Abstract
Biosourced carboxymethyl cellulose polymer electrolytes have been studied for potential application in electrochemical devices. The carboxymethyl cellulose was obtained by reacting cellulose derived from kenaf fibre with monochloroacetic acid. Films of the biosourced polymer electrolytes were prepared by solution-casting technique using ammonium acetate salt and (1-butyl)trimethyl ammonium bis(trifluoromethylsulfonyl)imide ionic liquid as charge carrier contributor and plasticizer, respectively. The shift of peak of carboxyl stretching in the Fourier transform infrared spectra confirmed the interactions between the host biosourced polymer with the ionic liquid. Scanning electron microscopy indicated that the incorporation of ionic liquid changed the morphology of the electrolyte films. The room temperature conductivity determined using impedance spectroscopic technique for the film without ionic liquid was 6.31 × 10−4 S cm−1 while the highest conductivity of 2.18 × 10−3 S cm−1 was achieved by the film integrated with 20 wt% (1-butyl)trimethylammonium bis(trifluoromethanesulfonyl) imide. This proved that the incorporation of ionic liquid into the salted system improved the conductivity. The improvement in conductivity was due to an increase in ion mobility. The results of linear sweep voltammetry showed that the electrolyte was electrochemically stable up to 3.07 V.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In these recent years, numerous investigations on biosourced polymers such as chitin, cellulose, carrageenan and chitosan as alternatives to synthetic polymers have attracted the attention of academicians and researchers all over the world. Biosourced polymers possess advantageous properties such as eco-friendly, non-toxic, cost-effective, abundant and have high potential to replace some petroleum-based polymers [1–4]. New types of polymer electrolytes (PEs) based on these green polymers have shown potential for application in solid-state electrochemical devices such as solid-state batteries [5–10], dye-sensitized solar cells [11, 12], capacitors [13] and sensors [14]. Among these biosourced polymers, carboxymethyl cellulose (CMC) is a cellulose derivative which exhibits biodegradable and non-hazardous properties. Pure CMC shows low ionic conductivity, but with addition of proper salt and plasticizer, its ionic conductivity can be enhanced.
Generally, addition of plasticizers to polymer electrolytes is an effective technique to improve their conductivity. The essence of plasticization is to boost the conductivity of polymer electrolytes using low molecular weight and high dielectric constant additives [15, 16]. In crystalline polymer electrolytes, the plasticization is also the alternative way to reduce crystallinity and improve the amorphous phase content of the polymer electrolytes because of the high polarity and low vapour pressure of the plasticizers [17, 18]. Furthermore, according to French et al. [19] and Koksbang et al. [20], the plasticizers help in dissolving and dissociating doping salts and thereby increase the number of ions. On the other hand, plasticizers such as N,N-dimethylformamide and N,N-dimethylacetamide are considered neither eco-friendly nor safe for application in electrochemical devices [21, 22].
As alternatives and because of their eco-friendly nature [23, 24], many ionic liquids (ILs) have been used. In fact, ILs have received keen interest for application in electrochemical devices due to their unique properties, such as negligible vapour pressure, good thermal stability and non-flammability, together with high ionic conductivity and a wide window of electrochemical stability [25]. ILs also meet the requirements of plasticizing salts and are able to improve thermal and mechanical properties of polymers. Polymer electrolytes containing ILs have been reported to possess good ionic conductivities [26–28]. Khanmirzaei et al. [29] reported that the incorporation of 1-methyl-3-propylimidazolium iodide into rice starch-sodium iodide-based electrolytes enhanced the room temperature conductivity from ~10−4 S cm−1 to 1.20 × 10−3 S cm−1. So, due to high ionic conductivity, the incorporation of ILs into polymer electrolytes distinctively improves their electrochemical stability [30].
