DA to dopamine o-quinone, and UA to dehydrourate.
Simultaneous detection of AA, DA, and UA
The analytical performance of CQDs-rGO/GCE sensor was further studied with DPV in the mixture of AA, DA, and UA.
As Fig. 6 shows, when the concentration of one analyte was altered and the other two substances were kept constant, three well separated peaks were observed, and good linear relationships between AA, DA, and UA and peak current in the concentration range of 60 μM–1 mM (R2 = 0.99) and 2–7 mM (R2 = 0.98), 1–10 μM (R2 = 0.99) and 27–80 μM (R2 = 0.99), and 20–90 μM (R2 = 0.99) and 100–500 μM (R2 = 0.99) were obtained, respectively. And the sensitivities of AA, DA, and UA were 0.025 and 0.005, 1.383 and 0.121, and 0.290 and 0.058 μA μM−1, respectively.
DPV graphs of the CQDs-rGO/GCE in 0.1 M PBS (pH 7.0) containing 10 μM DA, 50 μM UA, and different concentrations of AA (60 μM–7 mM) (a); containing 0.5 mM AA, 50 μM UA, and different concentrations of DA (0.1–110 μM) (c); containing 50 μM AA, 10 μM DA, and different concentrations of UA (20–500 μM) (e). Linear relationship between the peak current and the concentrations of AA (b), DA (d), and UA (f)
The analytical performances including linear range and sensitivity of CQDs-rGO/GCE toward AA, DA, and UA were compared with other electrochemical sensors that have been reported in recent years. From Table 1, we can see that CQDs-rGO/GCE showed improved or comparable properties with the previous works.
As can be seen from Fig. 7, simultaneous detection of AA, DA, and UA with different concentrations was carried out by DPV technology. The peak potential difference between AA and DA was calculated as 0.147 V, and that of DA and UA was 0.135 V, suggesting that the three compounds can be well distinguished. Moreover, with the increased of the concentration of AA, DA, and UA, the corresponding current on the CQDs-rGO/GCE was increased simultaneously, and the linear ranges of AA, DA, and UA were 0.2–0.9 mM (R2 = 0.99) and 1.4–4.2 mM (R2 = 0.99), 5–9 μM (R2 = 0.98) and 11–81 μM (R2 = 0.99), and 20–90 μM (R2 = 0.99) and 140–300 μM (R2 = 0.99), respectively.
The above results showed that CQDs-rGO/GCE can be well used for simultaneous detection of AA, DA, and UA.
Reproducibility, stability, and interference of the sensor
Under the same conditions, eighteen CQDs-rGO/GCE sensors were fabricated to detect 1 mM AA, 10 μM DA, and 500 μM UA with DPV, and each substance was detected with six sensors. The reproducibility of CQDs-rGO/GCE was analyzed by calculating the relative standard deviation (RSD) of the current of six electrodes of the same substance. The results are exhibited in Fig. 8a. The RSD of six sensors was calculated as 3.9% for AA, 1.95% for DA, and 0.88% for UA, which revealed that CQDs-rGO/GCE has good reproducibility.
Chronoamperometry was used to evaluate the long-term stability of CQDs-rGO/GCE under the condition of stirring, + 0.6 V and 2000 s (Fig. 8b–d). The current signals of the three substances remain basically unchanged within 2000 s, indicating that the sensor has good long-term stability [46, 47].
To test the anti-interference ability of CQDs-rGO/GCE, several possible interferential species such as BSA, HSA, Cu2+, Fe3+, and tryptophan were examined in 0.1 M PBS by chronoamperometry at a constant potential of + 0.6 V (see Fig. S1). It was found that the existence of aforementioned species (3 mM) showed negligible interferences for simultaneous detection of AA (900 μM), DA (80 μM), and UA (200 μM), revealing a good selectivity of CQDs-rGO/GCE.
Real samples
In order to verify the applicability of the proposed electrochemical sensor for real sample analysis, the application was evaluated by the determination of AA, DA, and UA in fetal bovine serum samples using standard addition method. The fetal bovine serum samples were diluted 10 times with 0.1 M PBS. The DPV experimental results are shown in Table S1. The recoveries of the spiked samples were detected within the range of 97.9–107.4%, indicating that the sensor has important practical application of significance.
