Abstract
Li2Fe1-x-yMnxTiySiO4/C (x = 0.05~0.20, y = 0.02~0.08), a porous spherical cathode material for lithium-ion batteries, has been prepared by combining the sol-gel, spray drying, and microwave synthesis method, and co-doped Li2FeSiO4 with the Mn2+ and Ti4+on the iron site. The effects of Mn2+ and Ti4+ co-doping on the crystal structure, microstructure, and electrochemical performance Li2FeSiO4 were investigated by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and electrochemical tests. The results showed that the modified sample (Li2Fe0.81Mn0.15Ti0.04SiO4/C), when x and y are 0.15 and 0.04, respectively, has the higher specific capacity and better cycle stability as well as lower impedance and better reversibility. Its electronic conductivity reached 6.85 × 10−4 S/cm, lithium-ion diffusion coefficient reached 1.65 × 10−13 cm2/s, and initial charge/discharge–specific capacity reaches 197.7/187.8 mAh/g and high capacity retention of 78% after 20 charge/discharge cycles at 2 C. According to the study, the Li2FeSiO4/C cathodes material of Mn and Ti co-substitution at the Fe site could partly make up the disadvantage of single Mn doping, and might provide an effective guide for the dopant incorporation to Li2FeSiO4 systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Silicate polyanion compound materials of lithium electrodes Li2MSiO4 (M = Co, Fe, Ni, Mn, etc.) are considered as alternative cathode materials for the next-generation lithium-ion battery (LIB), owing to their competitive advantages such as high theoretical capacity exceeding 330 mAh/g, safety, highly stability, environment friendly, abundant natural resources, and so forth [1,2,3,4,5,6,7]. Among several polyanionic compounds, orthorhombic metasilicate crystal (Pnm21, Pnmb, and P21/n), Li2FeSiO4 has become a hot spot of orthosilicate cathode materials because of some disadvantages of other materials, such as low electrochemical activity for Li2CoSiO4 [8, 9] and rapidly decreased capacity for Li2MnSiO4 and Li2NiSiO4 during charging and discharging [10,11,12]. However, the actual power density of Li2FeSiO4 (LFS) is low due to its low electronic conductivity and poor lithium ion mobility [13,14,15]. In order to solve these issues, researchers have made a number of efforts to enhance the electrochemical performance of Li-ion batteries such as carbon coating, [16, 17] cationic doping, [4, 6, 7, 18,19,20,21] and nano-crystallization [22]. For example, synthesized Li2FeSiO4/C/GO with citric acid and GO as carbon sources by Huang [23] et al. exhibited the better electrochemical properties than Li2FeSiO4, whose initial discharge capacity was 155 mAh/g and capacity retention achieved 96.8% after 30 cycles at 5 C. Nevertheless, carbon coating only increases the ionic conductivity of Li2FeSiO4 particles high on the surface and between particles, but not within Li2FeSiO4 particles, which limit its practical application to a certain degree. In addition, Kokal [24] et al. found that the radius of Mn2+ is similar to that of Fe2+ ion, and appropriate amount of Mn2+ substituted for Fe2+ can form solid solution in Li2FeSiO4 crystal and achieving a higher storage capacity because Mn2+/Mn3+ can be oxidized to Mn3+/Mn4+ to enable Li2Fe1-xMnxSiO4 to achieve more than one Li+-ion per formula unit extraction/insertion at a wide potential window. However, this process was usually accompanied with serious capacity fade and drastic poor rate capability due to the structural instability of Li2Fe1-xMnxSiO4 caused by Jahn-Teller effect during the first few cycles and subsequent upon further cycling [25]. Fortunately, Zheng [26] et al. showed that the n-type doping introduced by Ti4+ can not only improve the conductivity of Li2FeSiO4, but also enhance the coupling between SiO4 and FeO4 tetrahedrons through the hybridization of d-orbital electrons, so as to enhance the stability of cathode materials.
In this article, to overcome drastic capacity fade and poor rate capability of Mn-doped Li2FeSiO4, we report a porous spherical cathode material, which are Li2Fe0.81Mn0.15Ti0.04SiO4/C synthesized by sol-gel, spray drying, and microwave synthesis method for lithium-ion batteries. And flat charge/discharge plateau accompanied by the extraction/insertion of more than one Li+-ions in a narrow potential window 1.5–4.8 V for Li2Fe0.81Mn0.15Ti0.04SiO4/C (polymorph with orthorhombic structure, P21/n space group) was obtained [27]. Therefore, the co-doped Li2FeSiO4 with the Mn2+ and Ti4+on the iron site can not only show an obvious improvement in specific capacity, but also have some positive effect on the rate and cycling performance [28]. In addition, X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), atomic force microscope (AFM), and electrochemical tests were used to investigate systematically the effects of Mn2+ and Ti4+ co-doping on the crystal structure, morphology, and electrochemical performance.