In an earlier work [3], we investigated the role of the composition of ammonium acetate, CH3COONH4 to the properties of CMC based electrolytes. The highest conductivity of ~10−4 S cm−1 was shown by CMC-20 wt% CH3COONH4. To enhance its ionic conductivity, ionic liquid, (1-butyl)trimethylammonium bis(trifluoromethylsulfonyl)imide (BMATFSI), was incorporated to the system. We have tried to incorporate CMC with other types of ionic liquids; however, the ionic liquids did not contribute or help to enhance the properties of the electrolytes. BMATFSI comprised of (1-butyl)trimethylammonium cation (BMA+) and bis(trifluoromethylsulfonyl)imide anion (TFSI−). The conductivities of the molten salts based on ammonium ion are in the region of 10−4 to 10−3 S cm−1 at ambient temperature and electrochemical stability is up to 4.5 V at room temperature [31, 32]. The addition of BMATFSI into the polymer is expected to increase the value of conductivity due to their ability to disrupt the crystalline phase in the polymer to be more amorphous [33]. The presence of the BMATFSI would also act as a lubricant in the polymer electrolytes. Therefore, based on its compatibility with our materials, BMATFSI is proposed to serve as plasticizer in the biosourced polymer network of CMC.
A few polymer electrolytes based on biosourced polymers and ionic liquids are reported in the literature. To the best of our knowledge, there has been no work on biosourced polymer electrolyte (BPE) based on CMC derived from kenaf fibre plasticized with ionic liquid ever reported in the literature, except our published work [3, 34]. Although CMC is hydrophilic, and ionic liquid is hydrophobic, they can interact and form transparent biopolymer electrolyte films. This is an indication of good repartition of BMATFSI in CMC-based polymer and portends good physical properties. However, only conductivity and dielectric studies were discussed in that paper. Thus, in this paper, we report the interactions of components as well as thermal, morphological, electrical and electrochemical properties of the BMATFSI-incorporated CMC-CH3COONH4 systems investigated using Fourier transform infrared (FTIR) spectroscopy, thermogravimetric analysis (TGA), dynamic mechanical analysis (DMA), scanning electron microscopy (SEM), electrochemical impedance spectroscopy, transference number measurement and linear sweep voltammetry (LSV), respectively.
Experimental
Materials
Kenaf fibre was commercially obtained from KFI Sdn. Bhd., Malaysia while ammonium acetate and (1-butyl)trimethylammonium bis(trifluoromethylsulfonyl)imide were purchased from Sigma-Aldrich, Germany. All materials were used without further purification.
Preparation of biosourced polymer electrolytes
CMC used in the present work was derived from kenaf fibre as reported in our previous paper [3]. The value of degree of substitution (DS) for the obtained CMC is 1.49. The DS of the synthesized CMC is higher than that of commercial CMCs. This means that the CMC has a higher number of oxygens, thus providing more active sites for coordination with the cations of the doping salt, resulting in a higher conductivity value [3]. All of the BPE films were prepared by solution-casting technique. CMC was doped with 20 wt% of CH3COONH4, which was used as doping salt and varied amount of ionic liquid, BMATFSI (10–40 wt%). Pure CMC (without ammonium salt) film was also prepared as a reference sample. For the BPE films preparation, weighted amounts of CMC, doping salt and BMATFSI were dissolved in 40 mL of aqueous acetic acid (1 %) solution at room temperature. Complete dissolution was achieved after several hours of stirring using magnetic stirrer. The final clear solution was then poured into Petri dishes and left to dry at room temperature to form highly translucent and flexible thin films. The films were kept in a desiccator for further drying. Figure 1 shows one of the prepared CMC-CH3COONH4-20 wt% BMATFSI BPE films.
Sample characterization
FTIR spectroscopy
The FTIR measurement was performed by using a Perkin Elmer Frontier FTIR spectrometer equipped with an attenuated total reflection accessory. The sample was placed on top of a diamond surface with a pressure arm applying force onto the sample and infrared light was passed through the sample. FTIR spectra were recorded in the spectral range from 4000 to 500 cm−1 at a resolution of 2 cm−1. The FTIR data were recorded in the transmittance mode. The FTIR spectroscopy was carried out to study interactions between CMC, CH3COONH4 and BMATFSI.
Thermogravimetric analysis
TGA was carried out from ambient temperature up to 600 °C at a heating rate of 10 °C min−1 by using a Setaram EVO Labsys thermal analyser in argon atmosphere. TGA was performed in order to investigate thermal properties and transition behaviour of the studied BPE system. The first run was done up to 100 °C to remove absorbed water and the second and third runs were performed up to 600 °C.
