Abstract
Sulfonated silica particles are admixed with sulfonated poly (ether ether ketone) (SPEEK)/sulfonated poly (vinylidene fluoride-co-hexafluoropropylene) (SPVdF-HFP), with various ratios by means of solvent casting. X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), scanning electron microscope (SEM), atomic force microscopy (AFM), and energy-dispersive X-ray spectroscopy (EDAX) were employed for characterizing the polymer electrolytes. Physicochemical and electrochemical characterizations such as ion exchange capacity, water uptake, swelling ratio, lambda values, temperature-dependent proton conductivity, and performance for prepared polymer composites are also analyzed. From the XRD and FTIR confirms the phase analysis and complex formation of the prepared polymer electrolytes. For 6 wt% S-SiO2, incorporated polymer membrane shows the high water uptake (36.5%), swelling ratio (15.9%), and ion exchange capacity (1.70 meq g−1) values compared to the respective samples. The highest proton conductivity value obtained for the 6 wt% S-SiO2 incorporated polymer membrane of 80 wt% SPEEK-20 wt% SPVdF-HFP is 7.9 × 10−2 S cm−1. The current density and power density value of 354 mA cm−2 and 110 mW cm−2 with an OCV of 0.95 V at 90 °C under the 100% RH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the worldwide rising attentiveness for clean and ecological power sources, fuel cells attract an increasing attention in one of the promising renewable energy technologies. Among the different kinds of fuel cells, proton exchange membrane fuel cells (PEMFCs) are the important candidate for both stationary and portable power applications [1, 2]. PEMFCs have numerous physicochemical advantages over other types of fuel cells. They can always function at low temperature with high current densities. PEMFCs have extended load life owing to solid electrolyte, fast start-ups owing to lean alignment, high energy competence, and ability for irregular operation (tolerant to many starts and stops) [3,4,5,6,7]. Following, PEMFC does not manufacture pollutants such as NOx or CO, and when hydrogen is used as fuel, the only chemical by product is water. These are the reasons; PEMFC becomes a perfect power source for a zero emission vehicle (ZEV). Each of these aspects make PEMFCs, a valuable alternative for a series of power utilization, ranging from watt stage for portable micropower to kilowatt levels for taking to megawatts for large-scale stationary power systems in inhabited and spread generation. The membrane electrode assembly (MEA) setup is considered as the core of the fuel cell, where all useful electrochemical reactions take place and determine the whole-cell performance. An MEA setup is assembled by the sandwich of the polymer electrolyte membrane between the two electrodes. Among the components of MEA, the polymer electrolyte membrane (PEM) is a pivotal component and governs the performance of the fuel cell [8,9,10].
Although, the sulfonation stage of too high degree could lead to excess swelling [11]. So, changes are taken out on SPEEK owing the absorption of inorganic fillers. Roelofs et al. [12] studied a hybrid membrane of SPEEK/ dihydrogen imidazole-modified silica. They utilized in the membrane resides of a hydrolysable inorganic fraction and a functional organic group. This choice led to an elevated water-to-ethanol membrane selectivity. Rangasamy et al. [13] calculated the result of functionalized sulfonic acid groups on the fuel cell performance using silica-doped SPEEK and pure SPEEK. They exposed that at a superior degree of sulfonation, the ion swap ability, water uptake, and ionic conductivity amplified.
Newly, there has been an increasing obligation for cost-efficient polymeric materials with developed properties such as proton conductivity, chemical stability, and mechanical strength [14]. Compound membranes comprising of poly (vinylidene fluoride) (PVDF), poly (vinylidene fluoride-co-hexafluoropropylene (PVdF-HFP), and an inorganic material arouses for much advantage in the fuel cell performance. PVdF-HFP has been used as a matrix material for the cause of its developed solubility in organic solvents, low glass transition temperature, and decreased crystallinity [15]. A PVdF-HFP film is typically slights than PVDF in mechanical strength owing to the formless character of the HFP component. Sulfonated poly(vinylidene fluoride-co-hexafluoropropylene) (SPVdF-HFP) have both hydrophilic sulfonated group and vinylidene fluoride blocks. Thus, the presence of these sulfonic acid group upsurges physicochemical properties. The reason for blending SPVdF-HFP is the proton-conductive sulfonated groups that further strengthen the proton migration pathways through hydrophilic water-mediated channels and nanofillers especially metal oxides giving better performance. Acid-acid interaction created by blending of SPEEK with SPVdF-HFP improves the stability of the electrolyte membrane for the efficient fuel cell application [16, 17]. This blending makes them cost-effective separation; high-quality of mechanical, chemical resistances; and huge liquid uptake that gives a fluid like conductivity [18, 19]. Silica is the important common inorganic filler used in the fuel cells, mainly for proton swap over membrane fuel cell, and it has participation and significant role in raising the act of fuel cells by improving their membrane properties [20]. At present, silica has been generally applied in different technique of membranes, like fluorinated membranes (Nafion), sulfonated membranes (SPEEK, SPS, SPAES, SPI), and other organic polymer matrixes [21, 22]. The merging of silica into membrane matrices has improved the thermal stability, mechanical strength, water retention capacity, and proton conductivity of the membrane. Although the adding of silica into the membrane matrices has carried lots of advantages to the fuel cell act, there are now some restrictions that must be addressed to obtain constant among fuel cell quality, possibility, and economics. Following the level of development reached by silica-based hybrid membranes is now not sufficient to source the membrane fuel cell to be commonly for profit. There is still obstruction creating the ideal membrane with high proton conductivity, low fuel crossover, and outstanding act without considering the temperature and the humidity level. A technique is essential for the mixture of a membrane that can profit from fuel cell utilization. Many studies have been preferred on PVdF-HFP-PEG-alumina, PVdF-HFP-stannous oxide, PVdF-HFP-poly (benzimidazole), and PVdF-HFP-poly (styrene) [23,24,25,26,27,28]. To the best of our knowledge, there has been no previous study of proton taken out polymer found on SPEEK, SPVdF-HFP, and sulfonated silica. Therefore, the important aim of this investigation on SPEEK/SPVdF-HFP and sulfonated SiO2 nanocomposite membrane for PEM fuel cell application.
