Abstract
The poor cycle performance and rate capability limit the wide application of lithium-rich cathode materials. Herein, we propose a one-step method for 'La3+' doping and LaMnO3 coating by co-precipitation to improve the electrochemical performance of the Li1.2Mn0.54Ni0.13Co0.13O2. XRD, EDS of the cross section, and TEM successfully confirm that 'La3+' is doped into the material structure and LaMnO3 is uniformly coated on the surface of Li1.2Mn0.54Ni0.13Co0.13O2. The Li1.2Mn0.54-xNi0.13Co0.13LaxO2 (x = 0.03) (abbreviated as LR-NMC@0.03) sample delivers a discharge capacity as high as 248.1 mAh g−1 at 1C compared with that of 212.3 mAh g−1 for the pristine sample. More importantly, the capacity retention for the LR-NMC@0.03 sample after 100 cycles can reach 92.5%, which is significantly higher than that of 76.8% for the pristine sample. The better rate capability and cyclic performance of the LR-NMC@0.03 sample are attributed to the double strengthening effect by both the 'La3+' doping and LaMnO3 coating with better host structural and surface stability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With continuous consumption of traditional non-renewable energy, new energy resources have attracted more and more researchers’ attention. Lithium-ion batteries have the advantages of high specific energy, low self-discharge rate, long cycle life, no memory effect and low environmental pollution. They have become important energy storage devices in the fields of renewable energy, smart grid, and new energy automotive technology [1,2,3,4]. Since LiCoO2 (LCO) was commercialized and marketed by Sony in 1991, it has been widely used in consumer electronics products. Nevertheless, its inherent low energy density and expensive raw materials limit its application in power vehicles, for example, the pure electric vehicles [5]. Owing to the continuous efforts of scientists, cathode materials such as Li [NixCoyMn1-x-y]O2 [6,7,8,9,10], LiFePO4 [11, 12], and LiMn2O4 [13, 14] have been continuously developed. Compared with LCO, although they have lower cost, they still cannot meet the consumers for high-capacity cathode materials [1, 2].
In recent years, a layered transition metal oxide cathode material with chemical formula of zLi2MO3·(1-z)LiMO2(0 < z < 1, M = Mn0.5Ni0.5, MnxNiyCo(1-x-y) 0 < x,y < 1,0 < x + y < 1) has attracted many researchers’ attention. The Li-rich Mn-based layered oxide, typical representative Li1.2Mn0.54Ni0.13Co0.13O2, has a high theoretical specific capacity (> 250 mAh/g) and low cost, which is regarded as the most promising cathode materials for the next-generation Li-ion batteries. However, the low initial irreversible capacity, poor cycle performance, poor rate capability, and potential dropping limit its further application [15,16,17,18,19,20].
So far, most of the current research work focuses on simple doping and coating to improve the inherent defects of lithium-rich cathode materials. Different modifications of lithium-rich cathode were carried out in early research works, for example, the Mg2+ [21], Al3+ [22], and Cr3+ [23] doping and Al2O3 [24], ZnO [25], and ZrO2 [26] coating. Although these works have improved the electrochemical performance of lithium-rich cathode materials to some extent, there is still much room for improvement. As known to us, the structure of the lithium-rich cathode material will collapse after being charged and discharged, and it is easily eroded by the electrolyte [27]. Simple doping can stabilize the structure of lithium-rich cathode materials to some extent; however, the erosion of the material by the electrolyte is difficult to be avoided. And the simple coating can suppress the side reaction of between the electrolyte and the lithium-rich cathode materials to some extent, but it is also difficult to maintain the structural stability in long charge-discharge cycles.
In order to improve the electrochemical performance of lithium-rich cathode more effectively, here we propose a one-step method for doping and coating. Super heterovalent cation 'La3+' was selected as the doping element to replace 'Mn4+' in layered lithium-rich cathode materials Li1.2Mn0.54Ni0.13Co0.13O2. The binding energy of La–O bond is higher than that of Ni–O or Mn–O bond. The Gibbs free energy of formation of La2O3, MnO2 and NiO is − 1763.54 kJ mol−1, − 465.1 kJ mol−1, and − 211.7 kJ mol−1, respectively. Based on the above analysis, 'La3+' doping can effectively improve the stability of layered lithium-rich cathode materials. Due to the radius of the trivalent, La-ion is obviously larger than that of the tetravalent Mn-ion [28]; as a result, such a huge ion doping into the layered structure will effectively broaden the lithium-ion channel in the lattice and further improve the rate performance of the lithium-rich cathode material. It is worth mentioning that due to the heterovalent doping, the doping element 'La3+' cannot be completely entered into the material structure; instead, a part of the undoping 'La3+' ion is coated on the surface of the lithium-rich cathode material in the form of La compound. Thus, the application of heterovalent 'La3+' ion in the modification of lithium-rich cathode materials can stabilize the layered structure of the material. At the same time, the La-containing compound formed on the surface of the material can effectively prevent the electrolyte from eroding the material, so as to effectively improve the electrochemical performance of lithium-rich cathode materials.