Experimental
Synthesis of Li2Fe1-x-yMnxTiySiO4/C composite
The synthesis of Li2Fe1-x-yMnxTiySiO4/C, which is porous spherical, was carried out by a novel method of sol-gel, spray drying, and microwave synthesis. The main product was 0.04 M Li2Fe1-x-yMnxTiySiO4/C. It was prepared as follow: Firstly, dispersing carbon source (starch) in de-ionized water to obtain an aqueous solution with a carbon content of 6 wt.% in a beaker, which 6 wt.% was the theoretical carbon content of Li2Fe1-x-yMnxTiySiO4/C (the actual coated carbon content of is 5. 47 wt.%). Secondly, taking iron source (FeNO3·9H2O) and manganese source (Mn (CH3COO)2·4H2O) (the molar mass of Fe and Mn ratio is 1-x:x, x = 0.05, 0.10, 0.15, 0.20) blended by mechanical stirring, then added 0.08 M lithium source (CH3COOLi) and 0.2 g of surfactant (PVP) with continually mechanical stirring, followed by adding 0.04 M silicon source (SiO2-sol) and a specific amount of titanium source (nano-TiO2) (the molar mass of Fe, Mn, and Ti ratio is 1-x-y:x:y, y = 0.02, 0.04, 0.06, 0.08), blended by sonication at room temperature for 20 min. Thirdly, the sol mixture was turned into a sphere morphology, by spray drying with the inlet temperature at 200 C and out let temperature at 100 °C. Then, the precursor was dried in vacuum at 110 °C for 8 h and pressed into piece with tablet press (30 MPa). Finally, the piece was placed in a tube furnace and heated at different heat treatment temperature and time in an argon atmosphere.
Physico-chemical characterization
The X-ray diffraction (XRD, Bruker/D2 PHASER) was used to characterize the phase composition of the materials in reflection mode with Cu-Kα1 radiation (λ = 1.5406 nm), in the 2θ range between 10° and 80° in steps of 0.02° with an integration time of 2 s per step. The morphology and structure were evaluated by a field emission scanning electron microscopy (FESEM, Quanta 450 FEG), a transmission electron microscopy (TEM, FEI Tecnai G2 F30), and an atomic force microscope (AFM, E-sweep). The actual carbon content was evaluated by an elemental analyzer (ELEMENTAR, Vario EL cube).
Electrochemical characterization
Electrochemical characterization was done to test the electrochemical performance of Li2Fe1-x-yMnxTiySiO4/C cathodes material, which was performed by CR2032 cion cell. The cathode electrode was prepared by blending 80 wt.% active material (Li2Fe1-x-yMnxTiySiO4/C), 10 wt.% conductive additives (Super-P), and 10 wt.% waterborne adhesive (LB132) with magnetic stirring to form a slurry. Then, we coated slurry on aluminum foil current collector via doctor blade processing, followed drying under vacuum at 110 °C for 12 h. After that, the electrode film was cut into circular disks, and the average loading of active material is 3 mg/cm2. Afterward, all the cells were assembled in an argon-filled glove box (Super1220/990/750) with lithium metal as the counter electrode and a polypropylene microporous film (Celgaed2400) in the middle as separator. The electrolyte was a solution of 1 M LiPF6, which dispersed in the mixture of DMC:EMC:EC (the ratio of 1:1:1 by volume). The cells were tested between 1.5 and 4.8 V (vs. Li+/Li) at room temperature by CT2001A Land battery testing system [29]. The cyclic voltammetry (CV) tests were carried out at room temperature by CH1604E electrochemical workstation with a scanning rate of 0.1 mV/s in the potential window of 1.5 V to 4.8 V. Electrochemical impedance spectroscopy (EIS) was measured at room temperature using CH1604E electrochemical workstation in the frequency range from 0.1 Hz to 100 kHz with an AC voltage of 5 mV.
Results and discussion
Effect of Mn2+ doping on Li2Fe1-xMnxSiO4/C performance
XRD patterns of Li2Fe1-xMnxSiO4/C (x = 0.00, 0.05, 0.10, 0.15, 0.20) samples are showed in Fig. 1. It can be seen that Li2Fe1-xMnxSiO4/C doped with different Mn2+ amounts exhibit no impurity phase (PDF#01-077-4374), diffraction peaks were all relatively sharply, and the crystallinity was high. Above all, different Mn2+ doping dosage did not change the crystal structure of Li2FeSiO4/C, and Li2Fe1-xMnxSiO4/C still belonged to P21/n structure [30, 31]. Doping the dopant ions to replace the position of original ions will form a solid solution and change the unit cell parameters, and thus cause the shift of diffraction peak. When 2θ is 24.3°, this diffraction peak which matches the (111) crystal face of Li2FeSiO4 shows obvious shift to the left when doped Mn2+ replaced the position of Fe2+ in Li2FeSiO4 lattice. With increasing the mixing amount of Mn2+, more the shift could be found from the XRD patterns. Moreover, the lattice parameters were obtain by Cell-Refinement function of the Jade5.0 software during the analysis of the XRD results and are listed in Table 1. From Table 1, with the doped dosage of Mn2+change, the lattice parameters vary significantly. It can be observed a constantly increasement of the unit cell volume with the increased amount of Mn2+. As a result, the diffusion rate and coefficient of lithium ions were boosted by the increase of crystal cell volume, which can expand the diffusion channel of lithium ions and reduce the resistance of lithium ion diffusion.
