Abstract
Composite solid electrolytes in the system (1 − x)Li2CO3–xAl2O3, with x = 0.0–0.5 (mole), were synthesized by a sol–gel method. The synthesis carried out at low temperature resulted in voluminous and fluffy products. The obtained materials were characterized by X-ray diffraction, differential scanning calorimetry, scanning electron microscopy/energy-dispersive X-ray, Fourier transform infrared spectroscopy and AC impedance spectroscopy. Structural analysis of the samples showed an amorphous feature of Li2CO3 and traces of α-LiAlO2, γ-LiAlO2 and LiAl5O8. The prepared composite samples possess high ionic conductivities at 130–180 °C on account of the presence of lithium aluminates as well as the formation of a high concentration of an amorphous phase of Li2CO3 via this sol–gel preparative technique.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Composite materials are formed by mixing two or more compounds with different chemical and physical properties. Generally, the composite materials are composed of a reinforcement-dispersed phase embedded in a matrix phase. The matrix or the less hard phase containing the reinforcement phase will form the desired shape. The dispersed phase is usually stronger than the matrix. This improves the overall mechanical properties of the matrix. Thus, the final product of this process results in stronger composite compounds than the single material. Composite materials in regard to solid electrolytes have drawn a great deal of interest for over 35 years. The enhancement of ionic conductivity in a composite material by a factor of almost 50 has triggered the development of such material [1]. Composite solid electrolytes made up from ionic salt doped with oxides constitute a class of ionic conductors with high ionic conductivity [2]. Conductivity enhancement was due to an increase in free cations in the samples corresponding to the appearance of amorphous phases of ionic salts at the interface between the host matrix and dispersoid [2–4].
Composites based on lithium salts such as LiI–Al2O3 [5], Li2SO4–Al2O3 [6], LiNO3–Al2O3 [7], LiClO4–SiO2 [8] and LiClO4–Al2O3 [9] have been reported to have high ionic conductivities and may be used as solid electrolytes in lithium batteries. In this work, alumina (Al2O3) particles reinforced Li2CO3 of an ionic salt matrix to give composite materials. This improves not only the ionic conductivity of the matrix but the hardness and strength of the materials. The aims of this work were to investigate the properties of the composites prepared via a sol–gel technique and to study their ionic conductivities at low and high temperatures.
In this work, composite solid electrolytes in the system (1 − x)Li2CO3–xAl2O3, with x = 0.0–0.5 (mole), were prepared by the sol–gel method at a lower temperature compared to the conventional mechanical milling. The studies include investigation on the morphology, chemical content and thermal effects on the ionic conductivity of the prepared composites. Composite ionic conductors are very promising materials because their transport characteristics and mechanical properties can be finely adjusted by variation of the chemical nature, microstructure and concentration additives [9].
Experimental
Composite powders with an Al2O3 composition (mole) of x = 0.1–0.5 were prepared by the sol–gel method. The samples prepared are described by the general formula of (1 − x)Li2CO3–xAl2O3. Li2CO3 (high purity grade) and Al2O3 (high purity grade, average grain size ∼10 μm) were used as the starting materials. The required amounts of lithium carbonate and alumina were mixed thoroughly in deionized water under magnetic stirring at room temperature. An equivalent mass of citric acid to the mass of alumina was added to the solution and heated at 60 °C with continuous stirring. After 20 min, the solution was left to stand at room temperature for 4 weeks. In the next step, the mixture was gradually dried at 80 °C in an oven until a white gel was formed. The wet gel was further heated at higher temperature (80–220 °C) until it was totally dried. Most of the moisture in the gel was evaporated at this temperature. The final product obtained was a voluminous, fluffy and white powder. Structural characterizations for X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy/energy-dispersive X-ray (SEM/EDX) were performed on a D8 Advanced-Bruker X-ray Diffractometer with Cu Kα radiation, a Perkin Elmer RX1 spectrometer and INCA Energy 200 (Oxford Ins.), respectively. The thermal properties of the samples were measured on a Mettler Toledo DSC 822 with continuous heating at a rate of 10 °C min−1. For conductivity studies, pellets were made by pressing the samples at a pressure of 6–8 tonnes/cm2 with a diameter of 13 mm and a thickness of 1.1 mm. The ionic conductance measurements were performed on a Solartron 1260 impedance analyser with an AC amplitude of 100 mV in the frequency range of 0.1 to 107 Hz.