Conclusions
In this study, ultrasound was used to fully mix CQD solution and GO solution to prepare the electrodeposition solution, and one-step co-deposition method was used to synthesize CQDs-rGO nanocomposite on the surface of bare GCE to fabricate CQDs-rGO/GCE electrochemical sensor. The characterization results of FESEM and EDS confirmed that the CQDs-rGO nanocomposite has been successfully synthesized, and CV and EIS studies proved that the combination of the CQDs and rGO can effectively improve the conductivity of the sensor. In addition to high electrocatalytic activities, CQDs-rGO/GCE also exhibited strong anti-interference ability, high stability, and high reproducibility. The sensor is expected to further apply for the simultaneous detection of AA, DA, and UA in real samples.
References
Zhang W, Liu L, Li Y, Wang D, Ma H, Ren H, Shi Y, Han Y, Ye B (2018) Electrochemical sensing platform based on the biomass-derived microporous carbons for simultaneous determination of ascorbic acid, dopamine, and uric acid. Biosens Bioelectron 121:96–103
Moreno M, Arribas AS, Bermejo E, Chicharro M, Zapardiel A, Rodriguez MC, Jalit Y, Rivas GA (2018) Selective detection of dopamine in the presence of ascorbic acid using carbon nanotube modified screen-printed electrodes. Talanta 80(5):2149–2156
Chen H, Liu Y, Li H, Zhang Y, Yao S (2019) Non-oxidation reduction strategy for highly selective detection of ascorbic acid with dual-ratio fluorescence and colorimetric signals. Sensors Actuators B Chem 281:983–988
Rostami S, Mehdinia A, Jabbari A (2017) Seed-mediated grown silver nanoparticles as a colorimetric sensor for detection of ascorbic acid. Spectrochim Acta A 180:204–210
Zhang L, Feng J, Chou K, Su L, Lei, Hou X (2017) Simultaneously electrochemical detection of uric acid and ascorbic acid using glassy carbon electrode modified with chrysanthemum-like titanium nitride. J Electroanal Chem 803:11–18
Mu C, Lu H, Bao J, Zhang Q (2018) Visual colorimetric ‘turn-off’ biosensor for ascorbic acid detection based on hypochlorite–3,3′,5,5′,-tetramethylbenzidine system. Spectrochim Acta A 201:61–66
Li X, Zhou C, Zi Q, Cao Q (2016) An electrochemical signal transduction amplification strategy for ultrasensitive detection of ascorbic acid. J Electroanal Chem 780:321–326
Tukimin N, Abdullah J, Sulaiman Y (2018) Electrodeposition of poly(3,4- ethylenedioxythiophene)/reduced graphene oxide/manganese dioxide for simultaneous detection of uric acid, dopamine and ascorbic acid. J Electroanal Chem 820:74–81
Chen Y, Zhang T, Gao X, Pan W, Li N, Tang B (2017) A highly selective and instantaneous upconversion fluorescent nanoprobe for ascorbic acid detection in biological samples. Chinese Chem Lett 28(10):1983–1986
Fang J, Xie Z, Wallace X, Wang X (2017) Co-deposition of carbon dots and reduced graphene oxide nanosheets on carbon-fiber microelectrode surface for selective detection of dopamine. Appl Surf Sci 412:131–137
Liu H, Li N, Zhang H, Zhang F, Su X (2018) A simple and convenient fluorescent strategy for the highly sensitive detection of dopamine and ascorbic acid based on graphene quantum dots. Talanta 189:190–195
He Q, Liu J, Liu XP, Li G, Chen D, Deng P, Liang J (2019) A promising sensing platform toward dopamine using MnO2 nanowires/electro-reduced graphene oxide composites. Electrochim Acta. 296:683–692
Cai Z, Ye Y, Wan X, Liu J, Yang S, Xia Y, Li G, He Q (2019) Morphology-dependent electrochemical sensing properties of iron oxide–graphene oxide nanohybrids for dopamine and uric acid. Nanomaterials. 9:835
Wan X, Yang S, Cai Z, He Q, Ye Y, Xia Y, Li G, Liu J (2019) Facile synthesis of MnO2 nanoflowers/N-doped reduced graphene oxide composite and its application for simultaneous determination of dopamine and uric acid. Nanomaterials. 9:847
Zhang X, Ma L, Zhang Y (2015) Electrodeposition of platinum nanosheets on C60 decorated glassy carbon electrode as a stable electrochemical biosensor for simultaneous detection of ascorbic acid, dopamine and uric acid. Electrochim Acta 177:118–127