The SEM has been adopted to detect the morphology of the Li2Fe1-xMnxSiO4/C composites, and images of the Li2Fe1-xMnxSiO4/C composites with the amount of doped Mn2+ range from x = 0.05 to x = 0.20 are shown in Fig. 2. As shown in Fig. 2, all the samples present similar morphology of porous spherical particles and show no obvious agglomeration. The particle size distribution of different samples has also been detected and listed in Table 2. It can be seen from Table 2 that particle size distribution (D10, D50, and D90) and tap density of the as-synthesized Li2Fe1-xMnxSiO4/C exhibit little wave range with the amount of Mn2+ doped vary. The result proved that the amount of Mn2+ doping has no effect on the morphology, particle size distribution, and tap density of the synthesized Li2Fe1-xMnxSiO4/C.
Figure 3 shows the typical charge/discharge curves of the cathode with the Li2Fe1-xMnxSiO4/C (x = 0.05, 0.10, 0.15, 0.20) at 1/16 C. It figures that Li2Fe1-xMnxSiO4/C synthesized by different dosage of doped Mn2+ (x = 0.05, 0.10, 0.15, and 0.20) reveals the initial charge/discharge capacity of 160.4/145.5 mAh/g, 171.1/152.2 mAh/g, 194.8/171.7 mAh/g, and 177.6/128.1 mAh/g, respectively. And the corresponding coulomb efficiency values were 90.7%, 89.0%, 88.1%, and 72.1%, respectively. With the increase of Mn2+ content, the charge/discharge capacity of Li2Fe1-xMnxSiO4/C increased initially and then decreased. When x > 0.05 (x = 0.10, 0.15, and 0.20), the Li2Fe1-xMnxSiO4/C reveals higher charge capacity than 166 mAh/g. During the charging process, Mn2+ is oxidized to Mn4+ and the incorporation of Mn2+ into Li2FeSiO4/C can incorporate more than one Li+ [27]. When x = 0.20, Li2Fe1-xMnxSiO4/C owns the lowest specific capacity and coulomb efficiency. After Mn2+/Mn3+ is oxidized to Mn3+/Mn4+, it is not reduced to Mn2+/Mn3+ and combined with O2− to generate MnO4 tetrahedron to restore to the original vacancy [30].
In Fig. 4, the curves show the cycling performance of Li2Fe1-xMnxSiO4/C cathode at 0.1 C. The Li2Fe1-xMnxSiO4/C synthesized by different Mn2+/Mn3+ quantity (x = 0.05, 0.10, 0.15, and 0.20) delivers a specific capacity of 131.8 mAh/g, 136.1 mAh/g, 147.8 mAh/g, and 93.8 mAh/g, respectively. And the related capacity retention ratio was 93.7%, 92.6%, 89.8%, and 76.2%, respectively. During the cycling process, Li2Fe1-xMnxSiO4/C synthesized with different amounts of Mn2+/Mn3+ exhibit different degrees of capacity decay, and Li2Fe0.8Mn0.2SiO4/C decays the most seriously. Since Li2Fe0.8Mn0.2SiO4/C has the highest content of Mn2+/Mn3+, it has a high chemical activity to be oxidized into Mn3+/Mn4+, and it may react with electrolyte but not with O2− to form tetrahedron, which results in the material maintaining a certain structure. Therefore, the Li+ fails to be re-embedded into Li2Fe0.8Mn0.2SiO4/C after extracted. Capacity decreases as the number of circle increases, and the number of Li+ that can insert into Li2Fe0.8Mn0.2SiO4/C has decreased [32].
