Abstract
In this study, we report on poly(vinyl alcohol) (PVA)/gum arabic (GA)/LiClO4 membranes as solid polymer electrolytes (SPEs) for possible application in electrochemical devices. The samples were prepared with 0 to 48 wt% of LiClO4 as a source of ions and subjected to electrochemical, structural, morphological, optical, and thermal characterizations. The best results were obtained for PVA/GA with 24 wt% of LiClO4. This sample exhibited the highest ionic conductivity of 1.6 × 10−4 and 8.6 × 10−4 S cm−1 at 25 and 80 °C, respectively, transparency of 80–87% in the 400 and 633 nm range, being thermally stable up to 150 °C. The PVA/GA electrolytes were predominantly amorphous according to X-ray diffraction (XRD) data, and formed homogeneous surfaces in scanning electron microscopy (SEM) and atomic force microscopy (AFM) images.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer electrolytes consist of salts or acids dissolved in a polymer matrix, thus resulting in solid or quasi-solid (gel) solutions that contain ions. These electrolytes are advantageous in replacing liquid ones as they solve electrolyte leakage problems, especially the gel polymer electrolytes that are inexpensive and easy to prepare. They also provide excellent contact between the electrodes and retain the liquid phase [1]. Wright et al. [2] reported in 1973 on complexes between poly (ethylene oxide) (PEO) and alkali-metal ion salts, which has marked the start of a new research field on polymer electrolytes. Since then, many synthetic polymers have been used in polymer electrolyte matrices, most of which based on PEO. For more than 20 years, natural macromolecules have also been studied because they possess heteroatoms, similarly to PEO [3]. Examples include polymer electrolytes made with hydroxyethyl cellulose [4], hydroxypropyl cellulose [5], chitosan [6, 7], agar [8, 9], gellan gum [10], and blends such as chitosan-poly(vinyl alcohol) [11] or carrageenan [12]. Though many polysaccharides were used as polymer electrolytes matrices, there is no report on gum arabic (GA). GA is a hardened sap that accumulates on trunks and branches of wild acacia tree species such as Acacia senegal and Vechellia (Acacia seyal) [13]. It contains mainly glycoproteins and polysaccharides (arabinose and galactose); being edible, it is used as an additive in pharmaceutical and food industry as a stabilizer, thickener, and emulsifier [14]. Therefore, it is biocompatible and biodegradable. GA is also used in glue formulations [15], so one can expect it to provide efficient contact with electrodes when used as polymer electrolyte. Furthermore, similarly to PEO, GA has oxygens that can complex lithium ions and promote ionic conduction.
Polymer blends are made by mixing two or more compatible polymers that can result in one miscible phase, two partially miscible phases, or immiscible systems [16]. They can also be obtained by the addition of plasticizers to the polymer, which results in ionically conducting systems [7], and have enhanced properties compared to their constituents alone [16, 17]. Electrolytes made with polymer blends were first reported in 1985 when an increase of one order of magnitude in electrical conductivity at room temperature could be reached [18]. Most polymer electrolytes made of natural macromolecules contain a plasticizer, but there are also electrolytes made with synthetic polymers (e.g., chitosan-PEO [19]) or obtained from natural polymers only, including chitosan-agar and chitosan-gelatin [20]. The synthetic polymer poly(vinyl alcohol) (PVA) obtained through polymerization of vinyl acetate or ethyl acetate has also been used in polymer electrolytes. Being water-soluble, biocompatible and biodegradable, it is interesting for use in medicine [11]. It has been employed as polymer electrolyte with sodium triflate (NaTf) [21] or as an additive to synthetic and natural polymers [11, 22]. PVA with 30 wt% of NaTf had ionic conductivity of 7.39 × 10−5 S cm−1 [22], while plasticized PVA with 24 wt% of chitosan and 40 wt% of NH4NO3 had the conductivity of 2.07 × 10−5 S cm−1. An addition of 70 wt% of ethylene carbonate enhanced the ionic conductivity to 1.60 × 10−3 S cm−1 [11].
In this paper, we report the preparation and characterization of solid polymer electrolytes based on PVA/GA blends. Since both polymers are water-soluble, biocompatible and biodegradable, they comply with green chemistry principles. They have oxygens to complex lithium cations and promote ionic conduction. The PVA/GA membranes with different LiClO4 quantities were obtained and characterized by spectroscopic, structural, morphological, and microscopic techniques for possible use in flexible electrochromic devices.