Experimental sections
Materials
Poly vinylidene fluoride-co-hexa fluoro propylene (PVdF-HFP), silica (SiO2) was obtained from Sigma-Aldrich. PEEK polymer powder was procured from Victrex, Mumbai. N-methyl-2-pyrrolidinone, (99%) (NMP), sulfuric acid (98%) (H2SO4), methanol (99%) (CH3OH), and dichloroethane (99%) (DCE) was obtained from Merck, India.
Sulfonation of PEEK
PEEK was sulfonated as reported in our previous work [29]. In brief, PEEK polymers were mixed into concentrated sulfuric acid in steady stirring condition at room temperature. Following that, the solution was stirred for 7 h under nitrogen atmosphere and next added into more ice water. The SPEEK was rinsed with deionized water until pH was 7 and then dried at 70 °C overnight. The DS was estimated to be 65% by the acid–base titration method, proton NMR method, and CHNSO analysis [29,30,31]. The sulfonation of PEEK scheme is shown in Scheme 1.
Sulfonation of PVdF-HFP polymer
Sulfonation of PVdF-HFP was brought out according to the following method [17, 32]: first, co-polymer pellets were dried in a vacuum oven for 12 h at 60 °C. Then, 20 ml of chloro sulfonic acid was heated at 60 °C in a round-bottom flask and then co-polymer pellets were added into the acid solution carefully in a constant stirring form. After 7 h, black pellets were obtained and washed with 1,2-dichloroethane, methanol, and deionized water in the same way and finally dried in vacuum oven at 60 °C. The DS was resolute to be 2.1% by acid–base titration method [33]. The sulfonation of PVdF-HFP scheme is shown in Scheme 2.
Sulfonation of SiO2
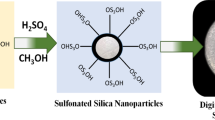
The sulfonated silica was ready by utilizing sulfuric acid according to the method reported by Selvakumar et al. [34]. One gram of SiO2 was mixed in 20 ml methanol solution having 1 M sulfuric acid under vigorous stirring for 12 h. The resultant product was mixed together by centrifugation and finally dried at 100 °C for 12 h to obtain the sulfonated silicon dioxide nanopowder. The sulfonation of SiO2 (S-SiO2) is shown in Scheme 3.
Preparation of polymer composite
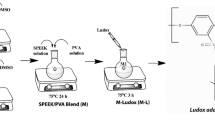
SPEEK (80 wt%) and SPVdF-HFP (20 wt%) were dissolved in NMP solvent and magnetically stirred for 12 h at room temperature. A suitable amount of sulfonated SiO2 (02, 04, 06 and 08 wt%) was added into the mixture and stirred for another 12 h at 60 °C and degassed to remove air bubbles. The resultant solution was poured on a petri dish, and the samples were exposed to drying (60 °C for 12 h) in a vacuum oven. After drying the polymer composites, they were stripped from the petri dish and kept in a desiccator. Thickness of the prepared composite film was estimated with the help of micrometer, and it is in the range of 50 μm. These proton-conducting polymer electrolytes were analyzed by various structural, morphological, physicochemical, and electrochemical studies.
Characterization of membranes
The water uptake (WU) of the composite membranes was determined in the accompanying way: a small piece from every prepared composite membrane was dipped in 24 h. The water uptake (WU) was calculated by the following equation:
where Wwet represents the weight of wet membranes, Wdry represents the weight of dry membranes. Swelling behavior was determined by means of change in surface area and thickness before and after hydration of the membrane. Membrane samples of 2 cm × 2 cm dimension were taken, and its thickness was measured using digital micrometer. The following equation helps for determining swelling ratio:
where Wwet in the weight of the membranes and Wdry belongs to the weight of the dry membrane.