Results and discussion
XRD analysis
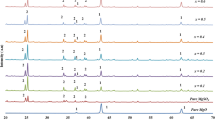
Figure 1 shows the X-ray diffraction spectra of pure Li2CO3 and doped with Al2O3. Pure Li2CO3 shows peaks at 2θ of 21°, 23.8°, 29.7°, 30.5°, 31.6°, 34°, 36°, 37°, 39.6° and 48.5°. Li2CO3 has a monoclinic crystal system with the space group C2/c. The monoclinic unit cell has parameters of a = 8.35900 Å, b = 4.97670 Å, c = 6.19400 Å and β = 114.72°. As can be seen from the spectra, all the composite samples gave the same diffraction pattern of Li2CO3. This similarity attested that the Li2CO3 phase was present in all the composite samples. However, upon doping with Al2O3 particles, some of the Li2CO3 diffraction peaks were found missing. The absence of the peaks (at 2θ = 23.7° and 48.5°) as well as the peak broadening were attributed to the formation of an amorphous phase of Li2CO3 in all the samples. The formation of the amorphous phase of Li2CO3 was initiated by chemical and physical contacts of both Li2CO3 and Al2O3 phases. This conclusion fully agreed with Neiman’s observations [10]. Therefore, Li2CO3 coexists as crystalline and amorphous states in the composite samples with x = 0.1–0.3. Consequently, at high Al2O3 content, the Li2CO3 salt was mainly in the amorphous state as shown by the composite sample with x = 0.5.
In this method, traces of crystalline α-LiAlO2, γ-LiAlO2 and LiAl5O8 (lithium aluminates) were also formed as detected in the X-ray diffraction spectra [11]. The crystallinity of these aluminates appeared to be low since the peak intensities of X-ray diffraction patterns corresponding to the lithium aluminates were small and their widths were also wide. However, the maximum peak intensity of α-LiAlO2 was observed at 2θ = 20.0° for composite samples with x = 0.1–0.3. The pathway of the formation of lithium aluminates can be represented by the following route [12]:
DSC analysis
DSC curves of pure Li2CO3 and prepared composite samples are shown in Fig. 2. The pure Li2CO3 showed an endothermic peak at 420 °C which corresponds to a solid–solid phase transition of the compound [13]. The phase transition temperature decreased with alumina content. This was due to the formation of the amorphous phase of Li2CO3 as indicated by XRD results. This effect was observed at ∼360 °C in the composite samples with x = 0.1–0.4. An exothermic peak due to crystallization of α-LiAlO2 was observed at ∼260 °C, while the endothermic peak at ∼285 °C was attributed to the decomposition of α-LiAlO2 and citric acid in the composite samples [14].
FTIR analysis
FTIR spectra in the wave number range 950–450 cm−1 for composite samples with x = 0.1 and x = 0.2 of mole fraction are presented in Fig. 3. The absorption bands in this range were found to be owed to the Al–O stretching vibrations [15]. These bands were assigned to the presence of the lithium aluminates (α-LiAlO2, γ-LiAlO2 and LiAl5O8) as indicated by the XRD results.
SEM/EDX analysis
Figure 4 shows SEM micrographs of pure Li2CO3 and the composite sample with x = 0.4. Different top surface morphologies can be observed for the composite sample with x = 0.4 after the sol–gel process. Prior treatment with alumina, the crystal structure of pure Li2CO3, was totally affected. The synthesis of composite solid electrolytes in the system (1 − x)Li2CO3–xAl2O3 led to the formation of the amorphous phase region of Li2CO3 in the sample with x = 0.4. This was further confirmed by the XRD results. Some spherical particles were also observed on the surface of the sample. These particles were attributed to the crystal phase of lithium aluminates due to a chemical reaction, as indicated by XRD and FTIR results. Figure 5 shows the spectrum of EDX point analysis (region selected with a square box) to confirm the chemical composition of the composite sample with x = 0.4. The presence of C, O and Al elements could be attributed to Li2CO3 and Al2O3 which is consistent with the XRD results.