Materials and methods
Preparation of solid polymer electrolytes
0.75 g (30 wt%) of PVA (Vetec; Brazil) and 0.25 g (10 wt%) of GA (Sigma-Aldrich; Brazil) were separately added to the beakers with 10 mL of distilled water at approximately 90 °C under magnetic stirring. After dissolution, these polymers were combined in a single beaker and stirred. Then, different amounts (0, 0.1, 0.3, 0.6, 0.9, and 1.2 g; 0–48 wt%) of anhydrous LiClO4 (Vetec, Brazil) as ion source, 0.5 g (20 wt%) of formaldehyde (J.T. Baker; Brazil) as a crosslinker, and 1 g (40 wt%) of glycerol (Synth; Brazil) as plasticizer, were added to this solution and mixed until complete homogenization. Finally, the solution was poured on a Petri dish and dried in an oven at 50 °C. The samples were stored in a desiccator for a week before analysis [23]. The samples were identified as PVA/GAx, where x is a weight percent of LiClO4.
Characterization techniques
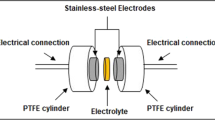
Electrochemical Impedance Spectroscopy (EIS) measurements were performed with an Autolab PGSTAT 302 N potentiostat/galvanostat in the frequency range from 101 to 106 Hz and amplitude potential of 5 mV. The round sample was pressed in between two well-polished stainless-steel electrodes of 1.2 cm of diameter, which were embedded in a cylindrical Teflon® holder. Measurements were taken at 25, 30, 40, 50, 60, 70, and 80 °C in a temperature-controlled homemade furnace equipped with a thermocouple connected to the stainless-steel electrode that allowed a direct reading of the system temperature through the software.
X-ray diffractograms were collected with a Rigaku, model ULTIMA IV diffractometer, containing a CuKα radiation source (λ = 1.5418 Å) and Bragg-Brentano geometry. The measurements were performed with a tube potential of 30 kV and current of 30 mA, scanning angle (2θ) variation from 10 to 80°, at room temperature. The morphological characterizations were performed using atomic force microscopy (AFM), with an Agilent Technologies model 5500 apparatus, and scanning electron microscopy (SEM), with a JEOL model JSM-6610LV microscope. The AFM images were taken by scanning 5 × 5 μm2 samples’ area. For the SEM analyses, films were previously dried at 50 °C and attached to the sample aluminum holder, with a carbon adhesive tape, and covered with gold. The beam current was 1 pA and the beam power was 10 kV. The images were registered at 1000× amplification. Thermogravimetric (TG) analyses were carried out with a TA Instruments SDT Q600. Twelve milligrams of the sample was heated from 25 to 550 °C at 10 °C min−1 under a dynamic N2 flux of 100 mL min−1. The UV-Vis spectra were registered with an Agilent Technologies Cary model 100 spectrophotometer in the wavelength range from 400 to 800 nm.
Results and discussions
Electrochemical impedance spectroscopy
The EIS analyses were aimed at determining the sample with highest ionic conductivity at ambient temperature, which depended on LiClO4 concentration. The measurements were performed in triplicate and Eq. 1 [24] was used to calculate the mean ionic conductivity for the samples with different mass percentages (wt%) of LiClO4.
where σ is the ionic conductivity; RP is the disruption resistance, which was determined by the intersection of the semicircle with the real axis (Z´) of the Nyquist graph; L is the polymer electrolyte thickness measured with a digital Gauge meter; and S is the superficial area of the sample.