Dynamic mechanical analysis
Glass transition temperatures of CMC films were determined by using Perkin Elmer DMA 8000 instrument. The analysis was done in tension mode. The samples used were of 2 cm length and 1 cm width. The thickness of the samples was around 0.01 cm to 0.05 cm. The temperature range was from −100 °C to 100 °C and the heating rate was 1 °C min−1 while the frequency was fixed at 1 Hz. Glass transition temperatures of BPE samples were determined from the peak of the tan δ-T curves.
Electrochemical impedance spectroscopy
The electrical study was performed on a Solartron 1260 impedance/gain phase analyser with frequency ranging from 10 to 4 MHz. Each BPE sample was sandwiched between two stainless steel electrodes of diameter 2.0 cm (area = 3.142 cm2). The impedance of the samples was measured at temperatures in the range from 0 to 75 °C. The dc conductivity, σ (S cm−1) was calculated using the equation,
where, t (cm) is the thickness of polymer electrolytes measured using Mitutoyo digital micrometre, R b is the bulk resistance obtained from the intercept of high frequency semicircle or the low frequency spike on the Zr-axis of Nyquist plots, and A (cm2) is the area of electolyte-electrode contact.
Scanning electron microscopy
The cross-sectional morphology of each of the BPE film was observed using Zeiss EVO MA10 scanning electron microscope at 1000× magnification with 10 kV electron beam. BPE samples were sputter-coated with gold using coating machine for 90 s before the analysis to prevent charging.
Transference number measurement
Ionic transference number measurement was carried out using a direct current polarization technique by monitoring the polarization current as a function of time. The BPE films were sandwiched between two stainless steel blocking electrodes connected to a 1.5-V voltage source.
Linear sweep voltammetry
The electrochemical stability window of the electrolytes, the selected BPE was measured using the technique of LSV which was performed on a Wonatech ZIVE MP2 multichannel electrochemical workstation. The LSV was carried out using stainless steel electrodes at a scanning rate of 1 mVs−1 from 0 to 4 V.
Results and discussion
Fourier transform infrared spectroscopic analysis
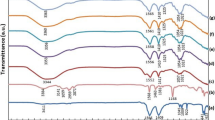
For investigating interactions between the host polymer, ionic salt and ionic liquid, FTIR spectroscopy was performed on the CMC-CH3COONH4-BMATFSI BPE films. Figure 2 shows FTIR spectra of pure CMC, CMC-20 wt% CH3COONH4 and CMC-CH3COONH4 containing 20 wt% BMATFSI in the 2000–800 cm−1 spectral range. The band at 1593 cm−1 is assigned to the stretching mode of COO− in the carboxylic group which is the main backbone in CMC [16]. Upon addition of CH3COONH4 to the CMC-salt system, this band shifted from 1593 cm−1 to 1586 cm−1. This suggests that the cation (H+) of the ammonium group [NH4 +] in CH3COONH4 interacted with the (C = O) of CMC. The weak band at 1040 cm−1 is ascribed to the characteristic of C–O stretching of polysaccharide skeleton [35, 36] and the small shifting of the band at 1058 cm−1 to lower wave numbers portends the contribution of CMC to the complexation of this ammonium groups. The bands observed at 1420 cm−1 and 1328 cm−1 are due to the symmetric vibration of –COO− and –C–O stretching, increased significantly after introducing the carboxymethyl substituents onto cellulose chain. However, the band at 1420 cm−1 might have overlapped with the bending vibration of O–H in CMC and the deformation band of NH in CH3COONH4 as reported by Kamarudin et al. [37].
The introduction of 20 wt% BMATFSI into the BPE system causes the shifting of the asymmetric stretching of COO− in the CMC-CH3COONH4 at 1586 cm−1 to a higher wavenumber (1592 cm−1). Shamsudin et al. reported a similar effect in their work where the asymmetric stretching peak of COO− in the carboxymethyl carrageenan was shifted to a higher wave number upon the inclusion of ionic liquid [38]. This shift in the wave numbers reveals that the BMATFSI has successfully complexed into the BPEs. The incorporation of BMATFSI theoretically weakens the dipole-dipole interactions in the polymer chains thus reducing the solvation of H+ by polymer matrix and induces an increase of segmental motion of the BPEs.
