Abstract
The high concentration of fluoride (F−) in water sources is the main challenge in major fluoride belts. Though capacitive deionization (CDI) with porous carbon electrodes is the promising alternative in removing charged species from aqueous solution, little has been presented on the usefulness of CDI with biomass-based electrodes in removing F− from natural water existing together with other ions such as Ca2+ and Mg2+. This study investigated the feasibility of using biomass-based electrodes for natural water defluoridation application. Porous carbon was synthesized from jackfruit peels (JFAC) through potassium hydroxide (KOH) activation. Surface morphology, pore structure, and electrochemical properties of the JFAC were investigated. The textural properties of the synthesized carbon and electrochemical characteristics of the fabricated electrodes were found to be influenced by activation temperature. Brunauer-Emmett-Teller (BET) surface area, pore diameter, pore volume, and pore surface area increased with an increase in activation temperature and KOH to carbon ratio. It was further confirmed that as the applied voltage increased from 1.2 to 2 V, the amount of adsorbed anions increased without significantly affecting the pH of the water. At 2.0 V, the electrodes showed a maximum F− adsorption efficiency and electrosorption capacity of 62% and 0.13 mg/g respectively. The electrosorption capacity depends on the initial concentration of the ion in the feed water. It was further observed that natural organic substances contained in the natural water might inhibit JFAC electrode surface and decrease its adsorption efficiency. This study provides cost-effective CDI electrode material prepared from biomass for water defluoridation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural water from various sources including ground and river water can contain many species such as disease-causing microorganisms, toxic metals, and chemicals which are harmful to human health. The water source may also be contaminated with fluoride (F−), nitrates, and heavy metals. These contaminants make the water not safe as it does not meet the required drinking water standards. Moreover, water may contain hardness-causing ions which may result in scaling problems and serious failures in water distribution systems [1]. Therefore, it is essential to measure the concentration levels of the contaminants and hardness-causing ions in the water to overwhelm their impacts.
The presence of F− in drinking water has either positive or negative effects on teeth and bones subject to the concentration and total amount consumed. For instance, F− concentrations between 1 and 1.5 mg/L make enamel stronger, 1.5–4 mg/L lead to dental fluorosis, higher concentrations (4–10 mg/L) with long-term exposure cause skeletal fluorosis while low concentration (approximately 0.5 mg/L) prevents occurrence of dental caries [2]. It is recommended that any drinking water should have a maximum permissible limit of F− of 1.5 mg/L and desirable highest limit of 1.0 mg/L [3]. Therefore, defluoridation of drinking water at the source or the household level is very important to prevent and control the occurrence of fluorosis [4] especially in major fluoride belts in the world, such as areas of China, India, and the Main Ethiopian Rift Valley located in Rift Valley of Africa [5].
Various methods including electrocoagulation [6], reverse osmosis, ion exchange process, adsorption, chemical precipitation, coagulation, nanofiltration, and electro-membrane systems such as electro-deionization reversal, electrodialysis reversal, and electrodialysis have been widely applied for effective drinking water defluoridation as reviewed by Ayoob et al. [7]. Some of these methods suffer limitations of high-energy expenditure, high operational cost, and low efficiency, limiting their real-world application. Adsorption is the most common method in which adsorbents such as born char [5], calcium phosphate systems [8], activated alumina [9], and activated carbon [10] are commonly used. However, availability of low-cost and sustainable adsorbent precursor at or near to areas affected by fluoride, simple methods for synthesizing adsorbent with high removal efficiency when using natural water, and regeneration of the adsorbent are the most challenging issues [10, 11]. Thus, low-energy, environmentally friendly, easy regeneration, and cost-effective electrochemical method (capacitive deionization (CDI) technology) may solve these challenges as efficient fluoride removal technology.
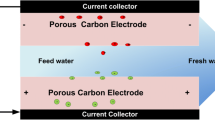
CDI uses electric fields to adsorb temporally charged species such as salt ions present in aqueous solution and store them in a polarized surface [12]. CDI operates under two main approaches, which are capacitive electrosorption and Faradaic ion storage [13]. Capacitive electrosorption is the most common approach in which the ions are stored within highly porous material due to the electrical double layer (EDL) formation [14]. Since CDI operates by forming EDL, its performance relies on electrode materials. Numerous carbons including activated carbon (AC) [15, 16], carbon aerogels [17, 18], carbon nanotubes [19, 20], carbon nanofibers [21], and graphene [22, 23] have been studied as promising CDI electrode materials. However, irrespective of excellent electrosorption performances of some of these carbon materials (carbon nanofibers, carbon nanotubes, and carbon aerogels), their high costs and complex synthetic procedures limit their practical application, particularly in water treatment [24]. Therefore, AC stands out since it is cost-efficient and commercially available than most of the carbon materials mentioned above. Generally, AC is produced from natural or synthetic materials through carbonization and activation processes [25]. Though AC from synthetic sources, for example, polymers, have shown excellent structures and properties for electrochemical capacitors [26], their production cost is relatively high, hence hinders their production for commercial purposes. Carbon derived from natural sources has received substantial consideration because of its low cost and excellent properties. Since most of commercial AC come from non-renewable and unsustainable natural sources such as coal, wood, petroleum residues, peat, and lignite, biomass waste can be used for AC production for its sustainability and renewability [27].
Various biomass have been utilized as a precursor for AC and studied for CDI electrodes, as reviewed by Elisadiki et al. [28]. Different biomass precursors, together with the synthesis conditions, determine the characteristics and the performance of the resulting carbon-based electrode. For example, Zhao et al. [29] prepared micro/mesoporous carbon sheets from watermelon peel precursor through KHCO3 activation and develop CDI electrode to remove NaCl from a solution of synthetic saltwater. Zhang et al. [30] employed waste biomass corncob to synthesize porous carbon with 3D honeycomb-like structure through hydrothermal carbonization and KOH activation and investigated its heavy metal removal ability when used as a CDI electrode. Furthermore, Li et al. [31] used micropore-dominant commercial AC as CDI electrode material for the removal of fluoride from the NaF solution. Most literature reported the electrosorption capacity of the material using simulated water (i.e., NaCl [29, 32] or NaF [31, 33,34,35,36]) solution with different concentrations. Little has been presented on the usefulness of CDI with biomass-based electrodes in removing F− from the natural/real water that exists together with natural organic substances and other ions such as Ca2+ and Mg2+. Therefore, this study investigated the feasibility of using biomass-based electrodes derived from jackfruit peels (JFAC) for natural water defluoridation application. The morphology, structure, and electrochemical properties of the JFAC carbon were also studied so as to realize the properties that improve the performance of JFAC electrodes in CDI.