Experimental
Materials synthesis
Carbonate co-precipitation method was adopted to synthesis La-doping Li-rich precursor of Li1.2Mn0.54-xNi0.13Co0.13LaxO2 (x = 0, 0.01, 0.03, 0.05) lithium-rich cathode materials. According to the stoichiometric ratio of Li1.2Mn0.54-xNi0.13Co0.13LaxO2 (x = 0, 0.01, 0.03, 0.05), MnSO4·H2O, CoSO4·7H2O, NiSO4·6H2O, and La (NO3)3·6H2O of different proportions were dissolved in deionized water to prepare a 2 mol/L sulfate mixed solution. Na2CO3 was selected as the precipitant and dissolved in deionized water to form a 2 mol/L Na2CO3 aqueous solution. At the same time, appropriate amount of NH3·H2O as a complexing agent was added to the Na2CO3 aqueous solution. The above two mixed solutions were dropped into the container simultaneously in the process of continuous stirring with the speed of 1000 rpm/min. During the reaction, the pH in the solution was controlled by NH3·H2O, and the pH was always maintained at about 7.8. In order to obtain precursors with better morphology, the reaction temperature and time were strictly maintained at 60 °C and 10 h, respectively. After aging for 12 h and stirring, filtering, and vacuum drying for 12 h, Li-rich precursor by La doping with spherical morphology can be obtained. Considering that lithium will volatilize during the sintering process, 5at% excess of lithium carbonate was added to the precursor. Li2CO3 was mixed with the precursor uniformly by ball mill, and then the above mixture was first calcined in a muffle furnace at 550 °C for 6 h and subsequently calcined at 900 °C for 12 h to obtain lithium-rich manganese-based cathode materials doped with different La content.
Characterization
The morphology of the samples and the distribution of the elements were observed by the scanning electron microscopy equipped with an energy dispersive (SEM/EDS, Sigma-H, Carl Zeiss Germany). X-ray diffraction (XRD, Rigaku IV, Nippon Science Corporation) was applied to analysis the samples’ phase composition and structure. The coating morphology on the particle surface was adopted by transmission electron microscopy (TEM, Philips Tecnai F30).
Electrochemical test
The electrochemical properties of the pristine and modified materials were tested by the CR2032-type coin cells. The average mass loading of the active materials is 2.5 mg cm−2. NMP (N-methylpyrrolidone) was used as a dispersant during the electrode sheet. The cathode material, PVDF (polyvinylidene fluoride), and acetylene black were mixed uniformly with a mass ratio of 80:10:10, and then the mixed slurry was evenly coated on the aluminum foil. The prepared cathode sheets were dried in a vacuum drying oven at 90 °C for 12 h. The coin cells were assembled in a fully dry Ar-filled glove box with water content and oxygen content lower than 0.1 ppm. Lithium metal sheets were used as the negative electrode, Celgard2400 microporous polypropylene membrane was used as the separator, and the electrolyte was 1 mol/L LiPF6 dissolved in 1:1 (V/V) vinyl carbonate (EC) and dimethyl carbonate (DMC). LAND (CT2001A) battery tester was used to test the galvanostatic charge-discharge. The voltage range was 2.0~4.6 V. AC impedance test was conducted by CHI660B electrochemical workstation with an amplitude of 10 mV from 100 KHz to 0.1 Hz.