Thermogravimetric analysis
TGA curves of the CMC-CH3COONH4-BMATFSI BPEs are presented in Fig. 3. Two distinct stages are observed. The first weight loss is about 24.11 to 26.77% in the temperature range between 30 and 135 °C for all BPE samples. The initial weight loss is credited to the presence of entrapped moisture in the samples [39]. A similar observation was reported by Lam et al. [40]. This also can be explained by the behaviour of biosourced polymer which tends to absorb moisture from its surroundings during loading or evaporation of solvents in the BPE samples [41].
In the present study, the CMC-CH3COONH4 complex integrated with BMATFSI shows an improvement in heat resistivity and thermal stability. This proves that the ionic liquid plays an important role in improving its thermal properties [42]. Apparently, with the incorporation of BMATFSI, the decomposition temperature, T d increases while the total weight loss of BPEs film decreases. It can be observed from Fig. 3 that the BPEs sample integrated with 20 wt% of BMATFSI achieved the highest T d of 363.38 °C with total weight loss of 62.43 % (second weight loss). This weight loss is due to the loss of COO− from the polysaccharide backbone [43]. At this stable range, the weight of polymer complexes is drastically reduced as the main contributor for this weight loss is attributed to COO−. Besides that, the presence of the amorphous phase in the BPE system results in an increase in heat sensitivity. Since it is thermally stable, less monomer was detached from the complex structure of BPE and reduced the total weight loss [44]. Among the plasticized BPEs, CMC-CH3COONH4 integrated with 20 wt% BMATFSI portrays the lowest total weight loss indicating that the film is the most thermally stable electrolyte.
Dynamic mechanical analysis
Figure 4 depicts variation of the loss factor (tan δ) with temperature for the CMC BPE-based films. The curve for the pure CMC sample shows a peak at −18 °C, which corresponds to the glass transition, T g. The introduction of ammonium acetate does not change the position of the tan δ peak but broadening toward lower temperature could be detected. This peak could be attributed to the plasticizing effect of ammonium acetate molecules owning the interactions that they developed carboxylated groups of CMC, as confirmed by FTIR analyses. The tan δ peak is clearly shifted toward very low temperature (−69 °C) after the introduction of BMATFSI. This reduction of T g indicates high plasticizing effect of the ionic liquid molecules that increases the CMC chain mobility. The plasticizing effect is expected to give favourable effect on ionic mobility of the resulting polymer electrolytes [45].
Electrochemical impedance spectroscopic analysis
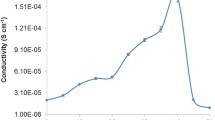
Presented in Fig. 5 is the variation of conductivity with ionic liquid content for the CMC-CH3COONH4 biosourced polymer electrolytes. The conductivity increases up to a maximum value at 20 wt% of BMATFSI and dramatically decreases at higher BMATFSI concentration. The conductivity of CMC is 7.51 × 10−5 S cm−1 while CMC-20 wt% CH3COONH4 has a conductivity value of 6.31 × 10−4 S cm−1. Addition of 10 wt% of BMATFSI increases the conductivity to 7.96 × 10−4 S cm−1. The conductivity increases further reaching a maximum value of 2.18 × 10−3 S cm−1 with 20 wt% loading of BMATFSI. This phenomenon is the result of BMATFSI plasticizing effect. The plasticizing effect weakens the dipole-dipole interactions in the polymer chains thus reduces the solvation of H+ by polymer matrix. This promotes ionic decoupling and enhances the dynamic free volume of the polymer system and thereby enhances the ionic conductivity [34, 46]. According to Norwati et al. [28], IL which also acts as a plasticizer does not only weakens the polymer-polymer chain interactions but also decreases the dipole-ion interactions in the dopant salt.
In addition, the plasticizing effect lowers the T g; therefore, it softens the polymer backbone and increases the segmental mobility. This observation is consistent with the T g-IL content behaviour discussed earlier. Another explanation for the increasing trend of conductivity with increase in BMATFSI concentration is the increase of ion mobility. This is evidenced from the dielectric studies which was reported in our earlier paper [34]. Upon addition of more than 20 wt% of BMATFSI, the ionic conductivity is drastically decreased. This drastic decrease phenomenon may be possibly due to formation of non-conducting ion pairs [46, 47]. The conductivity decrease is also caused by the creation of ion aggregates or ion multiples which reduces the number of mobile ions and impedes ion mobility in the BPEs [46, 48].