Materials and methods
Materials
Chemicals used in this study are potassium hydroxide (KOH), sodium hydroxide (NaOH), sodium chloride (NaCl), hydrochloric acid (HCl), glacial acetic acid, cyclohexanediaminetetraacetic acid (CDTA), polytetrafluoroethylene (PTFE, 60 wt% dispersion in water), and 99.9% ethanol all procured from Sigma-Aldrich chemicals company. Carbon black (TIMICA SUPPER C65) was purchased from MTI Corporation. All chemicals were of analytical grade and were used without further purification. Jackfruit peels were collected from local vendors at Kilombero market in Arusha Region, Tanzania.
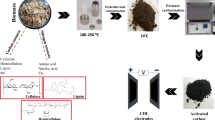
Preparation of activated carbon from jackfruit peels
Activated carbon from jackfruit peels was prepared as detailed in previous work [37] by changing the activating agent ratio. The KOH/carbon mass ratios of 2:1 and 3:1 were used and the samples were named as JFAC-x-y, where x is the mass ratio of activating agent and carbon while y is the activation temperature.
Physical characterization
High-resolution Zeiss Ultra Plus 55 field emission scanning electron microscopy (FESEM) operated at 2.0 kV was used to study the surface morphology of JFAC. Automated PANalytical X’PERT PRO X-ray diffractometer (XRD) system was used to measure the diffraction patterns of JFAC samples using Cu-Kα radiation (λ = 1.54060 Å) from the Bragg angle (2θ) 5° to 80°. Structural parameters including the interlayer spacing, (d002 and d100), and microcrystalline dimensions were evaluated by the application of Bragg’s law (Eq. (1)) and the Debye-Scherrer equation (2).
where n (1) is an integer determined by order of the reflection, λ is the wavelength (1.5406 Å) of the X-ray radiation, and θ is the Bragg angle representing the position of the (002) and (100) diffraction peaks. Lc is stack height, La is stack width, K is the shape factor equal to 0.89 and 1.84 for Lc and La, respectively, and βc. a is the full width at half maximum of the symmetrically shaped diffraction peaks [38, 39]. The mean number of layer planes in the microcrystallites (Np) was obtained from the ratio \( \frac{L_c}{d_{002}} \) while the ratio \( \frac{L_c}{L_a} \) was used to calculate the relative density of edge and basal planes in the microcrystallites.
The vibrational mode of the JFAC samples was studied by using a T64000 micro-Raman spectrometer (HORIBA Scientific, Jobin Yvon Technology) at an excitation wavelength of 514 nm [40, 41]. The porosity of the material was studied by nitrogen adsorption-desorption isotherms measured at 77 K using Nova 4200e Quantachrome porosimeter (UK) at a relative pressure, p/p0, the range of 0 to 1. Surface area and pore size distribution were evaluated by Brunauer-Emmet-Teller (BET) and Barrett-Joyner-Halenda (BJH) methods, respectively, and the t-plot method was used to estimate the micropore surface area and volume.
Electrochemical properties of JFAC electrodes
Potentiostat/galvanostat (PGSTAT204, AUT50663 Metrohm) was used to study the electrochemical properties of the JFAC electrodes in 1 M NaCl and 1 M NaF solutions at room temperature. A 1 cm × 1 cm working electrode was prepared by pressing 80 wt% JFAC, 10 wt% conducting carbon, and 10 wt% PTFE on a current collector. A platinum wire was used as counter electrode and saturated Ag/AgCl (KCl) as a reference electrode.
By integrating the area of cyclic voltammetry (CV) curves, the specific capacitances were calculated by using Eq. (3):
where C is the specific capacitance (F/g), ∫IdV is the total sum of voltammetric charge obtained by integrating the positive and negative arc of cyclic voltammograms, I is current (A), S is the scan rate (V/s), ΔV is the potential window, and m is the mass of active material in the working electrodes (g) [42]. Electrochemical impedance spectroscopy (EIS) analysis was used to assess the electrical conductivity of the electrodes in the frequency range from 10 mHz to 100 kHz.
Electrosorption experiments
Electrosorption performance of JFAC electrodes was conducted with laboratory-scale CDI reactor in a batch mode as per previous study [37]. During desalination experiment, 30 mL of natural water with a conductivity of 760 μS/cm was circulated to CDI cell at a flow rate of 5 mL/min by using a peristaltic pump, and potentiostat/galvanostat was used to supply voltage to the CDI cell electrodes. Portable digital conductivity meter and pH meter (GMH 3400 series) were used to measure conductivity and pH changes after every 5 min.
The removal efficiency was calculated from Eq. (4) [6, 43]:
where Cf and Ci are final and initial concentrations in milligrams per liter.
The salt/ion adsorption capacity was calculated from Eq. (5) [44]:
where Γ is the salt adsorption capacity in mg/g, Ci and Cf are the initial and final concentrations in milligrams per liter, V is the volume in liters, and m is the total mass of the active material in both electrodes in grams. The mass of electrodes used in this study varied from 0.4 to 0.8 g.
Water characterization
Water samples were collected from the laboratory taps pumped from boreholes located at the Nelson Mandela African Institution of Science and Technology, Arusha Tanzania. Ion-selective electrode (ISE) coupled with Mettler Toledo Seven Compact pH/Ion S220 meter was used to measure the concentration F−. Before F− measurements, total ionic strength buffer (TISAB II) was mixed with treated and untreated water in the ratio of 1:1. Glacial acetic acid (57.0 mL), NaCl (58.0 g), and cyclohexanediaminetetraacetic acid (CDTA (4 g)) were mixed in 500 mL of distilled water to prepare TISAB II. Solution pH was adjusted to 5.3–5.5 by adding about 150 mL of 6 M NaOH and distilled water was then added to make it 1 L. Hanna HI 9829 Multi-parameter was used to measure pH, electro-conductivity (EC), and total dissolved solids (TDS). Total hardness as Ca–CaCO3 was determined using a titration method with ethylenediaminetetraacetic acid (EDTA) with Eriochrome Black T (EBT) indicator and ammonium buffer. Calcium hardness and magnesium hardness as CaCo3 and concentration of Ca2+ and Mg2+ were determined by method 8030 Calcium and Magnesium; Calmagite Colorimetric Method with DR 2800 spectrophotometer according to the Standard Methods for Water and Wastewater Analysis [45].