Effect of Ti4+ doping on Li2Fe0.85-yMn0.15TiySiO4/C performance
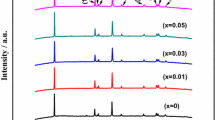
XRD patterns of Li2Fe0.85-yMn0.15TiySiO4/C (y = 0.02, 0.04, 0.06, 0.08) are shown in Fig. 5. According to the XRD result, Li2Fe0.85-yMn0.15TiySiO4/C doped by different Ti4+ quantities all belong to P21/n structure (PDF#01-077-4374) [29, 30]. Their diffraction peaks are sharp and with high crystallinity. The impurities of Fe, Mn, Ti, and Ti-compounds did not appear in the XRD patterns, indicating that different Ti4+ quantities have been doped into the lattice of Li2Fe0.85-yMn0.15TiySiO4/C. With the increase of Ti4+ doping, the diffraction peak of crystal plane (111) shifted significantly to the left, because the addition of Ti4+ to Li2Fe0.85-yMn0.15TiySiO4/C lattice changes the cell parameters. As shown in Table 3, the crystal lattice volume of Li2Fe0.85-yMn0.15TiySiO4/C increased by raising the Ti4+ dosage.
Figure 6 shows the SEM diagram of Li2Fe0.85-yMn0.15TiySiO4/C (y = 0.02, 0.04, 0.06, 0.08). According to the images, the morphology of Li2Fe0.85-yMn0.15TiySiO4/C shows no change with different amounts of Ti4+. It can be seen from Table 4 that theTi4+ doping has no influence on the particle size distribution and tap density of the synthesized Li2Fe0.85-yMn0.15TiySiO4/C.
In Fig. 7, the curves show that the Li2Fe0.85-yMn0.15TiySiO4/C (y = 0.02, 0.04, 0.06, 0.08) charge/discharge at 1/16 C. It can be seen that the initial charge/discharge capacity of Li2Fe0.85-yMn0.15TiySiO4/C with different Ti4+ content (y = 0.02, 0.04, 0.06, and 0.08) was 191.3/176.7 mAh/g, 197.7/187.8 mAh/g, 194.7/177.7 mAh/g, and 186.8/155.4 mAh/g, respectively. And the coulomb efficiency was 92.4%, 95.0%, 91.3%, and 83.2%, respectively. With the increase of Ti4+ content, the charge-discharge capacity of Li2Fe0.85-yMn0.15TiySiO4/C increased firstly and then decreased. When y = 0.04, a discharge specific capacity of Li2Fe0.81Mn0.15Ti0.04SiO4/C exhibits 187.8 mAh/g, which is equal to 1.13 Li+-ion de-intercalate from the crystal lattice. In addition, the coulomb efficiency reaches to 95.0%, suggesting that the incorporation of Ti4+ into the tetrahedron may make the Mn2+/Mn3+ and the MnO4 tetrahedron generated by O2− return to the original vacancy [27].
In Fig. 8, the curves show the cycle performance of the Li2Fe0.85-yMn0.15TiySiO4/C at 0.1 C. It can be seen that the Li2Fe0.85-yMn0.15TiySiO4/C synthesized by different mixing Mn2+ quantity y = 0.02, 0.04, 0.06, and 0.08 delivers a specific capacity of 168 mAh/g, 177.3 mAh/g, 159.2 mAh/g, and 138.1 mAh/g, respectively. The capacity retention ratio was 97. 8%, 98.3%, 96.0%, and 92.7%, respectively. The cycling stability of Li2Fe0.85-yMn0.15 TiySiO4/C improved firstly and then decreased with the increase of Ti4+ dosage. Therefore, the addition of appropriate amount of Ti4+ into Li2Fe0.85-yMn0.15 TiySiO4/C not only stabilizes the crystal structure, but also enhances cyclic performance of Li2Fe0.85-yMn0.15TiySiO4/C. And furthermore, the synergistic doped Ti4+ and Mn2+ improves the specific capacity of the material.
Characterization of Li2Fe0.81Mn0.15Ti0.04SiO4/C
The electrochemical performance of Li2Fe0.81Mn0.15Ti0.04SiO4/C is the most outstanding, when the Fe, Mn, and Ti mole ratio of Li2Fe1-x-yMnxTiySiO4/C is 1-x-y:x:y = 0.81:0.15:0.04. Figure 9 shows the SEM diagram of Li2Fe0.81Mn0.15Ti0.04SiO4/C. The figure shows that thickness of carbon coating layer on Li2Fe0.81Mn0.15Ti0.04SiO4 is about 15 nm, indicating Mn2+ and Ti4+ doped Li2Fe0.81Mn0.15Ti0.04SiO4/C after no change of starch of pyrolytic carbon Li2Fe0.81Mn0.15Ti0.04SiO4 coating effect.