Temperature dependence of ionic conductivity study was performed to investigate the influence of thermal treatment on the biosourced CMC polymer electrolytes. Temperature dependence of conductivity for the biosourced polymer electrolyte added with different compositions of BMATFSI was studied in the temperature range of 303–348 K. This measurement can be used to analyse the mechanism of ionic conduction of the BPEs [16]. Figure 6 illustrates the dependence of ionic conductivity on temperature for the BPE films.
The linear variation of log σ with 1000/T suggests Arrhenius behaviour or thermally assisted behaviour of ionic conductivity as expressed by
where, σ 0 is the pre-exponential factor, E a is the activation energy, k b is the Boltzmann constant and T is the absolute temperature. The regression values, R 2 obtained from linear fit of Arrhenius plots are close to unity (R 2~1). The conductivity does not show any abrupt change with temperature indicating that there was no phase transition in the polymer electrolytes within the selected temperature range. According to Nik Aziz et al. [49], this relation shows that the conductivity is thermally assisted which means that the variation of temperature used affected the conductivity of the samples. It can be assumed that the nature of ion transport, in this case H+, is quite similar to that occurring in ionic crystals, which is the ions jumping into the neighbouring vacant sites. As temperature increases, the rate of jumping increases resulting in an increase of conductivity [50, 51].
E a values were calculated using Equation (2) and the results are depicted in Fig. 7. Comparing Fig. 5 and Fig. 7, it can be seen that E a for conduction decreases gradually with increase in conductivity, implying that the ions in highly conducting samples require lower energy for migration. Samples with lower E a, provide smaller band gap which allows the conducting ions to excite more easily to free ion-like state, hence increases the conductivity of the samples [52]. The E a of the CMC-CH3COONH4 added with 20 wt% BMATFSI is 0.067 eV. This E a is considered low, thus it can be concluded that proton (H+) would break and re-bind the coordination bond easily with lower energy barrier.
Morphology study
Cross-sectional SEM micrographs of pure CMC, CMC-CH3COONH4, CMC-CH3COONH4–20 wt% BMATFSI biopolymer electrolyte films at ×1000 magnification are depicted in Fig. 8. Pure CMC shows the presence of large cracks. When CH3COONH4 is complexed with CMC, the cracks start to reduce, Fig. 8(b). With the addition of BMATFSI, the cracks are further decreased indicating that the sample become smoother (Fig. 8 (c)). This might be due to the plasticizing effect of the BMATFSI.
Ionic transference number measurements
Figure 9 presents the normalized polarization current-time plot for CMC-CH3COONH4 containing 20 wt% BMATFSI BPEs. The values of ionic transference numbers, t ion and electron transference number, t e were determined using the equations as follows:
where the I t is a total initial current at the time t = 0 (ionic and electronic) and I s is the current on saturation (electronic current only) which are determined from a normalized polarization current versus time plot.
The initial total current decreases with time due to the depletion of the ionic species in the BPEs and became constant in fully depleted situation. Ionic migration occurs until steady state is achieved. At the steady state, the cell is polarized and any residual current flow is due to electron migration across the electrolyte and interfaces. This phenomenon occurs if the BPEs is primarily ionic [50, 52].
Table 1 depicts the experimental values of t i for the samples of CMC containing 20 wt% CH3COONH4 and 20 wt% CH3COONH4 with 20 wt% BMATFSI BPEs. t i for CMC-CH3COONH4 is 0.99 which indicates an almost perfect ionic conductor. This means that the charge transport in the CMC-CH3COONH4 system is predominantly due to ions and expected to be protons with only a negligible electronic contribution [53].
The addition of BMATFSI into CMC-CH3COONH4 BPE leads to a decrease in t i. This result is found to be in agreement with that reported by Sharma et al. [54], who studied plasticized (PEMA + PVC + NaIO4) system. The decrease of t i may be attributed to the formation of ion pairs and neutral ion aggregate in CMC-CH3COONH4 upon addition of BMATFSI, which dilutes the ionic concentration. The t i of the BPE becomes smaller as the ion pairs and neutral ion aggregates do not give any contribution to ionic conduction.