Results and discussion
Structural and textural properties of JFAC
Figures 1 and 2 show the low- and high-magnification SEM images of the synthesized JFAC. From Figs. 1 and 2, the effect of the activating agent and activation temperature can be observed on the surface morphology of JFAC. When activating agent and temperature were introduced, vacancies, defects, and irregular pores which are responsible for enhancing the surface area and pore volume were formed (Figs. 1a–f and 2a–f). Significant differences in morphology were observed (as shown in low- and high-magnification images) in JFAC-3-600 (Fig. 2a, d), JFAC-3-700 (Fig. 2b, e), and JFAC-3-800 (Fig. 2c, f) in which enlarged pores appear on the surface of the material. This might be due to the breakage pores resulted from increasing activating agent ratio as previously observed by Zou et al. [46].
Figure 3 shows the nitrogen sorption isotherms and BJH pore size distribution plots of JFAC materials. From Fig. 2, it can be seen that adsorption occurs over the entire relative pressure indicating that the synthesized JFAC comprises of both micro- and mesopores. From Fig. 3a, it is observed that above the relative pressure p/p0 > 0.2, the adsorbed gas volume significantly increased linearly up to p/p0 = 1 for all samples indicating the absence of larger macropores or interparticle spaces. JFAC-2-800 and JFAC-3-800 samples showed very thin/strip hysteresis loop, corresponding to type H4 according to the IUPAC classification related to narrow slit-like pores, representing the presence of mesopores in the sample [47]. The strip/thin hysteresis loops generally show that the JFAC contained mostly mesopores. Figure 3b, c shows that most of the pores are less than 10 nm and are placed between 2 and 4 nm range signifying that mesopores are dominant in JFAC samples. The pore structure is important since the electrosorption capacity depends strongly on them. The micropores can enhance the specific surface area and offer additional ion adsorption sites while mesopores can decrease the ion resistance in porous carbon electrodes, therefore improving its performance.
Table 1 presents the BET surface area, micropore, and mesopore volume and surface area, average pore size, and the total pore volume of JFAC obtained from the nitrogen adsorption/desorption studies. From Table 1, it can be seen that BET surface area, micropore surface area (Smicro), mesopore surface area (Smeso), total pore volume (VT), and average pore diameter (Pd) of all JFAC increase with increasing activation temperature. This signifies that as activation temperature increases, the rate of reaction between carbon and KOH increases, consequently generating new pores. These results agree well with sorption isotherms presented earlier. Sample JFAC-2-800 exhibits a largest BET surface area of 2681 m2/g, the pore size of 2.23 nm, and a high pore volume of 2.6 cm3/g followed by JFAC-3-800 with a pore size of 4.16 nm, a BET surface area of 1993 m2/g, and a pore volume of 1.09 cm3/g. The BET surface area, pore size, and pore volume of JFAC-3-700 are 1350 m2/g, 3.47 nm, and 0.75 cm3/g respectively. JFAC-3-600 has a surface area of 1067 m2/g with a pore size of 2.93 nm and the least was unactivated carbon (JFC) with BET surface area of 607 m2/g and pore volume of 0.52 cm3/g. At KOH/C ratio of 2 to 3 with activation temperatures of 700 and 800 °C, the BET surface area and pore volume decrease while pore diameter increases. For JFAC-3-600 to JFAC-3-800, Vmicro increases 1.62 times (from 0.29 to 0.47 cm3/g), while the Vmeso increases 1.72 times (from 0.36 to 0.63). This is because at high activation temperature, the activation process occurs more completely [29], and the decomposition rate becomes faster, leading to more pore opening and widening of the pre-existing pores as previously reported by Deng et al. [48]. As KOH/C ratio increases, it induces breakage of the wall of the existing pore, mainly by destroying the mesopores, resulting in decrease in the mesopore content in JFAC. Furthermore, it can be observed from Table 1 that the Vmicro of JFAC-2-600 and JFAC-2-800 increased 2.16 times (from 0.19 to 0.41cm3/g), while the Vmeso increases 3.49 times (from 0.63 to 2.20cm3/g). This shows that KOH activation at high temperature generated mesopores in JFAC. This might be due to the fact that increasing activation temperature speeds up the process of widening of the pre-existing micropore or the transformation of micropores to mesopores in the carbon material due to the increase of the melting rate of K2CO3 and K2O as well as the rate of evaporating potassium (K) during the activation process as previously reported by Shen and Xue [49]. By varying KOH/C ratios from 2 to 3, the Vmicro increases while Vmeso decreases indicating that increasing KOH ratio generates micropores in the JFAC. From these results, the activation temperature and KOH/C ratio are the key factors in determining the surface and textural properties of the produced JFAC. Therefore, JFAC is anticipated to have a superior capacitance and electrosorption capacity due to their large surface area and pore size, which are among important factors in identifying good electrode materials for CDI.
The structure of the resulting JFAC carbon was analyzed using XRD. Figure 4 presents the XRD patterns of JFACs with two broad peaks. All JFAC samples possess the peak at around 2θ = 40°–45° allocated to the reflection (100) plane and the peak around 2θ = 20°–25° which characterizes disordered graphitic (002) planes. Presence of these peaks confirms the destruction of carbon matrix in the structure of lignocellulosic material as previously reported by Anton et al. [47]. Furthermore, the occurrence of the peak at around 2θ = 20°–25° and 40°–45° suggests that the structure of the formed JFAC is mainly amorphous. However, in JFAC-3-600 and JFAC-3-700 samples, some additional peaks (sparks) which are not attributed to carbon are observed at around 2θ = 33°–35°. These peaks can be associated with the presence of impurities/inorganic substances in the samples, or probably the samples were contaminated during sample preparation for characterization.