Figure 1 shows that the conductivity increases from 4.4 × 10−6 S cm−1 for the sample PVA/GA0 to 1.6 × 10−4 S cm−1 for PVA/GA24, and then decreases to 6.1 × 10−5 S cm−1 for the sample PVA/GA48. Therefore, PVA/GA samples behaved similarly to other SPE samples, i.e., their conductivity increased with salt concentration until an optimum value, after which it decreased [9]. The increase in ionic conductivity with the salt concentration in poly(vinyl alcohol)-lithium perchlorate-titanium oxide samples was attributed to an increased quantity of charge carriers [25]. At high salt concentrations, the ionic conductivity decreases owing to the salt saturation principle and crystallization. According to Fig. 1, the PVA/GA24 electrolyte had the highest ionic conductivity (1.6 × 10−4 S cm−1) at room temperature. This conductivity is similar to the values for a starch-based electrolyte (4.9 × 10−4 S cm−1) [26] or agar doped with acetic acid SPE (1.1 × 10−4 S cm−1). However, it is one magnitude higher than the 1.1 × 10−5 S cm−1 for agar-ionic liquid [27] or the 7.9 × 10−5 S cm−1 for the xanthan gum-acetic acid SPEs [28], and two orders of magnitude higher than the 1.43 × 10−6 S cm−1 of pectin-ionic liquid polymer electrolyte [29].
Measurements at different temperatures were performed to identify the possible ionic conductivity mechanism in PVA/GA. Figure 2 shows semicircles and spike straight lines in the Nyquist graphs for the samples containing 0, 4, 24, and 48 wt% of LiClO4, measured from room temperature to 80 °C. The intersection of those semicircles and straight lines with the real impedance (Z′) axis gives the sample resistance to calculate ionic conductivity. With increasing temperatures, the intersection points shifted to lower real impedance values, which means that the resistance tends to decrease with an increase in temperature.
The increase in ionic conductivity with the temperature is better illustrated in Fig. 3 in which the logarithm of the conductivity is plotted against the inverse of the temperature (K−1). The ionic conductivity values are listed in Table 1. The linear relation observed indicates an Arrhenius conduction mechanism expressed in Eq. 2 [4, 17], where ion movement occurs via jumps between adjacent solvation sites.
where Ea is the activation energy, A is the pre-exponential factor and R is the universal gas constant (8.31441 J mol−1 K−1).
The linear correlation between ionic conductivity and temperature is usually interpreted in terms of the Grotthuss mechanism [30]. However, in chitosan-acetic acid samples, this behavior resulted from the combination of Grotthuss and vehicle-type mechanisms [31]. The vehicle-type mechanism is possible because of the presence of glycerol, which acts as plasticizer to separate polymeric chains and provide a free volume for assisted ion movement. On the other hand, formaldehyde acts as cross-linker [30], which results in a polymeric network that suppresses chain movements, similarly to starch-NaI polymer electrolytes [32]. However, there are still oxygens from hydroxyl groups that can complex Li+ and support ionic movement through the main hopping mechanism [32]. Overall, the processes involved are jumps between coordination sites, local structural relaxations, and segmental movements of the complexes formed by the salt and the polymeric chain. As the temperature increased, the polymeric chain acquired faster internal rotations, favoring ionic movement inter- and intra-chain, resulting in the increase of the polymeric electrolyte conductivity [11]. The activation energy (Ea) could be calculated because the Arrhenius is a first-degree equation, where the term − Ea/2.303R is the angular coefficient of the straight line fitting the data. The lowest Ea of 21.1 and 26.3 kJ mol−1 were obtained for samples PVA/GA4 and PVA/GA24, while PVA/GA0 and PVA/GA48 had 30.7 and 36.4 kJ mol−1, respectively (Table 1). The increase in ionic conductivity is associated with a decrease in the energy of activation, so the ions can jump easily from one solvation site to another because they have enough thermal energy to overcome the energy barriers and move [8, 33].
Optical characterization
Transparency may be an important property for SPE applications, as in transmissive electrochromic windows (ECDs) where SPE should be transparent in the visible range but not in the UV to protect from harmful UV rays. Figure 4 shows that all PVA/GA electrolytes presented an increased transmittance from 0 to around 500 nm. For the sample PVA/GA24 the transmittance increased from 0 to 74% from 200 to 400 nm and to 80% from 400 to 633 nm. At 800 nm the transmittance of this sample was 81%. The transmittance at 633 nm varied by 16% between PVA/GA0 and PVA/GA48. The samples PVA/GA4 and PVA/GA24 had 86 and 80% of transparency at 633 nm, respectively (Table 2). Therefore, an increase in the LiClO4 concentration promoted a decrease in transparency probably because of an increase in the crystalline portion. Similar results were observed for polymer electrolytes made of xanthan gum with PVA [22] or agar-acetic acid [8].