Titration system was utilized to find the ion exchange capacity (IEC) of the prepared membranes. First, the membranes were engrossed in 1 M NaCl solution at 25 °C for 24 h to replace the H+ ions with the Na+ ions in the solution. After that, the solution was titrated with a 0.01 M NaOH solution using phenolphthalein as an indicator. The IEC values (meq g −1) can be resolute from the following formula:
Anywhere, volume and normality are taken for NaOH solution (ml) and mass of the dry weight of polymer membrane (g). The proton conductivities of membranes were tested by an AC impedance spectroscopy at RT to 80 °C using an Auto lab potentiostat/galvanostat with a frequency range of 0.1 Hz to 1 MHz with voltage amplitude of 50 mV. The proton conductivity of membranes was calculated from the exacting impedance data with the following formula:
where L is the thickness of samples (cm), A is the surface area of samples (cm2), and Rb is the bulk resistance of the samples (Ω). Zeta potential analysis was carried out by zeta sizer (Malvern instrument Ltd., UK). The structural property of the prepared membranes was analyzed through the X-ray diffraction technique by the (X’Pert PRO PANalytical) diffractometer instruments in the angular rotation of 5–80°. Fourier transform infrared (FTIR) spectrum was obtained by means of a Perkin Elmer 4000–400 cm−1. The morphology of the membranes was experiential by scanning electron microscope; energy-dispersive X-ray spectroscopy (EDAX) was scrutinized using EVO 18 Carl Zeiss from Germany. Topographical difference on SPEEK and prepared polymer composites were observed by AFM model 5500, Agilent Technologies Inc., PA. The INSTRON 3365 universal tensile testing machine is utilized (5 × 1 cm) to test the samples’ rigid property in room temperature at direction of movement of 1 cm/min.
Diffusion layer preparation for MEA (membrane electrode assembly) setup: The diffusion slurry ink was prepared with the proper mixing of 70 wt% Vulcan XC-72 (USA), 30 wt% Nafion solution, fixed amount of double-distilled water, and isopropyl alcohol subjected to ultrasonication for 2 h. The black ink coated carbon cloth was dried in box furnace at 380 °C for 7 h.
Anode and cathode preparation: The catalyst slurry inks for the anode and cathode were set up with the guide of carbon supported platinum black utilizing platinum loading 0.025 to 0.25 mg cm−2. Appropriate measures of double-distilled water and isopropyl alcohol were mixed to the slurry in an ultrasonicator. Obtained black catalyst slurry was coated onto the respective diffusion layers and dried in a vacuum oven at 100 °C for 3 h and 380 °C in box furnace for 7 h. Hot pressing the sample of 80 wt% SPEEK/ 20 wt% SPVdF-HFP/ 06 wt% S-SiO2 sandwiched between the prepared anode and cathode and then pressed at 90 °C with a pressure of 1.7 ton for 3 min.
MEA setup for PEMFC: The MEA was obtained by sandwiching the 80 wt% SPEEK/20 wt% SPVdF-HFP/06 wt% S-SiO2 membrane between the anode and cathode. Electrocatalyst of 40 wt% Pt:C (1:1) on Vulcan XC-72 in both sides of anode and cathode loading (0.5 mg cm−2). Systematizing the aforementioned setup by incorporating two gas diffusion layers on both sides of catalyzed membrane with the SerPentine channel-based bipolar plates. Flow rates for both hydrogen and oxygen gases were kept as 200 ml/min and 500 ml/min. The MEA was assembled in a 5-cm2 single-cell PEMFC with single serpentine flow fields [Anabond Sainergy Fuel Cell Private Limited, Chennai].
Results and discussion
Zeta potential analyses predict the charged surface in the effects of sulfonation on the surface of silica nanoparticles and unsulfonated nanoparticles by varying the different pH value, and analysis is tabulated in Table 1. From the observations, increasing the pH value, the negatively charged zeta potential is increased due to presence of polar molecules on the nanoparticle surface (sulfonic acids). This result confirms the presence of SO3H groups attached covalently to the silica surface through the condensation process.
The XRD spectra of PVdF-HFP, SPVdF-HFP, PEEK, and SPEEK are debits in Fig. 1. From Fig. 1a, the pure PVdF-HFP characteristic peaks such as 2θ = 17°, 19°, 26° and 39° corresponding to α (100), (α + β) (020), (α + γ) (110), and α (021) confirm the semicrystalline nature. After sulfonation of PVdF-HFP, the characteristic peaks indicate the amorphous nature of the prepared polymer electrolytes [32]. The pure PEEK of 2θ = 18.87°, 20.7°, 23°, and 28.9° which indicates the diffraction pattern of (110), (111), (200), and (211) corresponds to the crystalline planes [35]. After sulfonation, the 2θ value is changed to 20°, which indicates the mixture of both amorphous and crystalline nature of SPEEK. From Fig. 2, it can be seen that by increasing the amount of sulfonated silica from 2 to 6 wt%, the crystallinity of the prepared polymer electrolytes decreased which is confirmed by the presence of broad diffraction pattern with no distinct peak ascribed to the compatible effect of S-SiO2 and the blend. This makes the blended membrane of optimized S-SiO2 ratio to be more flexible and amorphous in nature.
