Abstract
This article presents the effect of nickel as dopant on the structural, morphological, and capacitance behaviors of SrTiO3 for supercapacitor application. Pure and Ni-doped SrTiO3 was synthesized via ball milling method. The phase structure and purity of the synthesized samples were confirmed by powder X-ray diffraction (XRD). The surface morphology showed the role of dopants in fixing the grain size of SrTiO3. The electrochemical performance of pure and Ni-doped SrTiO3 was investigated using cyclic voltammetry (CV) and Galvanostatic charge–discharge (GCD) in a 3-M KOH electrolyte solution. It was found that Ni-doped SrTiO3 exhibited the maximum specific capacity of 142 F/g at 1 A/g, which was significantly higher than that of pure SrTiO3 (105 F/g at 1 A/g). Electrochemical impedance spectroscopy (EIS) evidenced that there is the smallest charge transport resistance value for Ni-doped SrTiO3 sample.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide demand for energy conversion and energy storage technology is expected to high increase because of the depletion of fossil fuels [1]. The electrochemical energy storage devices of supercapacitors play an important role because of their charge storage mechanism, high power density, long life cycle, and safety [2]. Supercapacitors can be classified into two types such as (i) electrical double-layer capacitors (EDLCs) and (ii) pseudocapacitors. Among them, pseudocapacitors electrode materials show high capacitance values due to faradic reaction in both inner and outer surfaces of the electrode material. Usually, electrode material in the efficient supercapacitors may consist of metal oxide/hydroxide-based electrode. Due to very small electrical conductivity and poor cyclic stability at larger current density, these metal oxide/hydroxide and their composites have restricted their application [3]. It is well known that the drawbacks of metal oxides exclusively perovskites-based material have been used as the effective electrode due to their high specific capacitance and large operating potential wind [4, 5].
Karthick et al. [6] synthesized SrTiO3 with perovskite structure which exhibited a pseudocapacitive behavior of the electrodes. SrTiO3 is a typical perovskite-type oxide whose physical properties strongly depend on its chemical composition, structure, shape, size, and crystallinity. Strontium titanate (SrTiO3) is one of the most important multifunctional perovskites used in the fabrication of electronic devices, photoanode for quantum dot–sensitized solar cells, and electroceramics [7]. Moreover, for instance, SrTiO3 perovskite materials have applications dielectric, photocatalytic, thermistors, multilayer capacitors, electro-optical appliances, electromechanical devices, and field effect transistors [8]. SrTiO3 is an n-type semiconductor with cubic perovskite structure and it has an indirect band gap that varies from 3.2 to 3.4 eV, although some recent work shows that there is an increase in band gap to 3.77 eV causing a photon emission in the blue-green region of the spectrum [9, 10]. Recently, the metal elements like iron doped into the SrTiO3 leads to a various changes, which are closely joined to the valence state of a dopant and its relation to nearest oxygen vacancies and it has a possible application of memristive memories [11] .
In the present study, we have synthesized SrTiO3 compound using ball milling technique with nickel doping during short times with fine and homogeneous microstructures and studied their electrical properties. In the ball milling method of synthesis macro-crystalline, structured particles are broken down into nano-crystalline structures, but the original reliability of the material is retained. However, the nanoparticles can be altered into a new material, which involves breaking the original crystalline bonds. Apart from these, many research works tried to incorporate dopants into SrTiO3 which were focused on self-doping on oxygen vacancies and intrinsic defects and microstructural properties of SrTiO3 modified through ball milling process [12].
Materials and methods
Titanium dioxide (TiO2) (99.9% purity, Sigma–Aldrich), Strontium Nitrate Sr(NO3)2 (Sigma–Aldrich), urea, nickel nitrate Ni (NO3)2.6H2O (99.9% purity, Sigma–Aldrich),(98% purity, Merck). All chemicals used were of analytical grade. Deionized water was used throughout the experiment.
Synthesis of Ni-doped SrTiO3 nanoparticles
The stoichiometric proportions of TiO2 and Sr(NO3)2 were mechanically milled for 2 h at 300 rpm using a planetary ball milling apparatus (Pulverisette 5). The sample mass was 2.5 g and the appropriate ball-to-sample weight ratio was balanced at each milling process. The temperature maintained at room temperature. The as-produced precursor was calcinated at 500 °C for 5 h. The above procedure followed for the preparation of nickel-doped strontium titanate [13].