Structural characterization
Figure 5 displays diffractograms of PVA/GA electrolytes with 0–48 wt% of LiClO4. All the electrolytes presented a peak around 2θ = 20o, which corresponds to the plane (001). This peak shifted to higher 2θ with increasing LiClO4 concentration owing to a possible extension of the structural plane [32]. For PVA/GA0 there was a small shoulder at 2θ = 40.7°, which disappeared when the LiClO4 concentration increased. This suggested a predominantly amorphous structure of the samples. The X-ray diffractogram for PVA/GA48 exhibited some new narrow crystalline peaks at 2θ = 22, 24.7, 25.7, and 27.4o. Similarly to agar-LiClO4 polymer electrolytes, these peaks were probably due to LiClO4 crystallization in the matrix of the polymer electrolyte [9]. Moreover, some peaks assigned to LiClO4 for PVA/GA48 electrolyte in Fig. 5 are related to a preferential orientation of crystal growth [34].
The crystallinity of PVA/GA SPEs was obtained by fitting the X-ray diffractograms using Origin software and Levenberg-Marquardt gaussian mathematic model [10]. The degree of crystallinity of PVA/GA-salt phase was estimated from the ratio of total intensity of Bragg peaks over the total intensity of the whole spectrum, as shown by Eq. 3 [10, 33].
where Xc is the degree of crystallinity, Ic is the sum of the areas under the crystalline peaks, and Ia is the area under the amorphous peak. Figure 6 and Table 3 indicate that degree of crystallinity varied only slightly, between 28 and 30%, for the samples of PVA/GA with 0–48 wt% of LiClO4. The small increase in crystallinity upon adding 24 and 48 wt% of salt may be due complexation of Li+ with the oxygens from the samples’ network as formaldehyde was used in the synthesis [30]. Nevertheless, these samples are still considered as predominantly amorphous.
Topography characterization
Figure 7 shows the 3D AFM image of 5 × 5 μm2 area of PVA/GA samples with 0, 4 and 24 wt% of LiClO4. The images reveal a smooth sample surface, without any cracks or LiClO4 crystals. The root mean square (RMS) roughness varies from 1.23 to 11.8 nm for the samples of PVA/GA0 to PVA/GA24 (Table 4). The absence of cracks or bubbles was confirmed with SEM images (not shown here) for PVA/GA with different LiClO4 concentrations at 1000× amplification. The images were similar to each other, with smooth and homogeneous surfaces [9]. This smooth surface proved a good compatibility of the two polymers (PVA and GA), plasticizer and salt because no significant change was noted upon varying the LiClO4 concentration.
Thermal characterization
The thermal behavior of SPEs is important for their possible technological applications [35]. The results of thermogravimetric analysis for PVA/GA SPEs in Fig. 8 show only 2% weight loss up to 150 °C, followed by an additional 10% loss up to 255, 267 and 276 °C for PVA/GA0, PVA/GA24, and PVA/GA48, respectively, probably owing to trapped water or a random rupture of polymeric chains. The latter rupture may have caused release of low molecular mass components that vaporized, leading to the mass loss. Therefore, there was a small increase in sample stability by adding salt. Also, in Fig. 8 one notes a more abrupt and continued loss up to 550 °C in two stages. This weight loss corresponded to about 70% of the samples’ initial weight and was identified as SPEs degradation. This process started at slightly higher temperatures for the samples containing LiClO4, again pointing to increased thermal stability when LiClO4 was added. The thermograms are all similar, which suggests that the salt did not act as a catalyzer for polymer decomposition [36]. Above 450 °C, the weight loss was approximately 10% and explained as the polymer pyrolysis. The ash content at 550 °C was around 8% due to the products formed by LiClO4 oxidation [13].
Significantly for the possible applications, the thermal analysis indicated that PVA/GA polymer electrolytes are thermally stable up to 150 °C, which is much more than the 95 °C considered as the highest working temperature for electrochromic windows.