Electrochemical stability determination
LSV measurement was performed in order to determine the electrochemical stability window of the studied CMC based BPE film. The electrochemical stability study was done on the CMC-CH3COONH4 system incorporated with 20 wt% of BMATFSI since it is the highest ionic conducting BPE. The voltammogram of this BPE is shown in Fig. 10. The onset current of the BPE is detected at about 3.07 V. The onset current is assumed to be the biopolymer electrolyte’s breakdown voltage. This means that electrochemical stability window of the BPE is up to 3.07 V demonstrating that it is electrochemically stable to be used for fabrication of protonic batteries, since the electrochemical window of protonic battery is about ~1 V [55, 56].
Conclusions
In this work, BPEs of kenaf fibre-based carboxymethyl cellulose impregnated with CH3COONH4 and BMATFSI were prepared and characterized. BMATFSI influenced the ionic conductivity of the BPEs and the best value of 2.18 × 10−3 S cm−1 was exhibited by the system containing 20 wt% of BMATFSI. TGA analysis showed that incorporation of the IL improved the thermal stability of the BPE films. FTIR analysis confirmed the interaction between polymer host with ammonium salt and ionic liquid. The results also showed that by imparting ionic liquid into the biopolymer system, it decreased the roughness of the CMC-CH3COONH4 films’ surface which could help in enhancing contact at the electrolyte-electrode interface. Ionic transference number was found to be predominantly due to ions. Linear sweep voltammetry results showed that the electrochemical stability up to ~3.07 V indicating suitability of the BPEs for possible practical application in electrochemical devices.
References
Majid SR, Arof AK (2005) Proton-conducting polymer electrolyte films based on chitosan acetate complexed with NH4NO3 salt. Physica B 355:78–82
Mobarak NN, Ahmad A, Abdullah MP, Ramli N, Rahman MYA (2013) Conductivity enhancement via chemical modification of chitosan based green polymer electrolyte. Electrochim Acta 92:161–167
Rani MSA, Rudhziah S, Ahmad A, Mohamed NS (2014) Biopolymer electrolyte based on derivatives of cellulose from kenaf bast fiber. Polymers 6:2371–2385
Rudhziah S, Rani MSA, Ahmad A, Mohamed NS, Kaddami H (2015) Potential of blend of kappa-carrageenan and cellulose derivatives for green polymer electrolyte application. Ind Crop Prod 72:133–141
Jabbour L, Bongiovanni R, Chaussy D, Gerbaldi C, Beneventi D (2013) Cellulose-based Li-ion batteries: a review. Cellulose 20:1523–1545
Jeong SS, Böckenfeld N, Balducci A, Winter M, Passerini S (2012) Natural cellulose as binder for lithium battery electrodes. J Power Sources 199:331–335
Li J, Klöpsch R, Nowak S, Kunze M, Winter M, Passerini S (2011) Investigations on cellulose-based high voltage composite cathodes for lithium ion batteries. J Power Sources 196:7687–7691
Colò F, Bella F, Nair JR, Destro M, Gerbaldi C (2015) Cellulose-based novel hybrid polymer electrolytes for green and efficient Na-ion batteries. Electrochim Acta 174:185–190
Angulakhsmi N, Thomas S, Nair JR, Bongiovanni R, Gerbaldi C, Stephan AM (2013) Cycling profile of innovative nanochitin-incorporated poly (ethylene oxide) based electrolytes for lithium batteries. J Power Sources 228:294–299
Jabbour L, Gerbaldi C, Chaussy D, Zeno E, Bodoardo S, Beneventi D (2010) Microfibrillated cellulose–graphite nanocomposites for highly flexible paper-like Li-ion battery electrodes. J Mater Chem 20:7344–7347
Bella F, Mobarak NN, Jumaah FN, Ahmad A (2015) From seaweeds to biopolymeric electrolytes for third generation solar cells: an intriguing approach. Electrochim Acta 151:306–311
Chiappone A, Bella F, Nair JR, Meligrana G, Bongiovanni R, Gerbaldi C (2014) Structure–performance correlation of nanocellulose-based polymer electrolytes for efficient quasi-solid DSSCs. ChemElectroChem 1:1350–1358