Electrode preparation
A pure SrTiO3 electrode was fabricated by mixing 80 wt% of the active material, 10 wt% of activated carbon, and 10 wt% of polyvinylidene fluoride (PVDF) in N-methyl-2-pyrrolidone (NMP) solvent until homogeneous slurry was achieved. Then, the cleaned nickel foam was coated with the slurry and dried in an oven at 80 °C overnight. A standard three-electrode cell system was used to examine the electrochemical measurements. The pure SrTiO3-coated Ni foam was used as the working electrode, and Ag and platinum wire was used as the reference and counter electrodes, respectively. The measurements were conducted in 3 M KOH solution at room temperature. Cyclic voltammetry (CV), Galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS) with a frequency range from 0.01 Hz to 100 kHz were conducted using a SP-150 electrochemical workstation. For comparison, the same experiment was repeated using nickel-doped SrTiO3 as the active electrode materials.
Characterization details
The crystal structure and phase purity of SrTiO3 and nickel-doped SrTiO3 nanoparticles were analyzed by X-ray diffraction (XRD) (Bruker AXS D8 Advance). The average crystallite sizes calculated using Debye Scherrer’s equation i.e., d = 0.9λ/βcosθ, where β according to the full width at half maximum of the diffraction peak (FWHM), K = 0.89, θ is the Bragg diffraction angle, and λ is the X-ray wavelength corresponding to the Cu Kα radiation. The morphology and chemical composition were analyzed by field emission scanning electron microscopy (FESEM) (CAREL ZEISS) and energy dispersive X-ray spectrometer (EDAX) (OXFORD XMX N) analysis. The HRTEM images were recorded using a Jeol/JEM 2100 transmission electron microscope. The band gap energy values of the pure SrTiO3 and Ni-SrTiO3 nanoparticles were determined by UV-DRS measurements using Hitachi UV-365 spectrophotometer. The electrochemical measurements were carried out using SP-150 electrochemical workstation (BioLogic Science Instruments, France) for cyclic voltammetry (CV), Galvanostatic charge–discharge (GCD) and electrochemical impedance spectroscopy (EIS) tests with three-electrode systems in 3 M KOH electrolyte solution at room temperature.
Results and discussion
X-ray diffraction analysis
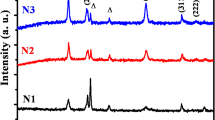
The XRD patterns of pure SrTiO3 and Ni-SrTiO3 are shown in Fig. 1 a, b. Both pure and Ni-doped strontium titanate showed sharp and intense XRD peaks indicating a high degree of crystallinity. All the X-ray diffraction patterns indicate the presence of cubic (Pm3m) symmetry phase of SrTiO3. The sharp peaks at 25.5°, 38.82°, 48.28°,54.09°,55.27°,62.89°, and 75.26° correspond to (101), (112), (200), (105), (211), (204), and (215) planes, and 32.5°, 40.8°, 46.10°, 57.86°, 67.87°, and 77.28° corresponding to (110), (111), (200), (211), (220), and (310) planes confirm the presence of anatase phase of titania and cubic (Pm3m) phase of strontium titanates respectively. The patterns match with JCPDS files available in the literature (JCPDS file no. 89-4921 and 86-0178) [14, 15]. The crystallite sizes of pure and Ni-doped SrTiO3 synthesized through ball milling method were calculated using the Scherrer formula [16]. A small value of FWHM implies a high crystallinity of the sample. The calculated average crystallite sizes are 12 nm and 10 nm corresponding to the samples of pure SrTiO3 and Ni-doped SrTiO3. The samples also contain a small amount of TiO2. The presence of TiO2 depends upon the heating duration and low calcination temperature [12]. Nickel metal of comparable sizes was used to prepare mixtures; therefore, the absence of reflections of the nickel metals may be due to a small crystallite size, low concentration, or low crystallinity. The minimal substitution of the Ni metal in the SrTiO3 was shown by the measured lattice parameters. The value of lattice parameter for SrTiO3 is found to be a = 3.990 Ǻ and for Ni-doped SrTiO3 is a = 3.970 Ǻ (cubic structure) [17]. The lattice parameter measured for SrTiO3 was comparable with JCPDS. For SrTiO3, it will cause a change in the original structure of the crystal depending upon the ionic radius and crystalline nature of the dopant. This makes a sharp increase in lattice constant which confirms the fusion of ion in the host lattice. Further adding of doping nickel may reduce the lattice constant due to variation of a microstrain of crystal.