Results and discussion
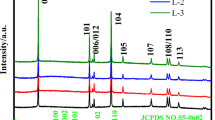
Figure 1 shows the XRD diffraction patterns of Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x = 0, 0.01, 0.03, 0.05) Li-rich cathodes. As shown in Fig. 1, all 'La3+'-doping samples show the characteristic diffraction peaks of layered lithium-rich manganese-based cathode materials. The pristine and 'La3+' modified samples present a layered R-3m LiCoO2 like α-NaFeO2 standard characteristic peak. All samples show weak diffraction peaks between 20° and 25°, which is mainly due to the existence of the material composition Li2MnO3 in the transition metal layer, resulting in the characteristic signs of LiMn6-type superlattice [29, 30]. The (006)/(012) and (018)/(110) characteristic peak pairs of all samples are obvious, which shows that all the samples have good crystallinity. With the increase of 'La3+' doping content, the peak of the main phase has not changed significantly, and the obvious characteristic peaks of the layered lithium-rich manganese-based cathode material are still maintained.
The previous research results show that the change of the lattice constant of the material can confirm whether the doping element has entered into the lattice of the material [31]. Jade software was used to calculate the lattice constant and (003)/(104) peak intensity ratio of the samples. The calculation results are shown in Table 1. It can be seen from the data in Table 1 that the lattice constants a and c of the 'La3+' modified samples are larger than those of unmodified samples. Therefore, it can be concluded that 'La3+' ion has been successfully diffused to the lithium-rich material structure. 'La3+' doping broadens the lithium intercalation diffusion channel of the cathode electrode material, which is mainly due to 'La3+' ion radius is larger than 'Mn4+' ion radius. The result is similar to those reported in the research [32, 33]. It is worth noting that as the doping amount of La increases, the lattice constant of the sample does not increase linearly. This may be due to the strong binding force between La and O. When the doping amount reaches a certain level, the strong binding force with O shrinks the interlayer spacing [28]. In general, the lattice constant c/a can be used as an index for evaluating the characteristics of layered structure materials. The higher the c/a, the better the layered structure characteristics. It can be seen from the data in Table 1 that the c/a value of 'La3+'-doping samples are higher than those of undoping samples, indicating that 'La3+'-doping samples have better layered structure characteristics than undoping samples. In addition, the peak intensity ratio of I(003)/I(104) is related to the mixed arrangement degree of Li+ and Ni2+ cations in the layered structure of the cathode material. All 'La3+'-doping samples have higher I(003)/I(104) peak intensity ratios than the undoping samples. Therefore, it can be concluded that the mixed arrangement of Li+ and Ni2+cations in the layered structure of the material can be inhibited by 'La3+' doping to a certain extent.
It should be noted that LaMnO3 second phase occurs in the XRD pattern of the La-doping samples shown in Fig. 1. Furthermore, as the amount of La-doping increases, the peak intensity of LaMnO3 second phase increases. The formation of LaMnO3 second phase can be ascribed to the reason that the 'La3+' ions were not fully doped into the layered structure of the lithium-rich cathode material. Combined with the abovementioned XRD structural analysis of La-doping, to further prove that 'La3+' ion has been entered the lithium-rich material structure and clarify the existence form of LaMnO3 second phase, the cross-section EDS analysis was applied in this paper. Figure 2a presents EDS analysis results of LR-NMC@0.03 Li-rich cathodes’ cross section. In the experiment, the data were collected in the diameter direction and on the surface of the lithium-rich cathode particle at a distance about 1 um. The typical EDS spectrum shown in Fig. 2b was established to demonstrate the presence of Mn, Co, Ni, O, and La. Experimental test results shown in Fig. 2c demonstrate that the La contents from point 1 to point 5, i.e., the interior to the surface of the LR-NMC@0.03 particle, are 0.46, 0.53, 0.48, 0.61, and 0.87at%, respectively. As shown in Fig. 2c, the variety trend of the La content from the interior to the surface of the particle can be clearly seen. It should be noted that the particle interior had been detected and the La contents were all lower than the theoretical La content (0.75at%), revealing that 'La3+' ion has been doped into the layered structure of the lithium-rich cathode material successfully. This result is consistent with the conclusion of the aforementioned XRD analysis.