The FTIR spectra of pure silica and sulfonated silica are depicted in Figs. 3 and 4 show the blended polymer composite of SPEEK/SPVdF-HFP-S-SiO2. The vibrational peak observed at 1050 cm−1 and 3400 cm−1 indicates the Si–O–Si bonds and OH groups of pure silica particle. The symmetric and asymmetric stretching vibrations of SO2 group of the S-SiO2 were found in peaks at 1171 and 1286 cm−1. At the same stretch, the sulfonation of silica is confirmed by the increased intensity at 1640 and 3400 cm−1 of OH molecules that bound with the sulfonic acid groups. The vibrational peaks observed at 1023 cm−1, 1074 cm−1, and 1247 cm−1 were assigned to the asymmetric and symmetric vibration of O=S=O and symmetric stretching vibration of S=O on sulfonic acid group present in the prepared polymer electrolytes. The vibrational band observed at 575 cm−1 is more intense for the prepared composites due to the sulfonic acid moieties of silica sulfuric acid [36,37,38]. The vibrational peak observed at 1724 cm−1 is attributed to the bending of protonated water molecule, and 1410 cm−1 confirms the absorbed water molecule.
The elemental presentation of the sulfonated silica nanoparticles is displayed in Fig. 5a. From the picture, the sulfur (S) group exists on the surface of sulfonated silica nanoparticles, which inveterate that the hydroxyl group of silica condensed with the organically grafted sulfonic acid groups (–SO3H) [39]. Morphology of SPEEK and prepared polymer electrolytes is portrayed in Fig. 5b–f. The SPEEK morphograph was ascribed for the smoothness in surface with no crack and pores present in the system. However, after blending with SPVdF-HFP and incorporation of sulfonated silica nanoparticle, there is an alteration in the morphology. The hydrogen bond between –OH of S-SiO2 and SO3H group facilitated better dispersion and improved the compatibility between the organic and inorganic components. It also witnessed the in situ grown sulfonated silica nanoparticles at low percentage of 02–06 wt% within the composite membranes which increase in the number of sulfonated silica particles rather than their size that increase for the better interlinking between the sulfonated silica particles. However, upon the addition of 08 wt% S-SiO2 particles, it starts agglomerating, which is liable for the lower performance in fuel cell test as shown in Fig. 5f [32, 40, 41]. Figure 6a–e represents the topographical images of SPEEK and prepared polymer composite electrolytes. SPEEK membrane exhibits the smooth with low surface defects. After incorporating SPVdF-HFP and sulfonated silica, it exhibited entirely different surface behavior in comparison to the SPEEK. Increasing the amount of sulfonated silica simultaneously increases the roughness value of the prepared polymer electrolytes. The bright and dark spots are due to the presence of hydrophilic/hydrophobic parts that creates proton conducting channels [42]. This is due to the hydrogen bond formation between the oxygen containing functional groups of sulfonated silica surface with SO3H group in SPEEK and SPVdF-HFP prepared polymer electrolytes [43].
The water uptake (WU) and swelling ratio (SR) are two indispensable parameters for PEM fuel cells [39, 44, 45]. Table 2 lists out the values for water uptake, swelling, IEC, and λ parameter of the pure and prepared polymer composite membranes. The water uptake of the prepared polymer membranes increases with the increase in sulfonated silica content in the membrane. This is due to the hydrophilic group (SO3H) of sulfonated silica interacts with water via electrostatic bond or hydrogen bond. The 6 wt% sulfonated silica membrane possessed the higher water uptake at 36.5% at room temperature owing to its most hydrophilic content. The higher water content of the polymer membranes ionizes the higher amount of sulfonic acid moieties and is responsible for proton conductivity. For 8 wt% of sulfonated silica, this validated for the decrease in water uptake parameter due to higher agglomeration of sulfonated silica in the prepared polymer electrolytes [46,47,48,49]. Simultaneously, the swelling ratio of the prepared polymer composite electrolytes also elevated with the increase of sulfonated silica content in accordance with water uptake [50]. The enhanced IEC values of the polymer composite electrolytes with increasing the sulfonated silica well agreed with water uptake parameter. The sulfonated silica contains –SO3H moieties responsible for the high mobility for the ionizable group that inherits the ease of movement of ions via hopping mechanism [51]. Hydration number or λ value which denotes the number of water molecules per ion exchangeable group and its value increases by the addition of sulfonated silica in the prepared polymer composite electrolytes [52, 53].
