Abstract
We have tailored the extraction solvent of natural anthocyanin pigments from Ixora coccinea flower to lower charge transport resistance and enhance effective electron diffusion coefficient as well as absorption properties in DSSC sensitization. Six different extraction solvents were used in this study, namely, absolute ethanol, 70 % ethanol, acetone, dimethyl formamide, dimethyl sulfoxide, and distilled water. Dimethyl formamide was found to be able to extract the most anthocyanins from the Ixora flower, followed by dimethyl sulfoxide, acetone, absolute ethanol, 70 % ethanol, and distilled water, respectively. Dye-sensitized solar cells were fabricated using these extracted dyes as sensitizers and the cell performances were measured and characterized. The dye-sensitized solar cell equipped with a dye sensitizer extracted in 70 % ethanol achieved the highest conversion efficiency (ɳ = 0.50 ± 0.04 %) owing to its highest electron diffusion coefficient and lowest transport resistance in the TiO2/dye/electrolyte interface. This was followed by the cell equipped a with dye sensitizer extracted in acetone (ɳ = 0.30 ± 0.04 %), absolute ethanol (ɳ = 0.27 ± 0.01 %), dimethyl formamide (ɳ = 0.17 ± 0.01 %), dimethyl sulfoxide (ɳ = 0.16 ± 0.01 %), and distilled water (ɳ = 0.09 ± 0.003 %) in conversion efficiency performances, respectively. This study provides an insight into the importance of choosing the right medium for both dye extraction as well as the favorable solvent medium for dye adsorption onto TiO2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The demand for energy increases with increasing world population. Current sources of energy that come from fossil fuels release hazardous and greenhouse gases to the atmosphere and, hence, pose a negative impact on climate, the environment as well as human health [1, 2]. Calculated solar energy that falls onto the Earth surface is at a rate of 120 PW (1 PW is equivalent to 1015 W). If this energy that comes from the Sun could be harnessed effectively, the world energy demand could be satisfied [3]. The benefits that come when harvesting light from the Sun would provide ample energy, thus making solar energy to be an important renewable energy resource [4].
Dye-sensitized solar cell (DSSC) has come up as a part of preferable photovoltaic devices that provide a solution toward harvesting of solar energy and convert it to electricity [5, 6]. This is due to its potential low cost, simple to fabricate, and use of nontoxic, recyclable materials [7–9]. However, its potentially high photovoltaic conversion efficiency still remains debatable and scientifically challenging to be opted practically [10–15]. A DSSC is comprised of a wide bandgap semiconductor thinly layered on a photoelectrode, electrolyte, and a counter electrode as well as dye sensitizers which are responsible for harvesting light and as a whole transforming solar energy into electrical energy [10]. The mechanisms of this photovoltaic device are based on the absorption of light by the adsorbed dye on the TiO2 surface, effective electron excitation from the dye which are transferred to the conduction band of the TiO2 passing through the electrode and out to the external circuit [16, 17].
Dye sensitizer remains one of the key elements in DSSC. High conversion efficiency has been achieved when DSSC is fabricated with ruthenium complex dye [18]. But the presence of heavy metals as well as their rarity, complicated synthesis, and expensive nature has inspired more studies to find alternative sensitizers from nature which do not pose threats to health and the environment [19–21]. Natural dyes which are utilized as dye sensitizers can easily be extracted from fruits, vegetables, and flowers with simple and direct chemical procedures at room temperature [22–24]. However, natural dye sensitizers have been reported to perform poorly in DSSC, and to date, the overall conversion efficiencies produced have been below 2 % [22, 25]. This low efficiency is due to the weak binding energy between dye molecules with the TiO2 film, low charge transfer with limited light absorption in the visible range, the structure of the dye molecules in relation to its stability, and the nature of the anchoring group itself [25–27].
Anthocyanins as dye sensitizers are able to bind with TiO2 which are mainly due to the presence of carboxyl and hydroxyl groups. This binding onto the surface of the TiO2 film will then facilitate electron transfer from anthocyanin molecules to the conduction band of TiO2 [5, 11]. Having said that, the sensitizing performance of anthocyanins differs from one another depending on the sources from where the anthocyanins are being extracted [10].
Solvents used to extract these natural dyes must be carefully chosen to achieve high effectiveness in the extraction process [28, 29]. This is because the solubility of different dyes varies depending on the polarity of the extraction solvents, hence affecting directly the solubility and anchoring behavior of the extracted dyes onto TiO2, which then affect cell overall efficiency [29, 30]. Polar aprotic solvents and polar protic solvents are two polar solvents commonly used to extract anthocyanins, and the polarity of these solvents can influence in terms of diffusion of dyes onto the TiO2 surface, and hence, careful selection of solvent for extraction is important [31].
The performance of DSSC using dyes extracted in different solvents has been studied, and the reported results show that the performance does depend on the solvent properties such as polarity, acidity, dye combination, and temperature [21, 31]. However, such studies focused on a few extraction solvents. For example, DSSC fabricated with curcumin dye in acetone, dimethylformamide, dimethyl sulfoxide, and ethanol yielded cell conversion efficiencies of 0.63, 0.44, 0.38, and 0.31 %, respectively [31]. Dyes extracted from Lawsonia inermis (henna) extracted in ethanol and a mixture of ethanol and water (4:1) showed conversion efficiencies of 0.66 and 0.52 %, respectively [25], whereas the dye extract of Melastoma fruit in deionized water and ethanol generated conversion efficiencies of 1.37 and 0.72 %, respectively [29].