SEM image of LR-NMC@0.03 Li-rich cathode cross section exhibiting the areas marked with the red dots where the point EDS analyses were performed, for a SEM image of cross section. b Representative EDS test spectrum. c La content from the interior to the surface of the cross section. d La content on the surface of cathode particle
One can find that the surface La contents of the LR-NMC@0.03 particle are significantly higher than those of the interior. Figure 2d exhibits La content distribution curve of the particle surface; as shown in Fig. 2d, La contents of the particle surface vary little, and the calculation results show that the average La content of the particle surface is about 0.90at%. Combined with the LaMnO3 second phase appeared in XRD pattern shown in Fig. 1 after La doping, it can be concluded that 'La3+' ions that are not fully doped into the layered structure of the lithium-rich cathode material exist on the surface of the material particles in the form of LaMnO3. In order to better identify the existence details of LaMnO3 phase on the particle surface, TEM characterization was adopted to analysis morphology and coating situation of LaMnO3 phase. The coating layer of about 2~3 nm shown in Fig. 3 can be clearly seen on the surface of the LR-NMC@0.03 Li-rich cathode particle. Meanwhile, the coating layer on the particle surface is dense and uniform. According to the phase analysis result in Fig. 1 and the EDS test result in Fig. 2band d, the coating layer can be determined as LaMnO3.
SEM was adopted to analyze the morphology and particle size of the pristine and the 'La3+' modified samples. Figure 4 presents the SEM images of the Pristine and the 'La3+' modified samples. As shown in Fig. 4, all the samples with the particle size about 10 μm are secondary particles formed by stacking primary particles, and the particles are dispersed evenly. The samples still maintain good spherical morphology after modified by 'La3+' ion. It is noteworthy that the surface of 'La3+' modified samples is more smooth and compact. However, the surface of the pristine sample is coarse, and there are many randomly distributed holes on the surface. Figure 5 presents the EDS mapping patterns of LR-NMC@0.03 Li-rich cathodes. As shown in Fig. 5, Ni, Co, Mn, and La elements were detected successfully on the particle surface by the EDS test. Meanwhile, all the detected elements distribute uniformly. The La element that distributes uniformly can be considered that LaMnO3 coating layer is well coated on the surface of positive particles. This result is well in accord with the analysis in Fig. 3.
The charge-discharge profiles of Li1.2Mn0.54-xNi0.13Co0.13LaxO2(x = 0, 0.01, 0.03, 0.05) Li-rich cathodes in the first cycle conducted from 2.0 V to 4.6 V at C/10 (1C = 250 mA g−1) are shown in Fig. 6. All the samples exhibit similar charge-discharge curve characteristics, i.e., the charge profiles of all the samples consist of a slant line below 4.5 V and a plateau around 4.5 V, corresponding to the oxidation of nickel and cobalt and the activation of Li2MnO3, respectively [34,35,36]. The charge-discharge capacity values, the irreversible capacity (Cirr) values, and the coulombic efficiency for all the samples in the first cycle are listed in Table 2. With the increase of La doping content, the charge capacity of the sample decreases in turn; however, the discharge capacity of the sample increases first and then decreases. It is important to note that the initial discharge capacity of Li1.2Mn0.54-xNi0.13Co0.13LaxO2 when x = 0.03 can reach as high as 286.7 mAh g−1 compared with that of 260.6 mAh g−1 for the pristine sample (x = 0). Furthermore, the Cirr value reduces from 77.0 to 36.4 mAh g−1, and the initial coulombic efficiency also increases from 77.2 to 88.7%. Appropriate La doping cannot only broaden the transport channel of lithium-ion but also stabilize the structure of the material. LaMnO3 formed by La that has not entered the material structure was coated on the surface of the material which can effectively prevent the electrolyte from eroding the surface of the cathode electrode material. Meanwhile, the LaMnO3 coating layer can protect oxygen vacancies generated during the first charge and suppress the evolution of O2 gas to a certain extent [27, 37]. Thus the discharge performance of the lithium-rich cathode material has been effectively improved. However, too little doping of La has limited effect on improving the structure and electrochemical performance of the material. Excessive doping of 'La3+' does not completely allow 'La3+' to enter the lattice of the layered material and generates too much two-phase LaMnO3. The excessive LaMnO3 may hinder the transport of lithium-ions, which worsens the electrochemical properties of the cathode material.
To evaluate the rate performance of the 'La3+' modified samples, the rate capacity voltage of the pristine for Fig. 7a and LR-NMC@0.03 for Fig. 7b at C/10, C/5, C/2, 1C, and 2C had been conducted. It can be seen that the LR-NMC@0.03 sample delivers a higher discharge capacity than that of the pristine at all the tested rates. For instance, LR-NMC@0.03 sample exhibits a discharge capacity of 248.1 mAh g−1 at 1C in comparison with that of 212.3 mAh g−1 for the pristine. Interestingly, all the spacing between adjacent capacity-voltage curves for the LR-NMC@0.03 sample are narrower than those of the pristine. The above analysis indicates that the LR-NMC@0.03 sample has a better rate performance.