Rahman MM, Lopa NS, Ju ML, Lee J-J (2017) Highly sensitive and simultaneous detection of dopamine and uric acid at graphene nanoplatelet-modified fluorine-doped tin oxide electrode in the presence of ascorbic acid. J Electroanal Chem 792:54–60
Li Q, Xia Y, Wan X, Yang S, Cai Z, Ye YB, Li G (2020) Morphology-dependent MnO2/nitrogen-doped graphene nanocomposites for simultaneous detection of trace dopamine and uric acid. Mat Sci Eng C-Mater 109:110615
Li G, Xia Y, Tian Y, Wu Y, Liu J, He Q, Chen D (2019) Review-recent developments on graphene-based electrochemical sensors toward nitrite. J Electrochem Soc 166:881–895
Oghli AH, Soleymanpour A (2020) Polyoxometalate/reduced graphene oxide modified pencil graphite sensor for the electrochemical trace determination of paroxetine in biological and pharmaceutical media. Mat Sci Eng C-Mater. 108:110407
Mutyala S, Mathiyarasu J (2016) A reagentless non-enzymatic hydrogen peroxide sensor presented using electrochemically reduced graphene oxide modified glassy carbon electrode. Mat Sci Eng C-Mater 69:398–406
Tan F, Cong L, Saucedo NM, Gao J, Li X, Mulchandani A (2016) An electrochemically reduced graphene oxide chemiresistive sensor for sensitive detection of Hg2+ ion in water samples. J Hazard Mater 320:226–233
Kurt Urhan B, Demir Ü, Öznülüer ÖT, Öztürk Doğan H (2020) Electrochemical fabrication of Ni nanoparticles-decorated electrochemically reduced graphene oxide composite electrode for non-enzymatic glucose detection. Thin Solid Films 693:137695
Ghanbari MH, Khoshroo A, Sobati H, Ganjali MR, Rahimi-Nasrabadi M, Ahmadi F (2019) An electrochemical sensor based on poly (l-cysteine)@AuNPs @ reduced graphene oxide nanocomposite for determination of levofloxacin. Microchem J 147:198–206
Öztürk Doğan H, Kurt Urhan B, Çepni E, Eryiğit M (2019) Simultaneous electrochemical detection of ascorbic acid and dopamine on Cu2O/CuO/electrochemically reduced graphene oxide (CuxO/ERGO)- nanocomposite- modified electrode. Microchem J 150:104157
Hu L, Sun Y, Li S, Wang X, Hu K, Wang L, Liang X-J, Wu Y (2014) Multifunctional carbon dots with high quantum yield for imaging and gene delivery. Carbon 67:508–513
Liu C, Zhang P, Zhai X, Tian F, Li W, Yang J, Liu Y, Wang H, Wang W, Liu W (2012) Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials. 33(13):3604–3613
Shao K, Yang Y, Ye S, Gu D, Wang T, Teng Y, Shen Z, Pan Z et al (2020) Talanta 208:120460
Guo W, Pi F, Zhang H, Sun J, Zhang Y, Sun X (2017) A novel molecularly imprinted electrochemical sensor modified with carbon dots, chitosan, gold nanoparticles for the determination of patulin. Biosens Bioelectron 98:299–304
Shereema RM, Rao TP, Sameer Kumar VB, Sruthi TV, Vishnu R, Prabhu GRD, Sharath Shankar S (2018) Individual and simultaneous electrochemical determination of metanil yellow and curcumin on carbon quantum dots based glassy carbon electrode. Mat Sci Eng C-Mater. 93:21–27
Hu S, Li X, Wang K, Wu Q, Zhang G, Liu X (2020) Sensor array based on carbon dots for ATP-related physiological phosphates detecting and ATP hydrolysis monitoring. Sensors Actuators B Chem 310:127851
Aftab S, Kurbanoglu S, Ozcelika G, Bakirhan NK, Shah A, Ozkan SA (2019) Carbon quantum dots co-catalyzed with multiwalled carbon nanotubes and silver nanoparticles modified nanosensor for the electrochemical assay of anti-HIV drug Rilpivirine. Sensors Actuators B Chem 285:571–583
Li L, Liu D, Shi A, You T (2018) Simultaneous stripping determination of cadmium and lead ions based on the N-doped carbon quantum dots-graphene oxide hybrid. Sensors Actuators B Chem 255:1762–1770
Chen D, Zhuang X, Zhai J, Zheng Y, Lu H (2018) Preparation of highly sensitive Pt nanoparticles-carbon quantum dots/ionic liquid functionalized graphene oxide nanocomposites and application for H2O2 detection. Sensors Actuators B Chem 255:1500–1506