FESEM and EDAX analysis
The surface morphology and elemental analysis of SrTiO3 and nickel-doped SrTiO3 are carried out employing scanning electron microscopy coupled with energy dispersive spectroscopy and resultant FESEM images and typical EDAX spectra are depicted in Fig. 2a–d that clearly shows an anomalous morphology. However, the undoped samples revealed more irregular structure and a spherical particle with agglomeration than that of doped SrTiO3. It seems reasonable decrease in particle size and rougher surface morphology are responsible for the increase of surface area for doped samples, which is shown in Fig.2a, c respectively [18, 19]. The particles also exhibited irregular small voids with the different sizes among the aggregated nanoparticles. The elements Sr, Ti, O, and Ni peaks are found in the EDAX spectra, which confirmed the presence of the compounds as shown in Fig. 2b, d [20].
HRTEM analysis
The HRTEM analysis of the Ni- SrTiO3 sample with various magnifications shown in Fig. 3a–d further confirms the spherical morphology and the corresponding selected area of electron diffraction (SAED) pattern shows more bright spots which indicate that the particles are polycrystalline in nature.
Measurement of electrochemical performance of supercapacitor
CV analysis
The electrochemical properties of the pure and Ni-doped SrTiO3 were evaluated using three-electrode systems. Figure 4 a, b depicts the cyclic voltammetry (CV) curves tested at different scan rates of product in the three-electrode system. In the three-electrode system reference electrode is Ag, Pt is the counter electrode, and the working electrode is fabricated using pure and Ni-doped SrTiO3 samples. For electrochemical measurements, 3 M KOH is used as the electrolyte solution and the CV measurements were carried out in the potential range of 0–0.7 V at different scan rates of 5 to 100 mV/s. The anodic peaks were observed in the potential range of 0.3–0.6 V for pure and 0.32–0.61 V for Ni- SrTiO3 and the cathodic peaks were observed in the potential range of 0.01–0.3 V for pure and 0.01–0.28 V for Ni-SrTiO3 electrodes which is mainly due to the faradaic redox reaction. Hence, the observed CV curves show the pseudocapacitive characteristic of the pure and Ni-doped SrTiO3 electrodes [21].
Charge–discharge analysis
The measurement of the charge–discharge curve was carried out between the potential ranges of 0 to 0.5 V at current densities from 1 to 10 A/g (Fig. 5 a, b).The observed charging curve is nearly analogous to discharging curve but vaguely vary from the charging time. The shape of the curves look likes an ideal pseudocapacitive performance with a sharp response. The specific capacitance (Cs) is calculated by using the relation (Eq. 1)
where I is the discharge current, Δt is the discharging time, m is the mass of active material, and ΔV is the applied potential window. Figure 6 a, b shows that the capacitance value decreases from the lower current density to higher current density for both the electrodes. The pure SrTiO3 shows the capacitance value of 105 F/g for the current density of 1 A/g decreases to 61 F/g at 10 A/g, and Ni-SrTiO3 electrode shows the capacitance value of 142 F/g for the current density of 1 A/g decreases to 70 F/g at 10 A/g. At higher current density, the specific capacitance attributed to the low value attributed due to the hydrate ions block the dynamic accommodation of the electrode surface. As well as in low current density, the specific capacitance attributed to a high value is due to the rapid charge transfer at the edge of the electrode and the electrolyte [22]. Cyclic stability was one of the essential parameter for supercapacitor electrode materials; the fabricated asymmetric Ni-doped SrTiO3 was subjected to continuous 3000 cycles of charging–discharging at a current density of 10 A/g. Figure 6c shows the capacitance retention of the fabricated asymmetric super capacitor. On the other hand, there is an increase in capacitance retention up to around 96% before 200 cycles which shows full activation process of the device. After 3000 cycles, the capacitance retention slightly reduced to 86%. The number of cycles was obtained which were due to swelling and shrinkage of Ni-doped SrTiO3 during the long-term charge and discharging process. This result suggested that the Ni-doped SrTiO3 may be suitable for supercapacitance applications.