To establish better understanding of the origin of the better rate capability for the LR-NMC@0.03 sample, the differential capacity versus voltage (dQ/dV) plotted in Fig. 8 had been performed at 1C rate for both the pristine sample and the LR-NMC@0.03 sample. As we know, the origin of the redox species comes from Ni2+↔Ni3+↔Ni4+ and Co3+↔Co4+ oxidation/reduction reaction simultaneously accompanied with different phase transitions during the electrochemical processes as reported in the researches [38,39,40]. The two obvious reduction peaks displayed in Fig. 8 shift to higher potential in the LR-NMC@0.03 sample compared with that in the pristine sample. Previous study [40] shows that the peak potential is closely related to the total polarization of the experimental samples including ohmic polarization, charge transfer polarization, and diffusion polarization. Specifically, the higher the peak potential value, the smaller the total polarization value, which is under the discharge process of cathode electrode. In other words, the LR-NMC@0.03 sample possesses higher the peak potential value than the pristine, indicating lower total polarization value. Therefore, the LR-NMC@0.03 sample exhibits better rate capability.
To further clarify the origin of the enhanced rate capability for the LR-NMC@0.03 sample comprising the pristine sample, electrochemical impedance spectra (EIS) measurement had been conducted at 25 °C after the first cycle and 100 cycles. All the samples were charged to 4.6 V at 1C before tested. The inset is the equivalent circuit used to fit the Nyquist plots listed in Fig. 9. In general, a Nyquist plot for the lithium-rich cathode material consists of two semicircles and one slope. Specifically, the intercept of the first semicircle with real axis in the highest frequency region represents the ohmic resistance (Rs) of electrolyte, separator, and electrode. The first semicircle in the high frequency region and the second semicircle in the middle frequency region refer to the resistance (Rf) of lithium-ion diffusion in the solid-electrolyte interface (SEI) and the charge transfer resistance (Rct) in the electrode and electrolyte interface, respectively. The slope in the low frequency region belongs to Warburg impedance (Wo) of lithium-ion diffusion in the bulk electrodes [41]. In this study, each Nyquist plot of both the samples exhibit an overlapped semicircle in the high-to-medium frequency region and a slope straight line in the low frequency region. It should be pointed that the semicircle of the high frequency region can be mainly attributed to the Rct. Generally, the Rs for the cathode materials is far less than the Rct. As shown in Fig. 9, the charge transfer resistance (Rct) values of both the samples no matter in which cycle have contributed the main part of the total impedance. However, the ohmic resistance (Rs) values as a tiny part of the total impedance are less than 6 Ω. Furthermore, there is very little difference in the Rs values between the pristine samples and the LR-NMC@0.03 samples, indicating that the modification of 'La3+' does not change the conductivity of the cathode electrode material. The details of the fitting impedance data are recorded in Table 3. It’s remarkable that the Rct values for the pristine sample at the 1st and 100th cycle are both greater than that of the LR-NMC@0.03 sample. Moreover, the Rct values for the pristine sample increase from 177.8 Ω at the 1st cycle to 713.9 Ω at the 100th cycle, while the Rct values for the LR-NMC@0.03 sample increase from 98.7 Ω at the 1st cycle to 226.2 Ω at the 100th cycle. The lower Rct values for the LR-NMC@0.03 sample at the 1st and 100th cycle can be ascribed to the effective inhibition of side reaction on the cathode/electrolyte interface by the LaMnO3 coating. Previous research results [42, 43] show that the dominant factor of the rate performance for the cathode electrode materials was the Rct value. Specifically, the electrode polarization decreases with the decrease of the Rct value, resulting in a better rate performance. It is worth mentioning that 'La3+' doping can effectively broaden the lithium-ion channel in the lattice as shown in Table 1 and further facilitates the transport of lithium-ions, thus improving the rate performance of the 'La3+' modified sample. Overall, the double strengthening effect of the 'La3+' modification promotes the LR-NMC@0.03 sample’s own better rate capability.