Conclusions
Solid polymer electrolytes of PVA/GA with different concentrations of LiClO4 were prepared and characterized. The highest ionic conductivity of 1.6 × 10−4 S cm−1 at 25 °C was obtained for the electrolyte of PVA/GA with 24 wt% of LiClO4. The ionic conductivity increased linearly with the temperature for all samples, pointing to a conduction via the Grotthuss mechanism. The activation energy for the best electrolyte PVA/GA24 was 26.3 kJ mol−1. The transmittance of this sample was 80% in the visible region, proving its suitability for transmissive electrochromic windows. The X-ray diffractograms of SPEs evidenced their predominantly amorphous character. This was explained in terms of LiClO4 saturation, which was added in excessive amounts. The SEM and AFM micrographs showed homogeneous electrolytes, without any cracks, bubbles, or granules. Finally, the thermal stability according to TGA analyses pointed to almost no difference in decomposition temperature that started at about 150 °C for the electrolytes with different LiClO4 concentrations. In summary, PVA/GA24 presented satisfying results, which make them promising electrolytes for electrochromic devices.
References
Sarwar MS, Niazi MBK, Jahan Z, Ahmad T, Hussain A (2018) Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohydrate Polym. 184:453
Fenton DE, Parker JM, Wright PV (1973) Complexes of alcali-metal ions with poly(ethylene oxide). Polymer 14:589
Regiani AM, Curvelo AAS, Pawlicka A, Gandini A, Le Nest JF (2000) Polymer electrolytes derived from hydroxiethylcellulose/polyether films. Mol Cryst Liq Cryst 353:181
Lopes LS, Machado GO, Pawlicka A, Donoso JP (2005) Nuclear magnetic resonance and conductivity study of hydroxyethylcellulose based polymer gel electrolytes. Electrochim Acta 50:3978
Ledwon P, Andrade JR, Lapkowski M, Pawlicka A (2015) Hydroxypropyl cellulose-based gel electrolyte for electrochromic devices. Electrochim Acta 159:227
Arof AK, Osman Z, Morni NM, Kamarulzaman N, Ibrahim ZA, Muhamad MR (2001) Chitosan-based electrolyte for secondary lithium cells. J Mater Sci 36:791
Pawlicka A, Danczuk M, Wieczorek W, Zygadlo-Monikowska E (2008) Influence of plasticizer type on the properties of polymer electrolytes based on chitosan. J Phys Chem A 112:8888
Raphael E, Avellaneda CO, Manzolli B, Pawlicka A (2010) Agar-based films for application as polymer electrolytes. Electrochim Acta 55:1455
Raphael E, Avellaneda CO, Aegerter MA, Silva MM, Pawlicka A (2012) Agar-based gel electrolyte for electrochromic device application. Mol Cryst Liq Cryst 554:264
Noor ISM, Majid SR, Arof AK, Djurado D, Pawlicka A (2012) Characteristics of gellan gum - LiCF3SO3 polymer electrolyte. Solid State Ionics 225:649
Kadir MFZ, Majid SR, Arof AK (2010) Plasticized chitosan-PVA blend polymer electrolyte based proton battery. Electrochim Acta 55:1475
Arof AK, Shuhaimi NEA, Alias NA, Kufian MZ, Majid SR (2010) Application of chitosan/iota-carrageenan polymer electrolytes in electrical double layer capacitor (EDLC). J Solid State Electrochem 14:2145
Mariod AA (2018) Gum Arabic: structure, Properties, Application and Economics. Academic Press
Patel S, Goyal A (2015) Applications of natural polymer gum Arabic: a review. Int J Food Prop 18:986
Dauqan E, Abdullah A (2013) Utilization of gum Arabic for industries and human health. Am J Appl Sci 10:1270
Chanda M, Roy SK (2006) Plastics technology handbook. CRC Press, p 896
Kaczmarek H, Dabrowska A, Vukovic-Kwiatkowska I (2011) Accelerated weathering of pectin/poly(vinyl alcohol) blends studied by spectroscopic methods. J Appl Polym Sci 122:1936
Fauteux D, Massucco A, McLin M, Van Buren M, Shi J (1995) Lithium polymer electrolyte rechargeable battery. Electrochim Acta 40:2185
Idris NH, Majid SR, Khiar ASA, Hassan MF, Arof AK (2005) Conductivity studies on chitosan/PEO blends with LiTFSI salt. Ionics 11:375