Figure 10 shows the rate and cycling performance of Li2Fe0.81Mn0.15Ti0.04SiO4/C cathode. The long-term cycling performance of the Li2Fe0.81Mn0.15Ti0.04SiO4/C cathode has been performed at 0.5 C for 50 circles. The specific capacity is 161.8 mAh/g after 50 cycles at 0.5 C times. And the rate performance of the batteries has been tested by a gradual raising current density from 0.2 C to 2 C. A high specific capacity can be observed from the rate performance test. The Li2Fe0.81Mn0.15Ti0.04SiO4/C cathode displays specific capacity of 172.4 mAh/g, 162.8 mAh/g, 147.1 mAh/g, and 126.0 mAh/g at 0.2 C, 0.5 C, 1 C, and 2 C, respectively. Moreover, when we lowered the current density to 0.2 C and cycled for 10 times, the Li2Fe0.81Mn0.15Ti0.04SiO4/C cathode still shows a specific capacity of 172.5 mAh/g. Even when the current density went back to 0.5 C, the specific capacity could return to 161.8 mAh/g. This outstanding rate performance indicates the high electrical conductivity and the fast Li+-ion conductivity of the Li2Fe0.81Mn0.15Ti0.04SiO4/C composite. The electrical conductivity characterization of Li2Fe0.81Mn0.15Ti0.04SiO4/C exhibits a result of 6.85 × 10−4 S/cm, thus three times higher than the Li2FeSiO4 coated by starch carbon. And the long-term cycling performance of the Li2Fe0.81Mn0.15Ti0.04SiO4/C cathode has been performed at 0.5 C for 50 circles. The specific capacity is 161.8 mAh/g after 50 cycles at 0.5 C times. The improved long-term cycling performance indicates that co-doped appropriate Mn2+ and Ti4+ into Li2Fe1-x-yMnxTiySiO4/C could keep the crystal structure of the material from collapsing. Although pure Mn2+doped can enhance the specific capacity, the cycling performance of the batteries will be worse. Overall, the Mn2+ and Ti4+co-doped, which is a novel method, demonstrates a feasible way to achieve a stable structure and good cyclic performance of this kind Li2FeSiO4 material.
Figure 11 is CV curves for the Li2FeSiO4/C and Li2Fe0.81Mn0.15Ti0.04SiO4/C cathode at initial three circles. The scanning rate was 0.1 mV/s in the potential window of 1.5–4.8 V. During the negative scanning process, the reduction peaks exhibits lithium implantation behavior concentrated at about 2.4 V, with good repeatability. During the scanning circle from second and third, the peak shifts to the left and increases the area. This is the structural rearrangement to a more stable structure after the first cycle, which means the amount of lithium embedded increases. In the forward scanning process, the oxidation peak Li2FeSiO4/C, which is manifested as lithium removal behavior, has a “transition” weak peak at 3.6 V in addition to the main peak type of 3.3 V, which can be attributed to the oxidation of Fe2+ to Fe3+ [15, 33,34,35]. The Li2Fe0.81Mn0.15Ti0.04SiO4/C obtained an overlapped oxidation peak curve at about 3.3 V, thus implying the stable structure during cycling. Li2Fe0.81Mn0.15Ti0.04SiO4/C with high potential of oxidation peak is a slowly weakening trend, suggesting that Mn2+ and Ti4+ doped are conducive to oxidation reaction of Fe3+/Fe4+, Mn3+/Mn4+, and Ti3+/Ti4+ [14, 36,37,38,39]. Thus, we speculated that the Mn2+- and Ti4+-doped Li2Fe0.81Mn0.15Ti0.04SiO4/C have increased structural stability during de/intercalation.
As shown in Fig. 12, the electrochemical impedance spectra (EIS) have been conducted to illustrate the electrochemical performance of Li2FeSiO4/C and Li2Fe0.81Mn0.15Ti0.04SiO4/C cathode after cycled 50 times at 0.5 C. The charge transfer resistance Rct in the high-frequency areas of Li2FeSiO4/C and Li2Fe0.81Mn0.15Ti0.04SiO4/C cathode is 130 and 35 Ω, respectively. The slope of Li2Fe0.81Mn0.15Ti0.04SiO4/C low-frequency area is significantly greater than that of Li2FeSiO4/C. This indicated that Mn2+ and Ti4+ co-doped increased the conductivity and significantly reduced the electrochemical impedance caused by the charge transfer on the surface of the electrode. According to formula (1) and (2), Li+ diffusion rate of the Li2FeSiO4/C and Li2Fe0.81Mn0.15Ti0.04SiO4/C cathode could be calculated, [40, 41] where D is the diffusion coefficient of lithium ion, R is the gas constant (8.314 J/K mol−1), T is the room temperature in our experiment (298 K), A is the surface area of the electrode (1.13 cm2), n is the number of the electrons per molecule attending the electronic transfer reaction which is 1, F is the Faraday constant (96,500 C/mol), C is the concentration of lithium ion in electrolyte (0.039 mol/cm3 for Li2FeSiO4) [38]. RD is the ohmic resistance, RL is the charge transfer resistance, ω is the angular frequency in the low-frequency region, and σ is the Warburg factor, which can be obtained from the slopes of linear fitting of Z″ versus ω-1/2 plots in Fig. 12.