The structural parameters calculated from X-ray diffraction data are shown in Table 2. It can be seen that there is no significant variation in d002 and d100 values of all JFAC samples, implying that the activating temperature and KOH/C ratio did not affect the microcrystalline orientation. The calculated interlayer spacing (d002) that varies from 3.63 to 3.75 is slightly higher than the typical graphitic dimensions of 3.35 Å. These results show that synthesized JFAC carbon consists of turbostratic (fully disordered) structures, as previously reported by Girgis et al. [50]. The JFAC samples show constant Lc value of 19.35 Å, La value ranging from 50.43 to 50.72 Å, and a constant Lc/La value of 0.38. Both parameters, i.e., microcrystalline size and orientation, are essential as they have a significant influence on the formed electric double layer and the specific capacitance value of the material [51].
Raman spectroscopy studies investigated the degrees of graphitization of the JFAC. Figure 5 shows the Raman spectra of JFAC carbon. Two characteristic peaks around 1360 and 1588 cm−1 representing the D band (defect band) and the G band (graphitic band), respectively, are observed. Presence of these two bands signifies that the synthesized JFAC is graphitized carbon. From Raman peaks, the ratio of the intensity of D band and G band (ID/IG) is proportional to the number of defect sites whereby a higher ratio reflects a lower degree of graphitization, which leads to a decrease in the electrical conductivity [52]. From Fig. 5a, it can be seen that the value of ID/IG increases slightly from 0.84 to 0.88 as activation temperature increases to 700 °C and then dropped to 0.85 as temperature increases further to 800 °C. Moreover, at KOH/C ratio of 3 and increased activation temperature from 600 to 800 °C, the ID/IG ratio has no clear correlation as it varied from 0.84, 0.87, to 0.84 for JFAC-3-600, JFAC-3-700, and JFAC-3-800 respectively (Fig. 5b). There is no clear explanation of the decrease of the ID/IG ratio at high activation temperature.
Electrochemical measurements
The electrochemical properties were evaluated by cyclic voltammetry (CV) and EIS measurements to characterize the capacitive properties of the JFAC electrodes. Figure 6 shows the CV curves of JFAC electrodes at scan rates of 5, 10, 20, 30, 50, and 100 mV/s when NaCl and NaF were used as electrolytes. All CV curves show quasi-rectangular shape, signifying that charge propagation is good within JFAC electrodes. Generally, a smooth rectangular voltammogram without redox peaks represents that electrodes are perfect electric double layer capacitance, and no Faradaic process occurs on the surface of the electrodes. At a scan rate of 100 mV/s, the cyclic voltammograms of all JFAC electrodes were distorted due to the decrease in the ion transfer rate in the electrolyte, which reduced the specific capacitance as previously reported by Quach et al. [53]
The specific capacitances of the JFAC electrodes in 1 M NaCl and 1 M NaF electrolytes at different activation temperatures and KOH/C ratios obtained from the CV curves are presented in Fig. 7a–d. It can be seen that specific capacitance decreased as scan rate increased. At a scan rate of 5 mV/s, JFAC-3-800 exhibited highest specific capacitance of 352 F/g, followed by JFAC-2-800 (302 F/g) and JFAC-2-700 (298 F/g), and JFAC-3-600 was the least with a specific capacitance of 167 F/g in NaCl solution. A similar trend was observed when NaF was used as electrolyte in which JFAC-3-800 exhibited the highest specific capacitance of 216 F/g followed by JFAC-2-800 with a specific capacitance of 172 F/g. The enhanced specific capacitance of JFAC-3-800 might be due to its appropriate structural properties with a well-balanced porosity between the micropores and mesopores and the highest micropore surface area (as shown in Table 1) and its high specific surface area (Fig. 8), which provides more electro-active sites for adsorption, thus increasing the electrical double layer charges. When the scan rate increased from 5 to 50 mV/s, the specific capacitance decreases to around 60–90 F/g and 12–55 F/g for all JFAC samples when NaCl and NaF solutions were used as electrolyte respectively. These results indicate that most of JFAC electrodes have good capacitance and rate capability. Literature reports that electrode material with high BET specific surface area exhibits higher specific capacitance values. From Fig. 8, it can be seen that JFAC-2-800 exhibits the largest specific surface area; however, its specific capacitance is not the highest. This might be due to the highest mesopore area and unbalanced micropore and mesopore volume and surface area compared to other samples (Table 1) together with its elevated charge transfer resistance as revealed by EIS studies in Fig. 9a. With these results, the best activation conditions for obtaining material with high specific capacitance from jackfruit peels are utilizing KOH/C ratio of 3 and activation temperature of 800.
Nyquist plots were used to analyze EIS data, as shown in Fig. 9. All JFAC electrodes display a similar Nyquist plot with two parts, a charge transfer resistance at the electrode-electrolyte interface region represented by a semicircle at high frequency and a slightly vertical capacitive line at low frequencies. For all samples, the semicircle at high frequency is observed to decrease as activation temperature increases from 600 to 800 °C, indicating decreased charge transfer resistance. However, for samples prepared with KOH/C ratio of 2, the semicircle is much larger (Fig. 9a) compared to samples prepared with KOH/C ratio of 3 (Fig. 9b). JFAC-3-600, JFAC-3-700, and JFAC-3-800 show a smaller charge transfer resistance in comparison with JFAC-2-600, JFAC-2-700, and JFAC-2-800 due to an increased degree of graphitization consistent with Raman studies previously discussed. Equivalent series resistance (ESR) of the electrode materials which show the conductivity of both active materials and the electrolyte is represented by the x-intercept of the Nyquist plot. From the Nyquist plots, one can see that ESR values of all JFAC samples are less than 1.5 Ω indicating that the material has high conductivity.
Water characterization
The physicochemical properties of the collected water sample along with their WHO permissible limits of drinking water quality are summarized in Table 3. The collected water sample has conductivity, TDS, and pH values of 762 μS/cm, 498 mg/L, and 7.4, respectively. Its F− concentration is 3.11 mg/L, which is above WHO drinking water permissible limit and total hardness of 112 mg/L (as CaCO3) which are classified as moderately hard water. Water is considered to be soft if the concentration of calcium carbonate is below 60 mg/L, moderately hard if it is between 60 and 120 mg/L, hard if the concentration is between 120 and 180 mg/L, and very hard if it is more than 180 mg/L [1]. According to WHO public drinking water standards, magnesium, calcium, and chloride level should not exceed the permissible limit of 50, 70, and 200 mg/L respectively. The concentration of hardness-causing ions in the water sample is 78 mg/L for Ca2+, which is above the WHO permissible limit, and 16 mg/L for Mg2+.