EIS measurement
Electrochemical behavior as charge/ion transportation process of the electrode materials, of the pure and Ni-SrTiO3 electrodes, was investigated through electrochemical impedance spectroscopy (EIS). The EIS plots for both the samples and the corresponding equivalent circuit were presented in Fig.7. Furthermore, Ni-SrTiO3 showed significantly a smaller semicircle as compared with pure SrTiO3 which corresponds to its smallest charge transfer resistance at the electrode-electrolyte interface [23].
Conclusions
The present work presents the synthesis and characterization of pure and Ni-SrTiO3 electrode material for supercapacitor applications through a simple, short duration, cost-effective, and environmentally friendly ball milling method. The structure, morphology, composition, and optical properties of the samples were characterized by powder XRD, FESEM, HRTEM, EDAX, and electrochemical studies. The results suggested that the prepared samples exhibited a spherical structure with agglomeration nature, which confirmed the role of dopant in fixing the crystallite size with a low band gap energy value. Doping of nickel in SrTiO3 as electrode material resulted in achieving high specific capacitance of around 142 F/g. The obtained experimental results proposed that the prepared electrode materials can be considered as a promising candidate for supercapacitor applications.
References
Kumar V, Mariappan CR, Azmi R, Moock D, Indris S, Bruns M, Ehrenberg H, Vijaya Prakash G (2017) Pseudocapacitance of mesoporous spinel-type MCo 2 O 4 (M = Co, Zn, and Ni) rods fabricated by a facile solvothermal route. ACS Omega 2:6003–6013. https://doi.org/10.1021/acsomega.7b00709
Arjun N, Pan G-T, Yang TCK (2017) The exploration of lanthanum based perovskites and their complementary electrolytes for the supercapacitor applications. Results Phys 7:920–926. https://doi.org/10.1016/j.rinp.2017.02.013
Palani NS, Kavitha NS, Venkatesh KS, Ashok Kumar K, Thirumal V, Pandurangan A, Sekar C, Ilangovan R (2018) Effect of NiO/Ni(OH)2 nanostructures in graphene/CNT nanocomposites on their interfacial charge transport kinetics for high-performance supercapacitors. J Solid State Electrochem 22:3273–3287. https://doi.org/10.1007/s10008-018-4032-x
Ghosh D, Giri S, Sahoo S, Das CK (2013) In situ synthesis of graphene/amine-modified graphene, polypyrrole composites in presence of SrTiO3 for supercapacitor applications. Polym-Plast Technol Eng 52:213–220. https://doi.org/10.1080/03602559.2012.727056
Rai A, Thakur AK (2017) Effect of Na and Mn substitution in perovskite type LaFeO3 for storage device applications. Ionics 23:2863–2869. https://doi.org/10.1007/s11581-017-1990-4
Karthick K, Ede SR, Nithiyanantham U, Kundu S (2017) Low-temperature synthesis of SrTiO 3 nanoassemblies on DNA scaffolds and their applications in dye-sensitized solar cells and supercapacitors. New J Chem 41:3473–3486. https://doi.org/10.1039/C7NJ00204A
Chen C, Dai Q, Miao C et al (2015) Strontium titanate nanoparticles as the photoanode for CdS quantum dot sensitized solar cells. RSC Adv 5:4844–4852. https://doi.org/10.1039/C4RA11960F
George CN, Thomas JK, Jose R et al (2009) Synthesis and characterization of nanocrystalline strontium titanate through a modified combustion method and its sintering and dielectric properties. J Alloys Compd 486:711–715. https://doi.org/10.1016/j.jallcom.2009.07.045