The obtained σ value of Li2FeSiO4/C and Li2Fe0.80Mn0.15Ti0.05SiO4/C is 31.5 Ω s-1/2 and 29.7 Ω s-1/2, respectively. Finally, the Li2Fe0.80Mn0.15Ti0.05SiO4/C has fast Li+ diffusion rate of 1.65 × 10−13 cm2/s and Li2FeSiO4/C of 1.47 × 10−13 cm2/s, which is consistent with the outstanding rate performance of Li2Fe0.80Mn0.15Ti0.05SiO4/C. As confirmed by the diffusion coefficient, the Li2Fe0.80Mn0.15Ti0.05SiO4/C cathode showed higher lithium ion conductivity. It could be attributed to that the co-doped Mn2+ and Ti4+ increase the cell volume and extending the Li+ diffusion path in and out of Li2Fe0.81Mn0.15Ti0.04SiO4/C particles [6, 26, 42].
To study the effect of structural stability of Li2FeSiO4/C and Li2Fe0.81Mn0.15Ti0.04SiO4/C composite electrode during cycling, SEM and AFM were used to observe the surface morphology of the electrode before and after cycling (Fig.S1 and S2). It can be seen that the electrode surface becomes smooth after charge/discharge testing, and the morphology has no obvious change. The Li2FeSiO4/C and Li2Fe0.81Mn0.15Ti0.04SiO4/C can still maintain spherical structure after cycling [43].
We also have compared our results with some recent reported heteroatom-doped Li2FeSiO4/C materials as shown in Table S1. The result demonstrates that the Li2Fe0.81Mn0.15Ti0.04SiO4/C with porous spherical structure prepared by combining the sol-gel, spray drying, and microwave synthesis method shows a higher initial discharge specific capacity and faster lithium ion diffusion rate.
Conclusions
Porous spherical Li2Fe1-x-yMnxTiySiO4/C (x = 0.05~0.20, y = 0.02~0.08) composite has been successfully prepared via a novel method of sol-gel, spray drying, and microwave synthesis for cathode material. This method carried out the bulk phase modified by co-doping Mn2+ and Ti4+ on the iron site and a thick layer carbon coating at the same time. Co-doping Ti4+ into Li2Fe0.85Mn0.15SiO4/C can make Mn2+ and O2− form tetrahedron again and return to the original vacancy to avoid the collapse of the crystal structure, and thus improves the stability of the material. In addition, Ti4+ can work with Mn2+ to increase the specific capacity of Li2Fe0.85-yMn0.15TiySiO4/C. When mole ratio of Fe and Ti in Li2Fe0.85-yMn0.15TiySiO4/C is 0.85-y:y (y = 0.04), Li2Fe0.81Mn0.15Ti0.04SiO4/C has a higher specific capacity and better cycle stability. The initial discharge specific capacity was 187.8 mAh/g, which is equal to 1.13 Li+ from Li2Fe0.81Mn0.15Ti0.04SiO4/C embedded in lattice. After cycling for 10 times at 0.2 C, the specific capacity was 172.5 mAh/g, and 161.8 mAh/g after cycled for 50 times at 0.5 C. In addition, by co-doping Mn2+ and Ti4+, the electric conductivity of Li2Fe0.81Mn0.15Ti0.04SiO4/C increased to 6.85 × 10−4 S/cm. Most importantly, co-doped appropriate Mn2+ and Ti4+ into Li2Fe1-x-yMnxTiySiO4/C could keep the stability of crystal structure from collapsing, and point out a feasible way to achieve a stable structure and good cyclic performance of Li2FeSiO4 material.