At low content of alumina, the crystalline and amorphous states of Li2CO3 were found to be coexisting. The SEM micrograph in Fig. 6 exhibits the crystalline region (spectrum 1) and amorphous region (spectrum 1) for the composite sample with x = 0.1. The EDX point analysis of spectra 1 and 2 showed high composition of C and O elements. The observation was in agreement with XRD results for the sample, and this could be attributed to the Li2CO3 phase. EDX analysis of spectrum 3 showed the presence of alumina particles in the sample.
Ionic conductivity
Complex impedance plots of composite samples with x = 0.1–0.5 and x = 0.3 are shown in Figs. 7 and 8, respectively. The absence of a semicircle portion at x = 0.1–0.5 of mole fraction implied that only resistive components prevailed in the materials. For the composite sample with x = 0.3, the plots showed semicircles at higher frequencies followed by a spike at a low-frequency region. The semicircles with the interception at the Z′-axis were assigned to bulk resistance (R b). The formation of spike represented interfacial effects between the electrode and electrolyte [16]. Bulk conductivities (σ b) of composite samples have been calculated using the relationship as shown below:
where d is the sample thickness (cm), R b is the bulk resistance (Ω), and A is the area (cm2) of the sample. Figure 9 illustrates the behaviour of conductivity with the composition of the system at different temperatures. The conductivity goes through two maxima, which takes place for composite samples with x = 0.2 and 0.4–0.5 with the values in the order of ∼10−3 S cm−1 (130–180 °C). The conductivity enhancement at x = 0.2 is due to the formation a new crystalline phase of lithium aluminates as confirmed by the XRD results. Consequently, in the second point at x = 0.4–0.5, the enhanced conductivity is attributable to the high concentration of the amorphous phase of Li2CO3 at high temperature. This phenomenon is in agreement with the literature where at sufficiently high oxide dispersity and concentration, the composite sintering is accompanied by dispersion of the ionic salt as a result of the solid-phase spreading of the ionic component over the oxide surface. A high-temperature surface-disordered state occurred in the ionic salt. As a result, adsorption of lithium cations to the oxide surface will take place, thus increasing the ionic conductivity [3]. The drop in conductivity may possibly be explained by the high concentration of alumina (dispersoid) grains in the composites. Blocking of conduction pathways by the alumina grains was observed by the fall of the ionic conductivity [17]. Higher ionic conductivity values in the order 10−3 S cm−1 at 130–180 °C are obtained compared to the conductivity of 10−8 S cm−1 at 150 °C for the same composite system prepared using the conventional solid-state sintering as reported by Bhoga and Singh [18]. This strongly demonstrates that the composite samples prepared using the sol–gel method in this work are able to produce materials with a much higher ionic conductivity.
Temperature dependencies of conductivity for the (1 − x)Li2CO3–xAl2O3 composites prepared are shown in Fig. 10. Conductivity seems to increase as the temperature increases. The Arrhenius plot shows a constant conductivity plateau beyond 140 °C in the system. This is due to the presence of the metastable amorphous phase formed at Li2CO3–Al2O3 interfaces as a result of chemical and physical interactions [7, 19–21]. The activation energy was obtained by fitting the ionic conductivity data with the Arrhenius equation:
where A is the pre-exponential factor, E a is the activation energy for conduction, k is the Boltzmann constant which is 8.52 × 10−5 eV/K and T is the temperature in Kelvin. The activation energy at 40–90 °C is shown in Fig. 10. The high value of activation energy at low temperatures indicates low mobility of ions in the samples.
Conclusion
Composite solid electrolytes in the system (1 − x)Li2CO3–xAl2O3 were successfully synthesized by a sol–gel method. The composite samples obtained were white, voluminous, fluffy and homogeneous. The water-based sol–gel method employed had produced composites with an amorphous feature of Li2CO3 and traces of α-LiAlO2, γ-LiAlO2 and LiAl5O8. The prepared composite samples possess high ionic conductivities at 130–180 °C on account of the presence of lithium aluminates as well as the formation of a high concentration of an amorphous phase of Li2CO3 via this sol–gel preparative technique.