Varzi A, Balducci A, Passerini S (2014) Natural cellulose: a green alternative binder for high voltage electrochemical double layer capacitors containing ionic liquid-based electrolytes. J Electrochem Soc 161:368–375
Yun S, Kim J (2010) Multi-walled carbon nanotubes–cellulose paper for a chemical vapor sensor. Sensors Actuat B-Chem 150:308–313
Ahmad A, Isa KBM, Osman Z (2011) Conductivity and structural studies of plasticized polyacrylonitrile (PAN)-lithium triflate polymer electrolyte films. Sains Malays 40:691–694
Ramlli MA, Chai MN, Isa MIN (2013) Influence of propylene carbonate as a plasticizer in CMC-OA based biopolymer electrolytes: conductivity and electrical study. Adv Mater Res 802:184–188
Johan MR, Fen LB (2010) Combined effect of CuO nanofillers and DBP plasticizer on ionic conductivity enhancement in the solid polymer electrolyte PEO–LiCF3SO3. Ionics 16:335–338
Cowie JMG, Martin ACS (1987) Ionic conductivity in poly (di-poly (propylene glycol) itaconate)-salt mixtures. Polymer 28:627–632
Frech R, Chintapalli S (1996) Effect of propylene carbonate as a plasticizer in high molecular weight PEO-LiCF3SO3 electrolytes. Solid State Ionics 85:61–66
Koksbang R, Olsen II, Shackle D (1994) Review of hybrid polymer electrolytes and rechargeable lithium batteries. Solid State Ionics 69:320–335
Egashira M, Todo H, Yoshimoto N, Morita M (2008) Lithium ion conduction in ionic liquid-based gel polymer electrolyte. J Power Sources 178:729–735
Lewandowski A, Świderska A (2003) Electrochemical capacitors with polymer electrolytes based on ionic liquids. Solid State Ionics 161:243–249
Zhao D, Liao Y, Zhang Z (2007) Toxicity of ionic liquids. Clean–soil, air, water 35:42–48
Sowmiah S, Srinivasadesikan V, Tseng MC, Chu YH (2009) On the chemical stabilities of ionic liquids. Molecules 14:3780–3813
Ye YS, Rick J, Hwang BJ (2013) Ionic liquid polymer electrolytes. J Mater Chem A 1:2719–2743
Cheng H, Zhu C, Huang B, Lu M, Yang Y (2007) Synthesis and electrochemical characterization of PEO-based polymer electrolytes with room temperature ionic liquids. Electrochim Acta 52:5789–5794
Ohno H, Yoshizawa M, Ogihara W (2004) Development of new class of ion conductive polymers based on ionic liquids. Electrochim Acta 50:255–261
Anuar NK, Subban RHY, Mohamed NS (2012) Properties of PEMA-NH4CF3SO3 added to BMATSFI ionic liquid. Materials 5:2609–2620
Khanmirzaei MH, Ramesh S, Ramesh K (2015) Polymer electrolyte based dye-sensitized solar cell with rice starch and 1-methyl-3-propylimidazolium iodide ionic liquid. Mater Design 85:833–837
Noda AWatanabe M (2000) Highly conductive polymer electrolytes prepared by in situ polymerization of vinyl monomers in room temperature molten salts. Electrochim Acta 45:1265–1270
Sun J, MacFarlane DR, Forsyth M (1997) Synthesis and properties of ambient temperature molten salts based on the quaternary ammonium ion. Ionics 3:356–362
Sun J, Forsyth M, MacFarlane DR (1998) Room-temperature molten salts based on the quaternary ammonium ion. J Phys Chem B 102:8858–8864
Huang H, He P, Hu N, Zeng Y (2003) Electrochemical and electrocatalytic properties of myoglobin and hemoglobin incorporated in carboxymethyl cellulose films. Bioelectrochemistry 61:29–38
Rani MSA, Dzulkurnain NA, Ahmad A, Mohamed NS (2015) Conductivity and dielectric behavior studies of carboxymethyl cellulose from kenaf bast fiber incorporated with ammonium acetate-BMATFSI biopolymer electrolytes. Int J Polym Anal Ch 20:250–260
Lii CY, Tomasik P, Zaleska H, Liaw SC, Lai VMF (2002) Carboxymethyl cellulose–gelatin complexes. Carbohyd Polym 50:19–26
Taleb MFA, El-Mohdy HA, El-Rehim HA (2009) Radiation preparation of PVA/CMC copolymers and their application in removal of dyes. J Hazard Mater 168:68–75
Kamarudin K, Isa MIN (2013) Structural and DC ionic conductivity studies of carboxy methylcellulose doped with ammonium nitrate as solid polymer electrolytes. Int J Phys Sci 8:1581–1587