References
Dominko R, Bele M, Kokalj A, Gaberscek M, Jamnik J (2007) Li2MnSiO4 as a potential Li-battery cathode material[J]. J Power Sources 174(2):457–461
Dompablo AD, Armand M, Tarascon JM et al (2006) On-demand design of polyoxianionic cathode materials based on electronegativity correlations: an exploration of the LiMSiO4 system (M=Fe, Mn, o, Ni)[J]. Electrochem Commun 8(8):1292–1298
Qu L, Luo D, Fang S, Liu Y, Yang L, Hirano SI, Yang CC (2016) Mg-doped Li2FeSiO4/C as high-performance cathode material for lithium-ion battery[J]. J Power Sources 307:69–76
Li L, Han E, Yang P, et al (2018) Study on electrochemical performance of Mg-doped Li2FeSiO4 cathode material for Li-ion batteries[J]. Ionics 1–10
Dominko R, Bele M, Gaberscek M et al (2006) Structure and electrochemical performance of Li2MnSiO4 and Li2FeSiO4 as potential Li-battery cathode materials[J]. Electrochem Commun 8(2):217–222
Li T, Jiang X, Gao K et al (2016) Electrochemical investigations of Mn and Al co-doped Li2FeSiO4/C cathodes for Li-ion battery[J]. J Chin Chem Soc 63(9):800–807
Sivaraj P, Abhilash KP, Nalini B et al (2018) Structure, dielectric, and temperature-dependent conductivity studies of the Li2FeSiO4/C nano cathode material for lithium-ion batteries[J]. Ionics:1–16. https://doi.org/10.1007/s11581-018-2685-1
Wu S, Zhang J, Zhu Z et al (2007) Structural and electronic properties of the Li-ion battery cathode material LixCoSiO4[J]. Curr Appl Phys 7(6):611–616
Zhang C, Chen Z, Zeng Y, Zhang Z, Li J (2014) Insights into changes of lattice and electronic structure associated with electrochemistry of Li2CoSiO4 polymorphs[J]. J Phys Chem C 118(14):7351–7356
Politaev VV, Petrenko AA, Nalbandyan VB, Medvedev BS, Shvetsova ES (2007) Crystal structure, relations and electrochemical properties of monoclinic Li2MnSiO4[J]. J Solid State Chem 180(3):1045–1050
Panda MR (2014) Investigations on structural and electrical propertise of Li2NiSiO4 [J]. Int J ChemTech Res 6(6):1962–1964
Guo HJ, Li LM, Li XH et al (2009) Effects of roasting temperature and modification on properties of Li2FeSiO4/C cathode [J]. J Power Sources 189(1):45–50
Bai J, Gong Z, Lv D, Li Y, Zou H, Yang Y (2012) Nanostructured 0.8Li2FeSiO4/0.4Li2SiO3/C composite cathode material with enhanced electrochemical performance for lithium-ion batteries[J]. J Mater Chem 22(24):12128–12132
Yang J, Kang X, He D, Peng T, Hu L, Mu S (2013) Hierarchical shuttle-like Li2FeSiO4, as a highly efficient cathode material for lithium-ion batteries[J]. J Power Sources 242(35):171–178
Nytén A, Abouimrane A, Armand M, Gustafsson T, Thomas JO (2005) Electrochemical performance of Li2FeSiO4 as a new Li-battery cathode material[J]. Electrochem Commun 7(2):156–160
Zhao Y, Wu C, Li J, Guan L (2013) Long cycling life of Li2MnSiO4 lithium battery cathodes under the double protection from carbon coating and graphene network[J]. J Mater Chem A 1(12):3856–3859
Shin HC, Chung KY, Cho BW, et al (2009) Improved electrochemical properties of the Li2MSiO4 (M=Mn, Fe) by carbon coating and doping[C]//Meeting Abstracts. The Electrochemical Society, Pennington, vol 3, pp 168
Zhang S, Deng C, Fu BL, Yang SY, Ma L (2010) Effects of Cr doping on the electrochemical properties of Li2FeSiO4 cathode materials for lithium-ion batteries [J]. Electrochim Acta 55(28):8482–8489
Zhang S, Deng C, Fu BL et al (2009) Doping effects of magnesium on the electrochemical performance of Li2FeSiO4 for lithium ion batteries [J]. J Electroanal Chem 644:150–154
Lv X, Zhao X, Wu S et al (2018) Fe–Si networks and charge/discharge-induced phase transitions in Li2FeSiO4 cathode materials[J]. Phys Chem Chem Phys 20(21):14557–14563
Zhang Y, Huo QY, Du PP et al (2012) Advances in new cathode material LiFePO4, for lithium-ion batteries[J]. Synth Met 162(13–14):1315–1326
Lv DP, Wen W, Huang XK et al (2011) A novel Li2FeSiO4/C composite: synthesis, characterization and high storage capacity [J]. J Mater Chem 21:9506–9512
Huang XB, You YY, Ren YR et al (2015) Spray drying-assisted synthesis of hollow spherical Li2FeSiO4/C particles with high performance for Li-ion batteries[J]. Solid State Ionics 278:203–208
Kokalj A, Dominko R, Mali G, Meden A, Gaberscek M, Jamnik J (2007) Beyond one-electron reaction in Li cathode materials: designing Li2MnxFe1-xSiO4[J]. Chem Mater 19(15):3633–3640