To evaluate the cycling stability of the pristine sample and the LR-NMC@0.03 sample, the charge-discharge cycle test had been performed at 1C with the potential window of 2.0–4.6 V. Figure 10 presents the details with regard to the relation between the cycle number and discharge capacity for both the pristine sample and the 'La3+' modified sample. The pristine sample delivers an initial discharge capacity and the discharge capacity after 100 cycles of 212.3 mAh g−1 and 163.1 mAh g−1, respectively, the discharge capacity retention of which is only 76.8%. Interestingly, the discharge capacity retention of LR-NMC@0.03 sample can reach 92.5%, corresponding to the initial discharge capacity is 248.1 mAh g−1 and the 100th cycle discharge capacity is 229.4 mAh g−1. The cyclic discharge curves at 1st, 25th, 50th, 75th, and 100th for both the samples are plotted in Fig. 11. It can be clearly seen that the discharge curves of the LR-NMC@0.03 sample at different cycles are noticeably denser compared with those of the pristine samples, indicating a lower capacity decay rate. The better cyclic stability of the LR-NMC@0.03 sample can be ascribed to the more stable host structure and surface structure for the double strengthening effect by both the 'La3+' doping and LaMnO3 coating. Firstly, the 'La3+' doping makes the cathode electrode material own a better layered structure. And the mixed arrangement degree of Li+ and Ni2+ cations has been lowered as analyzed in the second paragraph of the “Results and discussions” section. In addition, the binding energy of La–O bond is higher than that of Ni–O or Mn–O bond as described in the fourth paragraph of the “Introduction” section, which helps to retain oxygen vacancies in the structure of the cathode material and further improve the structure stability. Secondly, the LaMnO3 coating layer can suppress the corrosion of the cathode electrode material by the electrolyte, preventing the surface structure of the cathode electrode from severe collapse. Actually, one can find that the Rct value of the LR-NMC@0.03 sample only increases from 98.7 Ω at the 1st cycle to 226.2 Ω at the 100th cycle compared with that of the pristine; thus, the slow increase in Rct values during long cycles for the LaMnO3 coating facilitates the migration of lithium-ions across the cathode/electrolyte interface.
Conclusions
The one-step method for 'La3+' doping and LaMnO3 coating is successfully applied to modify the Li1.2Mn0.54Ni0.13Co0.13O2 cathode material. The electrochemical performances of the Li1.2Mn0.54Ni0.13Co0.13O2 for the double strengthening effect by 'La3+' doping and LaMnO3 coating had been improved effectively. The LR-NMC@0.03 sample exhibits lower irreversible capacity loss, higher initial coulomb efficiency, higher discharge capacity, better cyclic performance, and better rate capability compared with those of the pristine sample. The better electrochemical properties for the LR-NMC@0.03 sample can be attributed to stability improvement of its host structure and surface structure by 'La3+' doping and LaMnO3 coating, respectively. It is worth mentioning that one-step method by the 'La3+' modified can be extended to other kinds of cathode materials.
References
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium. Nature 414:359–367
Armand M, Tarascon JM (2008) Building better batteries. Nature 451:652–657
Melot BC, Tarascon JM (2013) Design and preparation of materials for advanced electrochemical storage. Acc Chem Res 46:1226–1238
Larcher D, Tarascon JM (2015) Towards greener and more sustainable batteries for electrical energy storage. Nat Chem 7:19–29
Xu B, Qian D, Wang ZY, Meng YS (2012) Recent progress in cathode materials research for advanced lithium ion batteries. Mater Sci Eng R 73:51–65