Muthusankar G, Sethupathi M, Chen S, Devi RK, Vinoth R, Gopu G, Anandhan N (2019) N, N-doped carbon quantum dots @ hexagonal porous copper oxide decorated multiwall carbon nanotubes: a hybrid composite material for an efficient ultra-sensitive determination of caffeic acid. Compos Part B-Eng 174:106973
Wei Y, Zhang D, Fang Y, Wang H, Liu Y, Xu Z, Wang S, Guo Y (2019) Detection of ascorbic acid using green synthesized carbon quantum dots. J Sensors 2019:1–10
Zhang D, Li L, Ma W, Chen X, Zhang Y (2017) Electrodeposited reduced graphene oxide incorporating polymerization of l-lysine on electrode surface and its application in simultaneous electrochemical determination of ascorbic acid, dopamine and uric acid. Mat Sci Eng C-Mater 70:241–249
Baytak AK, Aslanoglu M (2020) A novel sensitive method for the simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan using a voltammetric platform based on carbon black nanoballs. Arab J Chem 13:1702–1711
Puangjan A, Chaiyasith S, Taweeporngitgul W, Keawtep J (2017) Application of functionalized multi-walled carbon nanotubes supporting cuprous oxide and silver oxide composite catalyst on copper substrate for simultaneous detection of vitamin B2, vitamin B6 and ascorbic acid. Mat Sci Eng C-Mater 76:383–397
Sun D, Li H, Li M, Li C, Dai H, Sun D, Yang B (2018) Electrodeposition synthesis of a NiO/CNT/PEDOT composite for simultaneous detection of dopamine, serotonin, and tryptophan. Sensors Actuators B Chem 259:433–442
Rohani T, Taher MA (2018) Novel functionalized multiwalled carbon nanotube-glassy carbon electrode for simultaneous determination of ascorbic acid and uric acid. Arab J Chem 11(2):214–220
Zhang X, Zhang Y, Ma L (2016) One-pot facile fabrication of graphene-zinc oxide composite and its enhanced sensitivity for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. Sensors Actuators B Chem 227:488–496
Niu X, Yang W, Guo H, Ren J, Yang F, Gao J (2012) A novel and simple strategy for simultaneous determination of dopamine, uric acid and ascorbic acid based on the stacked graphene platelet nanofibers/ionic liquids/chitosan modified electrode. Talanta. 99:984–988
Selvarajan S, Suganthi A, Rajarajan M (2017) A facile approach to synthesis of mesoporous SnO2/chitosan nanocomposite modified electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. Surf Interfaces 7:146–156
Zhu Q, Bao J, Huo D, Yang M, Hou C, Guo J, Chen M, Fa H, Luo X, Ma Y (2017) 3D graphene hydrogel-gold nanoparticles nanocomposite modified glassy carbon electrode for the simultaneous determination of ascorbic acid, dopamine and uric acid. Sensors Actuators B Chem 238:1316–1323
Xie Y, Yuan J, Ye H, Song P, Hu S (2015) Facile ultrasonic synthesis of graphene/SnO2 nanocomposite and its application to the simultaneous electrochemical determination of dopamine, ascorbic acid, and uric acid. J Electroanal Chem 749:26–30
Yang H, Zhao J, Qiu M, Sun P, Han D, Niu L, Cui G (2019) Hierarchical bi-continuous Pt decorated nanoporous Au-Sn alloy on carbon fiber paper for ascorbic acid, dopamine and uric acid simultaneous sensing. Biosens Bioelectron 124-125:191–198
Savk A, Özdil B, Demirkan B, Nas MS, Calimli MH, Alma MH, Inamuddin, Asiri AM, Şen F (2019) Multiwalled carbon nanotube-based nanosensor for ultrasensitive detection of uric acid, dopamine, and ascorbic acid. Mat Sci Eng C-Mater 99:248–254
Funding
Financial supports from the National Natural Science Foundation of China (Grant Nos. 81973944, 81503636, and 81704146) and the National S&T Major Project (No. 2018ZX09201011) are acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 193 kb)
Rights and permissions
About this article
Cite this article
Wei, Y., Xu, Z., Wang, S. et al. One-step preparation of carbon quantum dots-reduced graphene oxide nanocomposite–modified glass carbon electrode for the simultaneous detection of ascorbic acid, dopamine, and uric acid. Ionics 26, 5817–5828 (2020). https://doi.org/10.1007/s11581-020-03703-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03703-5