Farrukh MA, Butt KM, Chong K-K, Chang WS (2019) Photoluminescence emission behavior on the reduced band gap of Fe doping in CeO2-SiO2 nanocomposite and photophysical properties. J Saudi Chem Soc 23(5):561–575. https://doi.org/10.1016/j.jscs.2018.10.002
Sharma M, Sen S, Jagannath G, Ghosh M, Pitale S, Vinay G, Gadkari SC (2018) Tunable blue-green emission from ZnS(Ag) nanostructures grown by hydrothermal synthesis. J Mater Res 33(23):3963–3970. https://doi.org/10.1557/jmr.2018.358
Siddheswaran R, Životský O, Hendrych A et al (2016) Structural and magnetic properties of the transition metals (TM Co, Ni) and Nb co-doped SrTiO 3 thin films. Mater Res Bull 83:193–200. https://doi.org/10.1016/j.materresbull.2016.06.012
Psiuk B, Szade J, Wrzalik R et al (2014) Milling-induced phenomena in SrTiO3. Ceram Int 40:6957–6961. https://doi.org/10.1016/j.ceramint.2013.12.019
Hu Y, Tan OK, Pan JS, Yao X (2004) A new form of nanosized SrTiO 3 material for near-human-body temperature oxygen sensing applications. J Phys Chem B 108:11214–11218. https://doi.org/10.1021/jp048973z
Budiman F, Kotooka T, Horibe Y et al (2018) Electric property measurement of free-standing SrTiO 3 nanoparticles assembled by dielectrophoresis. Jpn J Appl Phys 57:06HE07. https://doi.org/10.7567/JJAP.57.06HE07
OKAMOTO Y, SUZUKI Y (2014) Perovskite-type SrTiO3, CaTiO3 and BaTiO3 porous film electrodes for dye-sensitized solar cells. J Ceram Soc Jpn 122:728–731. https://doi.org/10.2109/jcersj2.122.728
Joseph AIJ, Thiripuranthagan S (2015) Metal doped titanate photo catalysts for the mineralization of Congo red under visible irradiation. RSC Adv 5:9792–9805. https://doi.org/10.1039/C4RA14722G
Subramanian V, Roeder RK, Wolf EE (2006) Synthesis and UV−visible-light photoactivity of noble-metal−SrTiO 3 composites. Ind Eng Chem Res 45:2187–2193. https://doi.org/10.1021/ie050693y
Wang D, Ye J, Kako T, Kimura T (2006) Photophysical and photocatalytic properties of SrTiO3 doped with Cr cations on different sites. J Phys Chem B 110:15824–15830. https://doi.org/10.1021/jp062487p
Qin Y, Wang G, Wang Y (2007) Study on the photocatalytic property of La-doped CoO/SrTiO3 for water decomposition to hydrogen. Catal Commun 8:926–930. https://doi.org/10.1016/j.catcom.2006.11.025
Jia A, Su Z, Lou L-L, Liu S (2010) Synthesis and characterization of highly-active nickel and lanthanum co-doped SrTiO3. Solid State Sci 12:1140–1145. https://doi.org/10.1016/j.solidstatesciences.2010.04.005
Manickam M, Ponnuswamy V, Sankar C, Suresh R, Mariappan R, Chandra bose A, Chandrasekaran J (2017) Structural, optical, electrical and electrochemical properties of Fe:Co3O4 thin films for supercapacitor applications. J Mater Sci Mater Electron 28:18951–18965. https://doi.org/10.1007/s10854-017-7849-7
Manikandan M, Dhanuskodi S, Maheswari N et al (2017) High performance supercapacitor and non-enzymatic hydrogen peroxide sensor based on tellurium nanoparticles. Sens Bio-Sens Res 13:40–48. https://doi.org/10.1016/j.sbsr.2017.02.001
Yadav AA, Lokhande AC, Pujari RB, Kim JH, Lokhande CD (2016) The synthesis of multifunctional porous honey comb-like La 2 O 3 thin film for supercapacitor and gas sensor applications. J Colloid Interface Sci 484:51–59. https://doi.org/10.1016/j.jcis.2016.08.056
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Priyadharsini, C.I., Marimuthu, G., Pazhanivel, T. et al. Electrochemical supercapacitor studies of Ni2+-doped SrTiO3 nanoparticles by a ball milling method. Ionics 26, 3591–3597 (2020). https://doi.org/10.1007/s11581-019-03412-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03412-8