Yaoa X, Xua Z, Yaoa Z, Chenga W, Gaob H, Zhaoa Q, Lia J, Zhoua A (2019) Oxalate co-precipitation synthesis of LiNi0.6Co0.2Mn0.2O2 for low-cost and high-energy lithium-ion batteries. Mater Today Commun 19:262–270
Chen S, He T, Su YF, Lu Y, Ban LY, Chen L, Zhang QY, Wang J, Chen RJ, Wu F (2017) Ni-rich LiNi0.8Co0.1Mn0.1O2 oxide coated by dual-conductive layers as high performance cathode for lithium-ion batteries. ACS Appl Mater Interfaces 9:29732–29743
Tsai YW, Hwang BJ, Ceder G (2005) In-situ x-ray absorption spectroscopic study on variation of electronic transitions and local structure of LiNi1/3Co1/3Mn1/3O2 cathode material during electrochemical cycling. Chem Rev 17(12):3191–3199
Wu F, Wang M, Su YF (2009) Effect of TiO2-coating on the electrochemical performances of LiCo1/3Ni1/3Mn1/3O2. J Power Sources 191(2):628–632
Liu W, Li X, Xiong D, Hao Y, Li J, Kou H, Yan B, Li D, Lu S, Koo A, Adair K, Sun X (2018) Significantly improving cycling performance of cathodes in lithium ion batteries: the effect of Al2O3 and LiAlO2 coatings on LiNi0.6Co0.2Mn0.2O2. Nano Energy 44:111–120
Xie HM, Wang RS, Ying JR, Zhang LY, Jalbout AF, Yu HY, Yang GL, Pan XM, Su ZM (2006) Optimized LiFePO4–polyacene cathode material for lithium-ion batteries. Adv Mater 18(19):2609–2613
Wang Y, Wang Y, Hosono E, Wang K, Zhou H (2008) The design of a LiFePO4/carbon nanocomposite with a core–shell structure and its synthesis by an in situ polymerization restriction method. Angew Chem Int Ed 47:7461–7465
Kim DK, Muralidharan P, Lee HW, Ruffo R, Yang Y, Chan CK, Peng HL, Huggins RA, Cui Y (2008) Spinel LiMn2O4 nanorods as lithium ion battery cathodes. Nano Lett 11:3948–3952
Hosono E, Kudo T, Honma I, Matsuda H, Zhou HS (2009) Synthesis of single crystalline spinel LiMn2O4 nanowires for a lithium ion battery with high power density. Nano Lett 3:1045–1051
Liu H, Chen C, Du C et al (2015) Lithium-rich Li1.2Ni0.13Co0.13Mn0.54O2 oxide coated by Li3PO4 and carbon nanocomposite layers as high performance cathode materials for lithium ion batteries. J Mater Chem A 3:2634–2641
Li B, Yan H, Ma J, Yu P, Xia D, Huang W, Chu W, Wu Z (2014) Manipulating the electronic structure of Li-rich manganese-based oxide using polyanions: towards better electrochemical performance. Adv Funct Mater 24:5112–5118
Nayak PK, Grinblat J, Levi M, Levi E, Kim S, Choi JW, Aurbach D (2016) Al doping for mitigating the capacity fading and voltage decay of layered Li and Mn-rich cathodes for Li-ion batteries. Adv Energy Mater 6:1502398
Iddir H, Benedek R (2014) First-principles analysis of phase stability in layered-layered composite cathodes for lithium-ion batteries. Chem Mater 26:2407–2413
Park YJ, Hong YS, Chang SH (2004) Structural investigation and electrochemical behaviour of Li [NixLi1/3-2x/3Mn2/3-x/3]O2 compounds by a simple combustion method. J Power Sources 129:288–295
Yabuuchi N, Yoshii K, Myung ST, Nakai I, Komaba S (2011) Detailed studies of a high-capacity electrode material for rechargeable batteries, Li2MnO3-LiCo1/3Ni1/3Mn1/3O2. J Am Chem Soc 133:4404–4419
Lee CW, Sun YK, Prakash J (2004) A novel layered Li [Li0.12NizMg0.32−zMn0.56]O2 cathode material for lithium-ion batteries. Electrochim Acta 49:4425–4432
Park SH, Sun YK (2003) Synthesis and electrochemical properties of layered Li [Li0.15Ni(0.275−x/2)AlxMn(0.575−x/2)]O2 materials prepared by sol-gel method. J Power Sources 119:161–165
Park C, Kim S, Nahm K et al (2008) Structural and electrochemical study of Li [CrxLi(1-x)/3Mn2(1-x)/3]O2 (0≤x≤0.328) cathode materials. J Alloys Compd 449:343–348
Zhang XF, Belharouak I, Li L, Lei Y, Elam JW, Nie A, Chen XQ, Yassar RS, Axelbaum RL (2013) Structural and electrochemical study of Al2O3 and TiO2 coated Li1.2Ni0.13Mn0.54Co0.13O2 cathode material using ALD. Adv Energy Mater 3(10):1209–1307
Kong JZ, Zhai HF, Qian X, Wang M, Wang QZ, Li AD, Li H, Zhou F (2017) Improved electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 cathode material coated with ultrathin ZnO. J Alloys Compd 694:848–854