Mattos RI, Tambelli CE, Raphael E, Silva IDA, Magon CJ, Donoso JP, Pawlicka A (2014) Proton mobility and copper coordination in polysaccharide and gelatin-based bioblends and polyblends. Cellulose 21:2247
Aziz SB, Abdullah OG, Rasheed MA (2017) Structural and electrical characteristics of PVA: NaTf based solid polymer electrolytes: role of lattice energy of salts on electrical DC conductivity. J Mater Sci: Mater Electron 28:12873
Caldeira I, Lüdtke A, Tavares F, Cholant C, Balboni R, Flores WH, Galio A, Pawlicka A, Avellaneda CO (2018) Ecologically friendly xanthan gum-PVA matrix for solid polymeric electrolytes. Ionics 24:413
Hossain KMZ, Felfel RM, Ogbilikana PS, Thakker D, Grant DM, Scotchford CA, Ahmed I (2018) Single solvent-based film casting method for the production of porous polymer films. Macromol Mat Eng 303:1700628
Malathi J, Kumaravadivel M, Brahmanandhan G, Hema M, Baskaran R, Selvasekarapandian S (2010) Structural, thermal and electrical properties of PVA–LiCF3SO3 polymer electrolyte. J Non-Cryst Solids 356:2277
Lim C-S, Teoh K, Ng H, Liew C-W, Ramesh S (2017) Ionic conductivity enhancement studies of composite polymer electrolyte based on poly (vinyl alcohol)-lithium perchlorate-titanium oxide. Adv Mater Lett 4:465
Liew C-W, Ramesh S (2015) Electrical, structural, thermal and electrochemical properties of corn starch-based biopolymer electrolytes. Carbohydrate Polym 124:222
Nadia S, Khanmirzaei M, Ramesh S, Ramesh K (2017) Quasi-solid-state agar-based polymer electrolytes for dye-sensitized solar cell applications using imidazolium-based ionic liquid. Ionics 23:1585
Tavares FC, Dorr DS, Pawlicka A, Avellaneda CO (2018) Microbial origin xanthan gum-based solid polymer electrolytes. J Appl Polym Sci 135:46229
Mendes JP, Esperança JMSS, Medeiros MJ, Pawlicka A, Silva MM (2017) Structural, morphological, ionic conductivity, and thermal properties of pectin-based polymer electrolytes. Mol Cryst Liq Cryst 643:266
Singh A, Narvi S, Dutta P, Pandey N (2006) External stimuli response on a novel chitosan hydrogel crosslinked with formaldehyde. B Mater Sci 29:233
Pawlicka A, Mattos RI, Tambelli CE, Silva IDA, Magon CJ, Donoso JP (2013) Magnetic resonance study of chitosan bio-membranes with proton conductivity properties. J Membrane Sci 429:190
Tiwari T, Pandey K, Srivastava N, Srivastava P (2011) Effect of glutaraldehyde on electrical properties of arrowroot starch+ NaI electrolyte system. J Appl Polym Sci 121:1
Nwanya A, Amaechi C, Udounwa A, Osuji R, Maaza M, Ezema F (2015) Complex impedance and conductivity of agar-based ion-conducting polymer electrolytes. Appl Phys A Mater Sci Process 119:387
Siddaiah T, Ojha P, Kumar NO, Ramu C (2018) Structural, optical and thermal characterizations of PVA/MAA: EA polyblend films. Mat Res 21
Saadiah M, Zhang D, Nagao Y, Muzakir S, Samsudin A (2019) Reducing crystallinity on thin film based CMC/PVA hybrid polymer for application as a host in polymer electrolytes. J Non-Cryst Solids 511:201
Pagot G, Bertasi F, Vezzù K, Nawn G, Pace G, Nale A, Di Noto V (2018) Correlation between properties and conductivity mechanism in poly (vinyl alcohol)-based lithium solid electrolytes. Solid State Ionics 320:177
Acknowledgments
The authors are grateful to Prof. Osvaldo Novais de Oliveira Jr. (ResearcherID A-1714-2008) and Sue Larsen for English revision.
Funding
The authors acknowledge Fundação de Amparo à Pesquisa do Estado de Rio Grande do Sul (FAPERGS, grant 12/2239-9), The Brazilian National Council for Scientific and Technological Development (CNPq, grants 308752/2017-1 and 307429/2017-2), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (Capes, grant 1780078) for the financial support given to this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cholant, C.M., Krüger, L.U., Balboni, R.D.C. et al. Synthesis and characterization of solid polymer electrolyte based on poly(vinyl alcohol)/gum Arabic/LiClO4. Ionics 26, 2941–2948 (2020). https://doi.org/10.1007/s11581-019-03433-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03433-3