CDI performance
When the potential was applied to the cell, ions in the water are adsorbed on the surface of JFAC electrodes, and thus, conductivity drops correspondingly (Fig. 10) due to the lowered concentration of ions. When the voltage of 1.2 V was applied to JFAC electrodes (Fig. 10a), the conductivity of water during the operation decreased sharply in the first 900 s and then slowly to 22, 11, 8, 6, and 3% of the initial value after 3 h. The same situation was observed when the potential of 2 V was applied to the JFAC electrodes (Fig. 10b), conductivity dropped abruptly for the first 900 s and then to 31 and 17% of the initial value when JFAC-3-700 and JFAC-3-600 electrodes were used respectively. For JFAC-3-800 electrode, conductivity dropped to 29% after 7200 s then rise slowly to a constant value. This indicates that the electrode saturates and cannot take more ions. For JFAC-2-800 electrode, conductivity dropped continuously to 33% of its initial value after 10,800 s without reaching saturation. Therefore, charge-discharge cycle stability and regeneration capacity were studied using JFAC-2-800 (Fig. 10c). Figure 10c shows the conductivity transient over 10 charge-discharge cycles. It can be observed that the ions were released back to the water during discharge at 0 V and the conductivity start to rise. For the first 5 cycles in discharge phase, the conductivity rise to its initial value while for the last 5 cycles, the conductivity of the water in each discharge phase did not reach its initial value indicating that not all ions were released back while discharging. Although not all ions were released back, there was no significant decline in conductivity change observed in the consecutive charge-discharge cycles indicating that JFAC-2-800 electrodes are stable and can be re-used for several hours without losing its adsorption capacity.
Fluoride removal with CDI cell
Table 4 presents the concentration of F− after electrosorption experiment for all prepared electrodes. From Table 4, it can be seen that at 2.0 V, JFAC-2-800, JFAC-3-700, and JFAC-3-800 reduce F− concentration to 1.47, 1.18, and 1.55 mg/L respectively. The values 1.47 and 1.18 mg/L are below the WHO permissible limit for drinking water quality of 1.5 mg/L. Thus, the CDI cell with JFAC biomass-based electrodes was used successfully for electrosorption of fluoride from tap water existing together with natural organic substances and other ions. The defluoridation efficiencies of JFAC electrodes calculated by Eq. (4) are represented in the bar diagram (Fig. 11). It is observed that defluoridation efficiency increased with increase in applied potential. JFAC-3-700 showed the best defluoridation efficiency (62%) when the potential of 2 V was applied. On the other hand, JFAC-3-800 exhibited highest specific capacitance (Fig. 7d), but it does not achieve the highest removal efficiency in the CDI test. This might be due to high charge transfer resistance and other intrinsic factors which may affect the performance of AC electrodes and limit its desalination performance as previously reported by Foo and Hameed [54].
Removal of other co-existing ions in the water
Fluoride is not the only ion present in water under study; other charged ions, such as hardness-causing cations (Ca2+, Mg2+), were also present. Even though water hardness has an insignificant health impact, it can lead to scaling and cause failures in heat exchangers, pipelines of boilers, and other electrical appliances as well as affecting the performance of cleaning detergents [55]. Therefore, it is important to remove/minimize hardness-causing ions from the water to the acceptable limits to avoid the aforementioned effects. In the charging step of the CDI process, these ions were adsorbed electrostatically by the oppositely charged JFAC electrodes and stored in EDLs on the electrode/solution interface. That is why conductivity was decreasing, as shown in Fig. 10. Figure 12a–c show removal efficiency of total hardness (TH), Ca2+, and Mg2+ for each electrode type at different applied voltages. It can be seen that removal efficiency increases with increasing applied potential, as presented in Fig. 12a. At 1.2 V, JFAC-2-700, JFAC-2-800, and JFAC-3-700 have the same hardness removal efficiency. At 2 V, the hardness removal efficiency increased for all electrodes whereby JFAC-3-700 outperforms others, and hardness concentration reduction of 80% was reached. Removal efficiency for Mg2+ and Ca2+ also increased with applied voltage (Fig. 12b, c). The removal efficiency of different ions at 2 V for each JFAC electrode is shown in Fig. 12d. The Mg2+ removal efficiency was higher than that of Ca2+ and F− for JFAC-2-700 and JFAC-3-800.
The electrosorption processes in reverse osmosis and ion exchange technologies are well known. In these processes, ionic charge and molecular size dictate the salt removal in which the larger multivalent ions are preferably removed than the smaller ones [56]. However, in CDI, it is not that much clear. Ion selectivity with CDI was firstly studied by Gabelich et al. [56] using carbon aerogel electrodes. Gabelich et al. [56] generally concluded that in a competitive environment, ions with different valences, the hydrated size of the ion in a solution controls the electrosorption preference. Smaller hydrated radii monovalent ions are removed more effectively than larger radii multivalent ions. Contrary, other studies revealed that ionic charge has a stronger effect on electrosorption ability than the hydrated ionic radius [57, 58] in which multivalent ions are electrosorbed first resulting to a higher electrosorption capacity than monovalent ions due to their larger ionic size and greater charge. However, for ions with the same initial concentrations and the charge, their hydrated radius dictates their selectivity and sometimes ions with higher feed concentration play a crucial role in ion electrosorption than the hydrated ionic radius [18, 59]. This is similar to what is observed in this study. The electrosorption capacity correlates well with the initial ion concentration in the feed water. An ion with higher hydrated radii, i.e., Mg2+ (4.28 Å), was removed in large quantity compared to Ca2+ (4.12 Å) and F− (3.52 Å) when JFAC electrodes were used. The electrosorption capacities of JFAC for different ions (shown in Table 5) nearly increased with increasing initial ion concentrations in the feed water except for JFAC-2-600, JFAC-2-700, and JFAC-3-800 in which EC of Ca2+ is small though its concentration is higher compared to that of Mg2+. The CDI electrode JFAC-3-700 shows improved electrosorption capacity for Ca2+ (0.59 mg/g) due to its high concentration in the water sample followed by Mg2+ (0.50 mg/g) and F− (0.13 mg/g). Hou and Huang [57] previously reported that ions with higher concentrations in the water/solution had a higher driving force and can easily be transported from the solution to the pore structure leading to higher electrosorption capacity.