The proton conductivity is a very essential property in fuel cell device. Proton conductivity is one of the major phenomena for PEM and it is attained to improve high voltage, current, and power densities. The Arrhenius behaviors of the prepared polymer electrolytes were calculated and shown in Fig. 8. Proton conductivity of the prepared polymer composite membranes in various temperatures is displayed in Table 3. The proton conductivity of the composite membranes is increased by two reasons: (i) excess concentration of SO3H moieties in the silica nanoparticle surface due to high surface area and (ii) intrinsic character of sulfonated silica nanoparticles retain bound water molecules both by the physically and chemically bonded. The SPEEK membrane obtained the value of 2.3 × 10−3 S/cm by the nature of intrinsic sulfonic acids in the polymer backbone. The SPVdF-HFP polymer was blended with SPEEK matrix; the conductivity decrease due to the acid–acid interaction led to consumption of protons or cations (H+) that forbids the hopping mechanism. Besides, increasing the sulfonated silica content in the SPEEK/ SPVdF-HFP polymer matrix increases the conductivity value. This phenomenon is due to interactions between the SO3H group of SPEEK, SPVdF-HFP polymer, and sulfonated silica nanoparticles. This confirms the hydrophilic nature of nanoparticles which observe more water molecules in the SPEEK/SPVdF-HFP blended membrane matrix which interconnected the hydrophilic domains. These interconnected hydrophilic clusters that facilitate the more efficient pathway for proton migration through the water mediator channels and results enhanced proton conductivity were observed. In the present study, increase in temperature increases the conductivity due to the more polymer chain segmental moment and favorable dissociation of sulfonic acid protons in the polymer backbone chains. These infer that the addition of silica sulfonic acid creates hydrophilic nature in the SPEEK and SPVdF-HFP polymers matrixes and well-interconnected channels for ion movement in the polymer electrolytes. The hygroscopic property of the sulfonic acid-coated silica nanoparticles more pronounced the increase of proton conductivity in the prepared polymer composite membranes at high temperatures. The proton conductivity of SPEEK (80 wt%)/SPVdF-HFP (20 wt%)/S-SiO2 (06 wt%) at 90 °C is 7.9 × 10−2 S/cm which is larger compared to bare SPEEK. Such a result denotes the rapid motion of proton-conducting sites and enhanced activity of hydronium ions at high temperature [54, 55]. At 8 wt% sulfonated silica-prepared polymer electrolytes, the proton conductivity was decreased by the filler agglomeration effect due to the poor dispersion in the matrix. The temperature-dependent proton conductivity of pure and nanocomposite of the prepared proton-conducting polymer electrolytes in the temperature region of 90 °C is shown in Fig. 7. The following equation can be explained by Arrhenius equation:
where σ0 is the pre-exponential factor, Ea is the activation energy, k is the Boltzmann constant, T is the absolute temperature, σ is the activation energy. As shown in Fig. 8, the activation energy values (Ea) were observed that are decreasing in the prepared nanocomposite membranes. The activation energy was decreased with increasing concentration of sulfonated silica nanoparticle compared to the pure SPEEK membrane. The SPEEK membrane obtained the value of 15.71 kJ/mol and nanocomposite membrane matrix in the range of 14.30 to 10.93 kJ/mol that indicate the proton conduction was transferred easier by the influence of sulfonated silica. At 8 wt% of sulfonated silica, nanoparticle presence in the SPEEK/SPVdF-HFP matrix pathways for proton conduction in the prepared polymer electrolytes means the blocking effect occurred in the system.
The mechanical properties of the pure SPEEK and prepared polymer electrolytes based on sulfonated silica are tabulated in Table 4. The SPEEK membranes showed the tensile strength and elastic modulus in order of the range of 33.7 MPa and 653 MPa respectively. The elongation at break is 41% that is higher compared to other prepared membrane. The increasing content of sulfonated silica in the SPEEK/SPVdF-HFP matrix increases the tensile strength and elastic modulus and decreases the elongation at break. The hydrogen bonding interaction between –SO3H moieties in SPEEK, SPVdF-HFP, and –OH group in sulfonated silica particle increases mechanical strength due to the rigid structure of the nanocomposite matrix. This rigid orientation of the polymer matrix favors the brittleness and the sequence produces the reduced elongation at break [56]. The tensile strength and elastic modulus was increased up to 6 wt% but at 8 wt% of silica content could cause inverse effect due to the agglomeration of silica in the prepared polymer electrolytes. The elongations at break of the prepared nanocomposite polymer membranes were lower than the pure SPEEK membrane and decrease with increase in the concentration of sulfonated silica in the SPEEK membrane. This is due to the reinforcing effect of sulfonated silica with the SPEEK/SPVdF-HFP matrix in the prepared polymer nanocomposite membranes [57].