Li YX, Gong ZL, Yang Y (2007) Synthesis and characterization of Li2MnSiO4/C nanocomposite cathode material for lithium ion batteries[J]. J Power Sources 174(2):528–532
Yang J, Zheng J, Kang X, Teng G, Hu L, Tan R, Wang K, Song X, Xu M, Mu S, Pan F (2016) Tuning structural stability and lithium-storage properties by d-orbital hybridization substitution in full tetrahedron Li2FeSiO4 nanocrystal[J]. Nano Energy 20:117–125
Devaraj S, Kuezma M, Ng CT, Balaya P (2013) Sol-gel derived nanostructured Li2MnSiO4/C cathode with high storage capacity[J]. Electrochim Acta 102(21):290–298
Arof AK (2008) Characteristics of LiMnO2 (M=Co, Ni, Ni0.2Co0.8, Ni0.8Co0.2) powders prepared from solution of their acetates[J]. J Alloys Compd 449(1–2):288–291
Yang J, Kang X, Lin HU et al (2013) Synthesis and electrochemical performance of Li2FeSiO4/C/carbon nanosphere composite cathode materials for lithium ion batteries[J]. J Alloys Compd 572(572):158–162
Zhong G, Li Y, Yan P, Liu Z, Xie M, Lin H (2010) Structural, electronic, and electrochemical properties of cathode materials Li2MSiO4 (M = Mn, Fe, and Co): density functional calculations[J]. J Phys Chem C 114(8):3693–3700
Nishimura S, Hayase S, Kanno R et al (2012) Structure of Li2FeSiO4[J]. J Am Chem Soc 130(40):13212–13213
Aravindan V, Karthikeyan K, Lee JW, Madhavi S, Lee YS (2011) Synthesis and improved electrochemical properties of Li2MnSiO4 cathodes [J]. J Phys D Appl Phys 44(15):152001–152004
Wu XZ, Jiang X, Huo QS, Zhang YX (2012) Facile synthesis of Li2FeSiO4/C composites with triblock copolymer P123 and their application as cathode materials for lithium ion batteries[J]. Electrochim Acta 80:50–55
Singh S, Mitra S (2014) Improved electrochemical activity of nanostructured Li2FeSiO4/MWCNTs composite cathode[J]. Electrochim Acta 123:378–386
Lv DP, Bai JY, Zhang P, Wu SQ, Li YX, Wen W, Jiang Z, Mi JX, Zhu ZZ, Yang Y (2013) Understanding the high capacity of Li2FeSiO4: in situ XRD/XANES study combined with first-principles calculations [J]. Chem Mater 25(10):2014–2020
Wang F, Wang YM, Sun DM, Wang L, Yang J, Jia HP (2014) High performance Li2MnSiO4 prepared in molten KCl-NaCl for rechargeable lithium ion batteries[J]. Electrochim Acta 119:131–137
Liu SK, Xu J, Li DZ, Hu Y, Liu X, Xie K (2013) High capacity Li2MnSiO4/C nanocomposite prepared by sol-gel method for lithium-ion batteries[J]. J Power Sources 232:258–263
Qiu H, Yue H, Wang X, Zhang T, Zhang M, Fang Z, Zhao X, Chen G, Wei Y, Wang C, Zhang D (2017) Titanium-doped Li2FeSiO4/C composite as the cathode material for lithium-ion batteries with excellent rate capability and long cycle life[J]. J Alloys Compd 725:860–868
Deng C, Zhang S, Yang SY, Fu BL, Ma L (2011) Synthesis and characterization of Li2Fe0.97M0.03SiO4 (M = Zn2+, Cu2+, Ni2+ ) cathode materials for lithium ion batteries[J]. J Power Sources 196(1):386–392
Shenouda AY, Liu HK (2008) Electrochemical behaviour of tin borophosphate negative electrodes for energy storage systems[J]. J Power Sources 185(2):1386–1391
Shenouda AY, Liu HK (2009) Studies on electrochemical behaviour of zinc-doped LiFePO4 for lithium battery positive electrode[J]. J Alloys Compd 477(1–2):498–503
Huang XB, Li X, Wang HY et al (2010) Synthesis and electrochemical performance of Li2FeSiO4/C as cathode material for lithium batteries [J]. Solid State Ionics 181(31–32):1451–1455
Yi L, Wang X, Wang G, Bai Y, Liu M, Wang X, Yu R (2016) Improved electrochemical performance of spherical Li2FeSiO4/C cathode materials via Mn doping for Lithium-ion batteries[J]. Electrochim Acta 222:1354–1364
Funding
The work was financially supported by the National Nature Science Foundation of China (grant no. 51572177), the Foundation of Sichuan Science and Technology department project (grant no. 2017JY0158), the Foundation of Sichuan science and technology project (grant no. 2017GZ0121), and the Foundation of Graduate Innovation Fund of Sichuan University of Science and Engineering (grant no. y2018048).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 1189 kb)
Rights and permissions
About this article
Cite this article
Liu, P., Gong, Y., Nie, S. et al. The porous spherical Mn and Ti co-doped Li2FeSiO4/C cathodes material for lithium-ion batteries. Ionics 25, 3611–3621 (2019). https://doi.org/10.1007/s11581-019-02953-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-02953-2