References
Liang CC (1973) Conduction characteristics of the lithium iodide – aluminium oxide solid electrolytes. J Electrochem Soc 120:1289
Uvarov NF, Ponomareva VG, Lavrova GV (2010) Composite solid electrolytes. Russ J Electrochem 46:722
Uvarov NF (2011) Composite solid electrolytes: recent advances and design strategies. J Solid State Electrochem 15:367
Yaroslavtsev AB (2009) Composite materials with ionic conductivity: from inorganic composites to hybrid membranes. Russ Chem Rev 78:1013
Poulsen FW, Andersen NH, Kindl B, Schoonman J (1983) Properties of LiI-Alumina composite electrolytes. Solid State Ionics 9–10:119
Uvarov NF, Bokhonov BB, Isupov VP, Hairetdinov EF (1994) Nanocomposite ionic conductors in the Li2SO4 – Al2O3 system. Solid State Ionics 74:15
Uvarov NF, Hairetdinov EF, Skobelev IV (1996) Composite solid electrolytes MeNO3 – Al2O3 (Me = Li, Na, K). Solid State Ionics 86–88:577
Vinod MP, Bahnemann D (2002) Materials for all-solid-state thin-film rechargeable lithium batteries by sol-gel processing. J Solid State Electrochem 6:498
Ulihin AS, Uvarov NF, Mateyshina YG, Brezhneva LI, Matvienko AA (2006) Composite solid electrolytes LiClO4 – Al2O3. Solid State Ionics 177:2787
Neiman AY, Uvarov NF, Pestereva NN (2007) Solid state surface and interface spreading: An experimental study. Solid State Ionics 177:3361
Khomane RB, Agrawal A, Kulkarni BD (2007) Synthesis and characterization of lithium aluminate nanoparticles. Mater Lett 61:4540
Kwon SW, Park SB (1997) Effect of Precursors on the Preparation of Lithium Aluminates. J Nucl Mater 246:131
Saito K, Uchida K, Tezuka M (1992) Lithium carbonate as a solid electrolyte. Solid State Ionics 53–56:791
Valenzuela MA, Téllez L, Bosch P, Balmori H (2001) Solvent effect on the sol–gel synthesis of lithium aluminate. Mater Lett 47:252
Chatterjee M, Naskar MK (2003) Novel technique for the synthesis of lithium aluminate (LiAlO2) powders from water-based sols. J Mater Sci Lett 22:1747
Sultana S, Rafiuddin (2011) Behaviour of electrical conductivity in CsI–Al2O3 and CsI–TiO2 systems. Arab J Chem. doi:10.1016/j.arabjc.2011.02.025
Uvarov NF, Hairetdinov EF, Bokhonov BB, Bratel NB (1996) High ionic conductivity and unusual thermodynamic properties of silver iodide in AgI – Al2O3 nanocomposites. Solid State Ionics 86–88:573
Bhoga SS, Singh K (1998) Li2CO3–ABO3 (A = Li, K, Ba and B = Nb, Ti) composite solid electrolyte systems. Solid State Ionics 111:85
Lavrova GV, Ponomareva VG, Uvarov NF (2000) Nanocomposite ionic conductors in the system MeNO3–SiO2 (Me = Rb, Cs). Solid State Ionics 136:1285
Uvarov NF, Brezhneva LI, Hairetdinov EF (2000) Effect of nanocrystalline alumina on ionic conductivity and phase transition in CsCl. Solid State Ionics 136:1273
Tadanaga K, Imai K, Tatsumisago M, Minami T (2000) Preparation of AgI–Al2O3 Composites with High Ionic Conductivity Using Al2O3 Aerogel and Xerogel. J Electrochem Soc 147:4061
Acknowledgments
The authors would like to thank the University of Malaya for granting the research grant (RG021/09AFR) to support this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sulaiman, M., Rahman, A.A. & Mohamed, N.S. Sol–gel synthesis and characterization of Li2CO3–Al2O3 composite solid electrolytes. Ionics 22, 327–332 (2016). https://doi.org/10.1007/s11581-015-1548-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1548-2