The single cell setup was carried out to study the polarization curve of the prepared polymer electrolyte membrane. The fuel cell tests were run up to 90 °C under the 100% RH with the required flow rate of hydrogen and oxygen gas (Fig. 9). The membrane electrode assembly was used to find out the open-circuit voltage. The prepared membrane of 80 wt% SPEEK/20 wt% SPVdF-HFP/06 wt% S-SiO2 obtained the current density and power density of 354 mA cm−2 and 110 mW cm−2, respectively. This composition displays the 0.95 V of OCV at 90 °C under the 100% RH. The highest open-circuit voltage was achieved in the membrane inferring that the membrane withstands long durability during the operation. This enriched stability shows that the membrane frees from the free radical reaction effect. The free radicals were created in the electrode/electrolyte interface during the incomplete reduction process in the cathode site [58]. The effective interplay of sulfonic acid of SPEEK, SPVdF-HFP, and OH of sulfonated silica produces the shielding effect of functional groups. These preserve the functional groups from degradation and in the same event, the metal oxide normally behaves as the free-radical scavenger. These consequences further promote the oxygen reduction reaction in the electrode site and create fewer barriers for the transport of the protons in the electrode/electrolyte interface. This result indicates that the composite membrane can be used as potential candidate for PEM fuel cell applications.
Conclusion
The composite membranes based on SPEEK and SPVdF-HFP with various concentrations of sulfonated silica were prepared by solvent casting technique. The hydrogen bonding and complexation between sulfonated SPEEK and SPVdF-HFP were confirmed by FTIR examination. SEM pictures showed that composite membranes have homogeneous morphology. The enhancement of proton conductivity is attributed to the presence of sulfonated SiO2. The hydrophilic nature promotes the ion channels and swells the membrane which results in enhanced proton conductivity. The maximum proton conductivity value was found to be 7.9 × 10−2 S/cm with the current density and power density of 354 mA cm−2 and 110 mW cm−2 at an OCV of 0.95 V in 90 °C under the 100% RH for 06 wt% S-SiO2within the blend of 80 wt% SPEEK-20 wt% SPVdF-HFP. It is concluded that the composite membrane (MR3) is a potential candidate for the development of PEM fuel cell.
References
Pandey J, Shukla A (2014) PVDF supported silica immobilized phosphotungstic acid membrane for DMFC application. Solid State Ionics 262:811–814
Zhang H, Shen PK (2012) Recent development of polymer electrolyte membranes for fuel cells. Chem Rev 112:2780–2832
Zhang J, Xie Z, Zhang J (2006) High temperature PEM fuel cells. J Power Sources 160(2):872–891
ChaSuk W, Colella W, Prinz FB (2006) Fuel cell fundamentals. John Wiley&Sons, New York
LeeJi Y, Yoo M, Cha K (2009) Lifecycle cost analysis to examine the economical feasibility of hydrogen as an alternative fuel. Int J Hydrog Energy 34:4243–4255
Peighambardoust SJ, Rowshanzamir S, Amjadi M (2010) Review of the proton exchange membranes for fuel cell applications. Int J Hydrog Energy 35:9349–9384
Handbook, fuel cell. EG & G technical services. Inc., Albuquerque, NM, DOE/ NETL-2004/1206;2004
Wee JH (2007) Applications of proton exchange membrane fuel cell systems. Renew Sust Energ Rev 11:1720–1738
Chandan A, Hattenberger M, Ahmad E-K (2013) High temperature(HT) polymer electrolyte membrane fuel cells (PEMFC)—a review. J Power Sources 231:264–278
Rayment C, Sherwin S (2003) Introduction to fuel cell technology. University of Notre Dame; 49–150
Jiang Z, Zhao X, Manthiram A (2013) Sulfonated poly (ether ether ketone) membranes with sulfonated graphene oxide fillers for direct methanol fuel cells. Int J Hydrog Energy 38:5875–5884
Roelofs KS, Hirth T, Schiestel T (2011) Dihydrogenimidazole modified silica-sulfonated poly (ether ether ketone) hybrid materials as electrolyte membranes for direct ethanol fuel cells. Mater Sci Eng B 176:727–735
Rangasamy VS, Thayumanasundaram S, Seo JW (2015) Vibrational spectroscopic study of pure and silica-doped sulfonated poly (ether ether ketone) membranes. Spectrochim Acta A Mol Biomol Spectrosc 138:693–699
Ren S, Sun G, Li C (2006) Sulphonated poly (ether ether ketone)/polyvinylidene fluoride polymer blends for DMFCs. Mater Lett 60:44
Stephan AM, Teeters D (2003) Characterization of PVdF-HFP polymer membranes prepared by phase inversion techniques I. Morphology and charge-discharge studies. Electrochim Acta 48:2143