It is further reported that EC is directly proportional to the BET surface area [60], but this was not observed in this study. From Table 5, it can be seen that carbon sample with highest surface area (JFAC-2-800) did not achieve the highest EC for all ions. Sample JFAC-3-700 with BET SSA of 1350 m2/g achieved the highest electrosorption capacity for F− and Ca2+. Therefore, we can say that not only high surface area required for efficient water defluoridation but also other material properties such as accessible pore size and the balanced structure between micropore and mesopore as previously reported by Seo et al. [58, 61]. Furthermore, the CDI cell configuration and the mass of active materials used to make the electrodes, size, and thickness of electrodes could also probably be important parameters to consider for effective CDI performance. Since natural water was utilized without prior treatment, the presence of natural organic substances may be another factor which inhibits the JFAC surface and limits its effectiveness in water defluoridation and desalination.
pH changes
When oxidation, reduction, or water-splitting reactions are taking place on the surface of electrodes, the pH of the effluent can change significantly [62]. To study if electrode reactions were taking place during the electrosorption process, the pH of the effluent was monitored throughout the experiment and presented in Fig. 13. From Fig. 13, the pH of the effluent during the electrosorption stage was observed to increase from 7.2 to 8.2 at all applied potentials. This range is within the WHO permissible limit for drinking water of 6.5–8.2. Considering that the pH of the effluent is retained within this range, it implies that no Faradaic reactions (e.g., redox reactions and water hydrolysis) were taking place when the voltage of 1.2 V (Fig. 13a) and 2 V (Fig. 13b) was applied to the JFAC electrodes. The CDI process generally occurs due to capacitive ion storage inside the pores and ion transport through the electrode material.
Conclusion
In this study, JFAC electrodes were studied in CDI for water defluoridation. It was found that JFAC-based electrodes remove fluoride from natural water to standard required for drinking water as specified by WHO. The presence of other ions in the water did not affect the defluoridation process, signifying that JFAC is a good CDI electrode for fluoride removal. The JFAC electrodes showed a higher EC for magnesium absorption as compared to calcium due to its high concentration in the water. Generally, the removal efficiency of JFAC electrode depends not only on the surface area and pore volume but also on other factors including applied voltage and initial concentration of an ion in the water. The applied voltage enhances the electrosorption capacity of Mg2+, Ca2+, and F−, and thus, electrical double layer adsorption dominate the electrosorption process since there was no significant change in pH observed during electrosorption stage. With a voltage of 2.0 V, the JFAC-3-700 electrode exhibits the highest electrosorption capacity for Ca2+, Mg2+, and F−. Therefore, the carbon derived from jackfruit peels may be considered as potential CDI electrode material for natural/real water defluoridation applications.
References
WHO (2010) Hardness in drinking-water: background document for development of WHO guidelines for drinking-water quality. Geneva
Mohapatra M, Anand S, Mishra BK, Giles DE, Singh P (2009) Review of fluoride removal from drinking water. J Environ Manag 91:67–77
WHO (2011) Guidelines for drinking-water quality. 104–108
Khairnar MR, Dodamani AS, Jadhav HC, Naik RG, Deshmukh MA (2015) Mitigation of fluorosis—a review. J Clin Diagn Res 9:5–9
Brunson LR, Sabatini DA (2013) Practical considerations, column studies and natural organic material competition for fluoride removal with bone char and aluminum amended materials in the Main Ethiopian Rift Valley. Sci Total Environ 488–489:580–587. https://doi.org/10.1016/j.scitotenv.2013.12.048
Hashim KS, Shaw A, Al Khaddar R, Ortoneda Pedrola M, Phipps D (2017) Defluoridation of drinking water using a new flow column-electrocoagulation reactor (FCER)—experimental, statistical, and economic approach. J Environ Manag 197:80–88. https://doi.org/10.1016/j.jenvman.2017.03.048
Ayoob S, Gupta AK, Bhat VT (2008) A conceptual overview on sustainable technologies for the defluoridation of drinking water. Crit Rev Environ Sci Technol 38:401–470. https://doi.org/10.1080/10643380701413310
Wagutu AW, Machunda R, Jande YAC (2018) Crustacean derived calcium phosphate systems: application in defluoridation of drinking water in East African rift valley. J Hazard Mater 347:95–105. https://doi.org/10.1016/j.jhazmat.2017.12.049
Tang Y, Guan X, Su T, Gao N, Wang J (2009) Fluoride adsorption onto activated alumina: modeling the effects of pH and some competing ions. Colloids Surf A Physicochem Eng Asp 337:33–38
Yadav AK, Abbassi R, Gupta A, Dadashzadeh M (2013) Removal of fluoride from aqueous solution and groundwater by wheat straw, sawdust and activated bagasse carbon of sugarcane. Ecol Eng 52:211–218. https://doi.org/10.1016/j.ecoleng.2012.12.069
Barathi M, Santhana Krishna Kumar A, Rajesh N (2013) Efficacy of novel Al–Zr impregnated cellulose adsorbent prepared using microwave irradiation for the facile defluoridation of water. J Environ Chem Eng 1:1325–1335. https://doi.org/10.1016/j.jece.2013.09.026
Jande YAC, Kim WS (2013) Desalination using capacitive deionization at constant current. Desalination 329:29–34. https://doi.org/10.1016/j.desal.2013.08.023