Wang ZY, Liu EZ, Guo LC, Shi CS, He CN, Li JJ, Zhao NQ (2013) Cycle performance improvement of Li-rich layered cathode material Li [Li0.2Mn0.54Ni0.13Co0.13]O2 by ZrO2 coating. Surf Coat Technol 235:570–576
Qiu B, Zhang MH, Wu LJ, Wang J, Xia YG, Qian DN, Liu HD, Hy S, Chen Y, An K, Zhu YM, Liu ZP, Meng YS (2016) Gas–solid interfacial modification of oxygen activity in layered oxide cathodes for lithium-ion batteries. Nat Commun 7:12108
Speight JG (2005) Lange’s handbook of chemistry [M]. Mc Graw-Hill, New York
Deng H, Belharouak I, Sun YK (2009) LixNi0.25Mn0.75Oy (0.5 ≤x ≤2,2 ≤y≤2.75) compounds for high –energy lithium –ion batteries. J Mater Chem 19(26):4510–4516
Jarvis KA, Deng Z, Allard LF (2011) Atomic structure of a lithium-rich layered oxide material for lithium-ion batteries: evidence of a solid solution. J Mater Chem 23(16):3614–3621
Myungs T, Izumi K, Komaba S (2005) Role of alumina coating on Li-Ni-Co-Mn-O particles as positive electrode material for lithium-ion batteries. J Mater Chem 17(14):3695–3704
Ding Y, Zhang P, Jiang Y (2007) Effect of rare earth elements doping on structure and electrochemical properties of LiNi1/3Co1/3Mn1/3O for lithium-ion battery. Solid State Ionics 178:967–971
Li N, An R, Su YF (2013) The role of yttrium content in improving electrochemical performance of layered lithium -rich cathode materials for Li-ion batteries. J Mater Chem A 1:9760–9767
Arunkumar TA, Wu Y, Manthiram A (2007) Factors influencing the irreversible oxygen loss and reversible capacity in layered Li [Li1/3Mn2/3]O2-Li [M]O2 (M=Mn0.5-yNi0.5-yCo2y and Ni1-yCoy) solid solutions. Chem Mater 19:3067–3073
Wu Y, Manthiram A (2006) High capacity, surface-modified layered Li [Li(1-x)/3Mn(2-x)/3Nix/3Cox/3]O2 cathodes with low irreversible capacity loss. Electrochem. Solid-State Lett 9:A221–A224
Liu W et al (2015) Countering voltage decay and capacity fading of lithium-rich cathode material at 60C by hybrid surface protection layers. Adv Energy Mater 5:201500274
Florian S, Hana B, Mudit D et al (2017) From surface ZrO2 coating to bulk Zr doping by high temperature annealing of nickel-rich lithiated oxides and their enhanced electrochemical performance in lithium ion batteries. Adv Energy Mater 7:1701682
Ghanty C, Markovsky B, Erickson EM et al (2015) Li+-ion extraction/insertion of Ni-rich Li1+x (NiyCozMnz)wO2 (0.005<x<0.03; y:z=8:1, w≈1) electrodes: in situ XRD and Raman spectroscopy study. ChemElectroChem 2:1479
Li J, Downie LE, Ma L, Qiu W, Dahn J (2015) Study of the failure mechanisms of LiNi0.8Mn0.1Co0.1O2 cathode material for Lithium ion batteries. J Electrochem Soc 162:A1401
Liu J, Yang Y, Yu P, Li Y, Shao H (2006) Electrochemical characterization of LaNi5-xAlx (x=0.1-0.5) in the absence of additives. J Power Sources 161:1435
Tan KS, Reddy MV, Subba GV, Chowdari BV (2005) Effect of AlPO4-coating on cathodic behaviour of Li (Ni0.8Co0.2)O2. J Power Sources 141:129
Jafta CJ, Ozoemena KI, Mathe MK, Roos WD (2012) Synthesis, characterisation and electrochemical intercalation kinetics of nanostructured aluminium-doped Li [Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for lithium ion battery. Electrochim Acta 85:411–422
Liu J, Jayan BR, Manthiram A (2010) Conductive surface modification with aluminum of high capacity layered Li [Li0.2Mn0.54Ni0.13Co0.13]O2 cathodes. J Phys Chem C 114:9528–9533
Funding
This work was supported by the Science and Technology Program of Sichuan Province under grant number 2019YJ0539.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tang, Y., Han, X., Zhang, W. et al. La doping and coating enabled by one-step method for high performance Li1.2Mn0.54Ni0.13Co0.13O2 Li-rich cathode. Ionics 26, 3737–3747 (2020). https://doi.org/10.1007/s11581-020-03551-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03551-3