Devi AU, Neelakandan S, Nagendra A (2016) Highly selective sulfonated poly(vinylidene fluoride-cohexafluoropropylene)/poly(ether sulfone) blend proton exchange membranes for direct methanol fuel cells. J Appl Polym Sci 133:43907
Neelakandan S, Rana D, Matsuura T, Muthumeenal A, Kanagaraja P, Nagendran A (2014) Fabrication and electrochemical properties of surface modified sulfonated poly(vinylidenefluoride-co-hexafluoropropylene) membranes for DMFC application. Solid State Ionics 268:35–41
Quartarone E, Carollo A, Tomasi C (2007) Relationship between microstructure and transport properties of proton- conducting porous PVDF membranes. J Power Sources 168:126
Neelakandan S, Ramachandran R, Fang ML, Wang L (2019) Improving the performance of sulfonated polymer membrane by using sulfonic acid functionalized hetero-metallic metal-organic framework for DMFC applications. Int J Energy Res 43:3756–3767
Ying YP, Kamarudin SK, Masdar MS (2018) Silica-related membranes in fuel cell applications: an overview. Int J Hydrog Energy 16068-16084
Lufrano F, Baglio V, Di Blasi O (2012) Solid polymer electrolyte based on sulfonated polysulfone membranes and acidic silica for direct methanol fuel cells. Solid State Ionics 216:90–94
Padmavathi R, Karthikumar R, Sangeetha D (2012) Multilayered sulphonated polysulfone/silica composite membranes for fuel cell applications. Electrochim Acta 71:283–293
Gnana kumar G, Kim AR, Nahm KS (2011) High proton conductivity and low fuel crossover of polyvinylidene fluorideehexafluoro propylene silica sulfuric acid composite membranes for direct methanol fuel cells. Curr Appl Phys 11:896–902
Krishnan NN, Henkensmeier D, Jang JH (2011) Sulfonated poly (ether sulfone)-based silica nanocomposite membranes for high temperature polymer electrolyte fuel cell applications. Int J Hydrog Energy 36:7152–7161
Gnana kumar G, Kim P, Nahm KS (2007) Structural and characterization of PVdF-co-HFP/PEG/Al2SO3proton conducting membranes for fuel cells. J.Membr.Sci 303:126–131
Gnana kumar G, Kim AR, Nahm KS (2010) High ion and low molecular transportation of the polyvinylidene fluoride-hexa fluoro propylene hybrid membranes for the high temperature and lower humidity direct methanol fuel cell applications. J Power Sources 195:5922–5928
Hazarika M, Jana T (2013) Novel proton exchange membrane for fuel cell developed from blends of polybenzimidazole with fluorinated polymer. Eur Polym J 49:1564–1576
Acar O, Sen U, Ata A (2010) Blend membranes from (2,5 benzimidazole) and poly (Styrenesulfonic acid) as proton conducting polymer electrolytes for fuel cells. J Mater Sci 45:993–998
Selvakumar K, Rajendran K, Ramesh Prabhu M (2018) Influence of barium zirconate on SPEEK-based polymer electrolytes for PEM fuel cell applications. Ionics 25:2243–2253. https://doi.org/10.1007/s11581-018-2613-4
Mai Z, Zhang H, Li X (2011) Sulfonated poly(etheretherketone) and sulfonated polyvinylidene fluoride – co- hexafluoropropylene based blend exchange membranes for direct methanol fuel cell applications. J Power Sources 196:482
Salarizadeh P, Javanbakht M, Pourmahdian S (2016) Enhancing the performance of SPEEK polymer electrolyte membranes using functionalized TiO2 nanoparticles with proton hopping sites. Solid State Ionics 6(57):51599–51608
Das S, Kumar P, Dutta K, Kundu PP (2014) Partial sulfonation of PVdF-co-HFP: a preliminary study and characterization for application in direct methanol fuel cell. Appl Energy 113:169–177
Selvakumar K, Ramesh Prabhu M (2018) Investigation on meta-polybenzimidazole blend with sulfonated PVdF-HFP proton conducting polymer electrolytes for HT-PEM fuel cell application. J Mater Sci Mater Electron 29:15163–15173. https://doi.org/10.1007/s10854-018-9658-z
Selvakumar K, Rajendran S, Ramesh Prabhu M (2017) A study of influence on sulfonated TiO2-poly (vinylidene fluoride-co-hexafluoropropylene) nano composite membranes for PEM fuel cell application. Appl Surf Sci 418:64–71
Johra FT, Lee JW, Jung WG (2014) Facile and safe graphene preparation on solution based platform. J Ind Eng Chem 20:2883
Sivasankaran A, Sangeetha D (2015) Influence of sulfonated SiO2 in sulfonated polyether ether ketone nanocomposite membrane in microbial fuel cell. Fuel. 159:689–696
Bagheri A, Javanbakht M, Hosseinabadi P, Beydaghi H, Shabaniki A (2018) Preparation and characterization of SPEEK/SPVDF-co-HFP/LaCrO3 nanocomposite blend membranes for direct methanol fuel cells. Polymer. 138:275–287
Bagheri A, Salarizadeh P, Hazerd MSA, Hosseinabadi P, Kashefi S, Beydaghi H (2019) The effect of adding sulfonated SiO2 nanoparticles and polymer blending on properties and performance of sulfonated poly ether sulfone membrane: fabrication and optimization. Electrochim Acta 295:875–890