Zhang C, He D, Ma J, Tang W, Waite TD (2018) Faradaic reactions in capacitive deionization (CDI)—problems and possibilities: a review. Water Res 128:314–330. https://doi.org/10.1016/j.watres.2017.10.024
Porada S, Zhao R, van der Wal A, Presser V, Biesheuvel PM (2013) Review on the science and technology of water desalination by capacitive deionization. Prog Mater Sci 58:1388–1442. https://doi.org/10.1016/j.pmatsci.2013.03.005
Zou L, Morris G, Qi D (2008) Using activated carbon electrode in electrosorptive deionisation of brackish water. Desalination 225:329–340
Oda H, Nakagawa Y (2003) Removal of ionic substances from dilute solution using activated carbon electrodes. Carbon 41:1037–1047
Farmer JC, Fix DV, Mack GV, Pekala RW, Poco JF (1996) Capacitive deionization of NH4ClO4 solutions with carbon aerogel electrodes. J Appl Electrochem 26:1007–1018
Xu P, Drewes JE, Heil D, Wang G (2008) Treatment of brackish produced water using carbon aerogel-based capacitive deionization technology. Water Res 42:2605–2617
Wang L, Wang M, Huang Z-H, Cui T, Gui X, Kang F, Wang K, Wu D (2011) Capacitive deionization of NaCl solutions using carbon nanotube sponge electrodes. J Mater Chem 21:18295–18299
Wang S, Wang D, Ji L, Gong Q, Zhu Y, Liang J (2007) Equilibrium and kinetic studies on the removal of NaCl from aqueous solutions by electrosorption on carbon nanotube electrodes. Sep Purif Technol 58:12–16
El-Deen AG, Barakat NA, Khalil KA, Kim HY (2014) Hollow carbon nanofibers as an effective electrode for brackish water desalination using the capacitive deionization process. New J Chem 38:198–205
Li H, Lu T, Pan L, Zhang Y, Sun Z (2009) Electrosorption behavior of graphene in NaCl solutions. J Mater Chem 19:6773–6779
Li H, Zou L, Pan L, Sun Z (2010) Novel graphene-like electrodes for capacitive deionization. Environ Sci Technol 44:8692–8697
Chen B, Ma Q, Tan C, Lim TT, Huang L, Zhang H (2015) Carbon-based sorbents with three-dimensional architectures for water remediation. Small 11:3319–3336
Feng C, Chen YA, Yu CP, Hou CH (2018) Highly porous activated carbon with multi-channeled structure derived from loofa sponge as a capacitive electrode material for the deionization of brackish water. Chemosphere 208:285–293. https://doi.org/10.1016/j.chemosphere.2018.05.174
Inagaki M, Konno H, Tanaike O (2010) Carbon materials for electrochemical capacitors. J Power Sources 195:7880–7903. https://doi.org/10.1016/j.jpowsour.2010.06.036
Chen Y, Zhu Y, Wang Z, Li Y, Wang L, Ding L, Gao X, Ma Y, Guo Y (2011) Application studies of activated carbon derived from rice husks produced by chemical-thermal process—a review. Adv Colloid Interf Sci 163:39–52
Elisadiki J, Kibona TE, Machunda RL, Saleem MW, Kim W-S, Jande YAC (2019) Biomass-based carbon electrode materials for capacitive deionization: a review. Biomass Convers Bior:1–30. https://doi.org/10.1007/s13399-019-00463-9
Zhao S, Yan T, Wang Z, Zhang J, Shi L, Zhang D (2017) Removal of NaCl from saltwater solutions using micro/mesoporous carbon sheets derived from watermelon peel via deionization capacitors. RSC Adv 7:4297–4305. https://doi.org/10.1039/c6ra27127h
Zhang XF, Wang B, Yu J, Wu XN, Zang YH, Gao HC, Su PC, Hao SQ (2018) Three-dimensional honeycomb-like porous carbon derived from corncob for the removal of heavy metals from water by capacitive deionization. RSC Adv 8:1159–1167. https://doi.org/10.1039/c7ra10689k
Li Y, Jiang Y, Wang T-J, Zhang C, Wang H (2017) Performance of fluoride electrosorption using micropore-dominant activated carbon as an electrode. Sep Purif Technol 172:415–421. https://doi.org/10.1016/j.seppur.2016.08.043
Zhao C, Liu G, Sun N, Zhang X, Wang G, Zhang Y, Zhang H, Zhao H (2018) Biomass-derived N-doped porous carbon as electrode materials for Zn-air battery powered capacitive deionization. Chem Eng J 334:1270–1280. https://doi.org/10.1016/j.cej.2017.11.069
Gaikwad MS, Balomajumder C (2017) Tea waste biomass activated carbon electrode for simultaneous removal of Cr(VI) and fluoride by capacitive deionization. Chemosphere 184:1141–1149. https://doi.org/10.1016/j.chemosphere.2017.06.074
Wu P, Xia L, Dai M, Lin L, Song S (2016) Electrosorption of fluoride on TiO2-loaded activated carbon in water. Colloids Surf A Physicochem Eng Asp 502:66–73. https://doi.org/10.1016/j.colsurfa.2016.05.020
Tang W, Kovalsky P, He D, Waite TD (2015) Fluoride and nitrate removal from brackish groundwaters by batch-mode capacitive deionization. Water Res 84:342–349. https://doi.org/10.1016/j.watres.2015.08.012
Tang W, Kovalsky P, Cao B, He D, Waite TD (2016) Fluoride removal from brackish groundwaters by constant current capacitive deionization (CDI). Environ Sci Technol 50:10570–10579. https://doi.org/10.1021/acs.est.6b03307
Elisadiki J, Jande YAC, Machunda RL, Kibona TE (2019) Porous carbon derived from Artocarpus heterophyllus peels for capacitive deionization electrodes. Carbon 147:582–593. https://doi.org/10.1016/j.carbon.2019.03.036
Enock TK, King’ondu CK, Pogrebnoi A, Jande YAC (2017) Biogas-slurry derived mesoporous carbon for supercapacitor applications. Mater Today Energy 5:126–137. https://doi.org/10.1016/j.mtener.2017.06.006
Mohd Nor NS, Deraman M, Omar R, Awitdrus, Farma R, Basri NH, Mohd Dolah BN, Mamat NF, Yatim B, Md Daud MN (2015) Influence of gamma irradiation exposure on the performance of supercapacitor electrodes made from oil palm empty fruit bunches. Energy 79:183–194. https://doi.org/10.1016/j.energy.2014.11.002
Barzegar F, Bello A, Momodu D, Madito MJ, Dangbegnon J, Manyala N (2016) Preparation and characterization of porous carbon from expanded graphite for high energy density supercapacitor in aqueous electrolyte. J Power Sources 309:245–253. https://doi.org/10.1016/j.jpowsour.2016.01.097