Shamsudin IJ, Ahmad A, Hassan NH, Kaddami H (2015) Biopolymer electrolytes based on carboxymethyl ҡ-carrageenan and imidazolium ionic liquid. Ionics. doi:10.1007/s11581-015-1598-5
Ramesh S, Liew CW, Morris E, Durairaj R (2010) Effect of PVC on ionic conductivity, crystallographic structural, morphological and thermal characterizations in PMMA–PVC blend-based polymer electrolytes. Thermochim Acta 511:140–146
Lam E, Leung AC, Liu Y, Majid E, Hrapovic S, Male KB, Luong JH (2012) Green strategy guided by Raman spectroscopy for the synthesis of ammonium carboxylated nanocrystalline cellulose and the recovery of byproducts. ACS Sustain Chem Eng 1:278–283
Stephan AM, Saito Y, Muniyandi N, Renganathan NG, Kalyanasundaram S, Elizabeth RN (2002) Preparation and characterization of PVC/PMMA blend polymer electrolytes complexed with LiN(CF3SO2)2. Solid State Ionics 148:467–473
Baranyai KJ, Deacon GB, MacFarlane DR, Pringle JM, Scott JL (2004) Thermal degradation of ionic liquids at elevated temperatures. Aust J Chem 57:145–147
Biswal DR, Singh RP (2004) Characterisation of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohyd Polym 57:379–387
Samsudin AS, Lai HM, Isa MIN (2014) Biopolymer materials based carboxymethyl cellulose as a proton conducting biopolymer electrolyte for application in rechargeable proton battery. Electrochim Acta 129:1–13
Ghiya VP, Dave V, Gross RA, Mccarthy SP (1996) Biodegradability of cellulose acetate plasticized with citrate esters. J Macromol Sci A 33:627–638
Woo HJ, Majid SR, Arof AK (2011) Conduction and thermal properties of a proton conducting polymer electrolyte based on poly (ε-caprolactone). Solid State Ionics 199:14–20
Rani MSA, Mohamed NS, Isa MIN (2015) Investigation of the ionic conduction mechanism in carboxymethyl cellulose/chitosan biopolymer blend electrolyte impregnated with ammonium nitrate. Int J Polym Anal Ch 20:491–503
Yahya MZA, Arof AK (2003) Effect of oleic acid plasticizer on chitosan–lithium acetate solid polymer electrolytes. Eur Polym J 39:897–902
Aziz NAN, Idris NK, Isa MIN (2010) Proton conducting polymer electrolytes of methylcellulose doped ammonium fluoride: conductivity and ionic transport studies. J Phys Sci 5:748–752
Ramesh S, Yahaya AH, Arof AK (2002) Dielectric behaviour of PVC-based polymer electrolytes. Solid State Ionics 152:291–294
Souquet JL, Levy M, Duclot M (1994) A single microscopic approach for ionic transport in glassy and polymer electrolytes. Solid State Ionics 70:337–345
Khiar ASA, Arof AK (2010) Conductivity studies of starch-based polymer electrolytes. Ionics 16:123–129
Linford RG (1988) Experimental techniques for studying polymer electrolytes. Solid state ionics devices. World Scientific, Singapore. 551–571
Ramamohan K, Sharma AK (2013) Effect of plasticizer on (PVC + PEMA + NaIO4) solid polymer blend electrolyte system for battery characterization studies. Adv in Polym Sci Technol 3:49–53
Pratap R, Singh B, Chandra S (2006) Polymeric rechargeable solid-state proton battery. J Power Sources 161:702–706
Ng LS, Mohamad AA (2008) Effect of temperature on the performance of proton batteries based on chitosan–NH4NO3–EC membrane. J Membrane Sci 325:653–657
Acknowledgments
M.S.A Rani would like to thank the Malaysian Ministry of Higher Education for awarding him MyBrain15 scholarship. Financial support from the University of Malaya (research grants RG255-13AFR and PG092-2014A) is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rani, M.S.A., Hassan, N.H., Ahmad, A. et al. Investigation of biosourced carboxymethyl cellulose-ionic liquid polymer electrolytes for potential application in electrochemical devices. Ionics 22, 1855–1864 (2016). https://doi.org/10.1007/s11581-016-1728-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1728-8