Yoo M, Kim M, Hwang Y (2014) Fabrication of highly selective PVA-g-GO/SPVA membranes via cross-linking method for direct methanol fuel cells. Ionics 20(6):875–886
Jun MS, Choi YW, Kim JD (2012) Solvent casting effects of sulfonated poly(ether ether ketone) for polymer electrolyte membrane fuel cell. J Membr Sci 396:32–37
Yu L, Shen HM, Xu ZL (2009) PVDF–TiO2 composite hollow fiber ultrafiltration membranes prepared by TiO2sol–gel method and blending method. J Appl Phys 113:1743–1763
Nagar H, Sahu N, Basava Rao VV, Sridhar S (2020) Surface modification of sulfonated polyethersulfone membrane with polyaniline nanoparticles for application in direct methanol fuel cell. Renew Energy 146:1262–1277
Salarizadeh P, Javanbakht M, Pourmahdian S (2017) Enhancing the performance of SPEEK polymer electrolyte membranes using functionalized TiO2 nanoparticles with proton hopping sites. RSC Adv 7(14):8303–8313
Siva sankaran A, Sangeetha D (2015) A study of influence on nano composite sulfonated TiO2 and sulfonated poly styrene-ethylene-butylene-poly styrene for microbial fuel cell application. Energy 88:202–208
Mondal S, Soam S, Kundu PP (2015) Reduction of methanol crossover and improved electrical efficiency in direct methanol fuel cell by the formation of a thin layer on Nafion 117 membrane: effect of dip-coating of a blend of sulphonated PVdF-co-HFP and PBI. J Membr Sci 474:140–147
Zhong S, Cui X, Gao Y (2014) Fabrication and properties of poly(vinyl alcohol)-based polymer electrolyte membranes for direct methanol fuel cell applications. Int J Hydrog Energy 39:17857–17864
Liang X, Pan G, Xu L (2015) A modified decal method for preparing the membrane electrode assembly of proton exchange membrane fuel cells. Fuel 139:393–400
Rambabu G, Bhat SD (2014) Simultaneous tuning of methanol crossover and ionic conductivity of SPEEK membrane electrolyte by incorporation of PSSA functionalized MWCNTs: a comparative study in DMFCs. Chem Eng J 243:517–525
Di Z, Xie Q, Li H (2015) Novel composite proton-exchange membrane based on proton-conductive glass powders and sulfonated poly (ether ether ketone). J Power Sources 273:688–696
Kumar P, Dutta K, Kundu PP (2014) Enhanced performance of direct methanol fuel cells: a study on the combined effect of various supporting electrolytes, flow channel designs and operating temperatures. Int J Energy Res 38:41–50
Kumar P, Dutta K, Das S (2014) Membrane prepared by incorporation of crosslinked sulfonated polystyrene in the blend of PVdF-HFP/Nafion: characterization and evaluation for application in DMFC. Appl Energy
Mayahi A, Ismail AF, Ilbeygi H (2013) Effect of operating temperature on the behavior of promising SPEEK/ cSMM electrolyte membrane for DMFCs. Sep Purif Technol 106:72–81
Dutta K, Das S, Kumar P (2014) Polymer electrolyte membrane with high selectivity ratio for direct methanol fuel cells: a preliminary study based on blends of partially sulfonated polymers polyaniline and PVdF-co-HFP. Appl Energy 118:183–191
Gumusoglu T, Ari GA, Deligoz H (2011) Investigation of salt addition and acid treatment effects on the transport properties of ionically cross-linked polyelectrolyte complex membranes based on chitosan and polyacrylic acid. J Membr Sci 376:25–34
Gnana Kumar G, Kim AR, Nahm KS (2011) High proton conductivity and low fuel cell crossover of poly vinylidene fluoride-co-hexa fluoro propylene-silica sulfuric acid composite membranes for direct methanol fuel cells. Curr Appl Phys 11:892–902
Pradeepa P, Edwinraj S, Sowmya G (2015) Optimization of hybrid polymer electrolytes with the effect of lithium salt concentration in PEO/PVdF-HFP blends. Mater Sci Eng B
Pradeepa P, Sowmya G, Ramesh Prabhu M (2016) Influence of barium titanate nanofiller on PEO/PVdF-HFP blend-based polymer electrolyte membrane for Li battery applications, J Solid State Electrochem
Thampan TM, Jalani H, Choi (2005) Systematic approach to design higher temperature composite PEMS. J Electro Chem Soc 152:316–325
Funding
Funded by DST-SERB (EEQ/2017/000033), New Delhi, dated 26 Mar 2018 and MHRD–RUSA PHASE–2.0 (Letter no. F.24-51/2014-U), New Delhi.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martina, P., Gayathri, R., Pugalenthi, M.R. et al. Nanosulfonated silica incorporated SPEEK/SPVdF-HFP polymer blend membrane for PEM fuel cell application. Ionics 26, 3447–3458 (2020). https://doi.org/10.1007/s11581-020-03478-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03478-9