Mirghni AA, Madito MJ, Masikhwa TM, Oyedotun KO, Bello A, Manyala N (2017) Hydrothermal synthesis of manganese phosphate/graphene foam composite for electrochemical supercapacitor applications. J Colloid Interface Sci 494:325–337. https://doi.org/10.1016/j.jcis.2017.01.098
Chen W, Fan Z, Gu L, Bao X, Wang C (2010) Enhanced capacitance of manganese oxide via confinement inside carbon nanotubes. Chem Commun 46:3905–3907
Hashim KS, Al Khaddar R, Jasim N, Shaw A, Phipps D, Kot P, Pedrola MO, Alattabi AW, Abdulredha M, Alawsh R (2019) Electrocoagulation as a green technology for phosphate removal from river water. Sep Purif Technol 210:135–144. https://doi.org/10.1016/j.seppur.2018.07.056
Leong ZY, Yang HY (2016) Porous carbon hollow spheres synthesized via a modified Stöber method for capacitive deionization. RSC Adv 6:53542–53549. https://doi.org/10.1039/c6ra06489b
Eaton AD, Clesceri LS, Greenberg AE, Franson MAH (2005) Standard methods for the examination of water and wastewater, 21st ed., American Public Health Association, American Water Works Association, Water Environment Federation
Zou R, Quan H, Wang W, Gao W, Dong Y, Chen D (2018) Porous carbon with interpenetrating framework from Osmanthus flower as electrode materials for high-performance supercapacitor. J Environ Chem Eng 6:258–265. https://doi.org/10.1016/j.jece.2017.11.080
Anton, Z, Marek, M, Slavomír, H, Michal, L, Zuzana, D, Milota, K, Jaroslav, B (2016) Preparation of chemically activated carbon from waste biomass by single-stage and two-stage pyrolysis. J Clean Prod 643–653. https://doi.org/10.1016/j.jclepro.2016.12.061
Deng J, Xiong T, Xu F, Li M, Han C, Gong Y, Wang H, Wang Y (2015) Inspired by bread leavening: one-pot synthesis of hierarchically porous carbon for supercapacitors. Green Chem 17:4053–4060
Shen Z, Xue R (2003) Preparation of activated mesocarbon microbeads with high mesopore content. Fuel Process Technol 84:95–103. https://doi.org/10.1016/s0378-3820(03)00050-x
Girgis BS, Temerk YM, Gadelrab MM, Abdullah ID (2007) X-ray diffraction patterns of activated carbons prepared under various conditions. Carbon Sci 8:95–100
Nabais JM, Teixeira JG, Almeida I (2011) Development of easy made low cost bindless monolithic electrodes from biomass with controlled properties to be used as electrochemical capacitors. Bioresour Technol 102:2781–2787. https://doi.org/10.1016/j.biortech.2010.11.083
Zubrik A, Matik M, Hredzák S, Lovás M, Danková Z, Kováčová M, Briančin J (2016) Preparation of chemically activated carbon from waste biomass by single-stage and two-stage pyrolysis. J Clean Prod 143: https://doi.org/10.1016/j.jclepro.2016.12.061
Quach NKN, Yang W-D, Chung Z-J, Tran HL (2017) The influence of the activation temperature on the structural properties of the activated carbon xerogels and their electrochemical performance. Adv Mater Sci Eng 2017:1–9. https://doi.org/10.1155/2017/8308612
Foo KY, Hameed BH (2009) A short review of activated carbon assisted electrosorption process: an overview, current stage and future prospects. J Hazard Mater 170:552–559. https://doi.org/10.1016/j.jhazmat.2009.05.057
Gabrielli C, Maurin G, Francy-Chausson H, Thery P, Tran TTM, Tlili M (2006) Electrochemical water softening: principle and application. Desalination 201:150–163. https://doi.org/10.1016/j.desal.2006.02.012
Gabelich CJ, Tran TD, Suffet IHM (2002) Electrosorption of inorganic salts from aqueous solution using carbon aerogels. Environ Sci Technol 36:3010–3019. https://doi.org/10.1021/es0112745
Hou C-H, Huang C-Y (2013) A comparative study of electrosorption selectivity of ions by activated carbon electrodes in capacitive deionization. Desalination 314:124–129. https://doi.org/10.1016/j.desal.2012.12.029
Seo SJ, Jeon H, Lee JK, Kim GY, Park D, Nojima H, Lee J, Moon SH (2010) Investigation on removal of hardness ions by capacitive deionization (CDI) for water softening applications. Water Res 44:2267–2275. https://doi.org/10.1016/j.watres.2009.10.020
Ying TY, Yang KL, Yiacoumi S, Tsouris C (2002) Electrosorption of ions from aqueous solutions by nanostructured carbon aerogel. J Colloid Interface Sci 250:18–27. https://doi.org/10.1006/jcis.2002.8314
Wang G, Qian B, Dong Q, Yang J, Zhao Z, Qiu J (2013) Highly mesoporous activated carbon electrode for capacitive deionization. Sep Purif Technol 103:216–221
Alfredy T, Jande YAC, Pogrebnaya T (2019) Removal of lead ions from water by capacitive deionization electrode materials derived from chicken feathers. J Water Reuse Desal. https://doi.org/10.2166/wrd.2019.074
He D, Wong CE, Tang W, Kovalsky P, Waite TD (2016) Faradaic reactions in water desalination by batch-mode capacitive deionization. Environ Sci Technol Lett 3:222–226. https://doi.org/10.1021/acs.estlett.6b00124
Acknowledgments
The authors would like to acknowledge Prof. Marta Hatzell and Daniel A. Moreno from Georgia Institute of Technology for manufacturing the CDI cell.
Funding
This work was supported by the research grant from European Union’s Horizon 2020 research and innovation program under FLOWERED project grant agreement number 690378, The World Academy of Sciences (TWAS) through grant number 16-529 RG/CHE/AF/AC_G–FR3240293305 dated 12 December 2016, and the University of Dodoma.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elisadiki, J., Jande, Y.A.C., Kibona, T.E. et al. Highly porous biomass-based capacitive deionization electrodes for water defluoridation. Ionics 26, 2477–2492 (2020). https://doi.org/10.1007/s11581-019-03372-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03372-z