This paper discusses a study on the effect of six different extraction solvents potentially used in natural dye extraction. The objectives of the study are to investigate the effect of extraction solvent to overall cell conversion efficiency and, hence, identify a suitable extraction solvent for DSSC application. Analyses include absorption properties of the dye extracts which were analyzed using a UV-Vis spectrophotometer, incident photon-to-current efficiency, calculation of anthocyanin dye concentrations using pH differential method, determination of aggregation of dye particles in the dye extracts, current-to-voltage characterization, and electrochemical impedance spectroscopy measurement of working DSSCs.
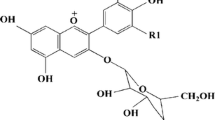
In this study, the natural anthocyanin dye sensitizer was extracted from Ixora coccinea flower which is abundant in Brunei Darussalam. It is a shrub with a height commonly growing up to about 4–6 ft. This flower has several cultivars which come in different shapes, sizes, and colors. The flower often appeared in clusters at the end of branches, with four petals. A study has reported that the major anthocyanin present in Ixora is pelargonidin-3-glucoside (Fig. 1) [32].
Materials and method
Extraction, characterizations, and preparation of natural dye sensitizer
The natural anthocyanin dyes were extracted in six different solvents: distilled water, absolute ethanol, 70 % ethanol, dimethyl formamide (DMF), dimethyl sulfoxide (DMSO), and acetone. Fresh petals (10 g) of I. coccinea were crushed using a pestle and mortar in the respective solvents and left overnight (refrigerated at 3 °C), to make 50 mL of the dye extract. The extract was then stirred for 2 h and then filtered to remove the solid residue which was then purified using petroleum ether. To further remove any remaining small solid residues, the resultant extract was centrifuged at 4500 rpm for 15 min and the extract (50 mL) was stored and refrigerated in an enclosed bottle.
The dye extracts were then characterized for their light absorption properties using the UV-Vis spectrophotometer. The initial concentration for anthocyanin presence in each extract was determined with the pH differential method, where all dye extract concentrations were then adjusted and leveled equally by dilutions. In determining the degree of possible aggregations of dye molecules in the extract, sizes of aggregated dye molecules were analyzed using Zetasizer (Nano MAN 0317) after shaking the extract using a vortex mixer for 30 s.
Fabrication of photoelectrode
Photoelectrodes were fabricated using a TiO2 paste Solaronix (nanoxide-T, colloidal anatase particles size ~13 nm, ~120 m2 g−1 (BET), Switzerland). The TiO2 was coated on precleaned fluorine-doped conducting tin oxide (FTO) glasses (Nippon sheet glass ~7 Ω sq−1) by the doctor blade method. Electrodes were preheated (~50 °C) using a hair dryer and sintered at 450 °C for 30 min. The thickness of the TiO2 electrodes used for this investigation was ~9 μm (scanning electron microscope, SEM) [33].
Dye-synthesized solar cell preparation, incident photon-to-current efficiency, and current-voltage measurements
The prepared photoelectrodes were dipped in the dye extracts overnight allowing adsorption of dye molecules onto the TiO2 surface. Overnight dipping is necessary to allow dye molecules to attach onto the TiO2 surface efficiently. The dyed electrode was then rinsed with respective solvents, which was then assembled after introducing an electrolyte containing tetrabutylammonium iodide (TBAI, 0.5 M)/I2 (0.05 M), in a mixture of acetonitrile and ethylene carbonate (6:4, v/v) in between the dyed electrode and the platinum counter electrode. The assembled cell was then exposed to irradiation of 100 mW cm−2 for 4 h prior to the current-voltage measurement, allowing optimum incorporation of the electrolyte into the TiO2 layer [33]. Measurement on incident photon-to-current efficiency was carried out using PVE 300 system (BENTHAM: photovoltaic characterization), with xenon arc lamp and quartz halogen bulb as the light source, which cuts at 700 nm. The photoelectric current-voltage (I-V) measurement was carried out by placing the assembled cell under solar simulator (model: DYESOL LP-156B).
Electrochemical impedance measurement
The electron kinetics of the cell was investigated using electrochemical impedance spectroscopy. This measurement was carried out using a computer-controlled electrochemical interface (SI 1287, Solatron) and impedance/gain-phase analyzer (SI 1260, Solatron). The frequency range and the amplitude of the alternative voltage used were from 0.01 to 106 Hz and 10 mV, respectively. The impedance measurements were performed at open circuit condition under irradiation of 100 mW cm−2. Impedance parameters and equivalent circuits were then obtained by fitting the spectra with ZView software (v3.3, Scribner Associate Inc.).
Results and discussion
Absorption spectra and aggregation behavior of the dye
The absorption of light was analyzed using the UV-Vis spectrophotometer in the wavelength range from 300 to 800 nm and is shown in Fig. 2. Figure 2a shows the absorption spectrum of the dye extracted in 70 % ethanol, absolute ethanol, acetone, DMF, DMSO, and distilled water. Figure 2b illustrates the absorbance spectra of the dye adsorbed onto the TiO2 layer. The dye extracted in distilled water exhibits the highest absorbance followed by 70 % ethanol, absolute ethanol, acetone, DMF, and DMSO in pure dye solution. As shown in Fig. 2b, the absorbance was broader when the dye was adsorbed onto the TiO2 layer compared to the absorbance of TiO2 anode only. This indicates the binding of the dye molecules with TiO2 surfaces. Dye extracted in distilled water that adsorbed onto the TiO2 layer tends to have a broad absorption range compared to the other extraction solvents. Similar findings by Hong et al. mentioned that the broad absorption range is due to that distilled water contained ions which are able to absorb light and thus broaden the absorption range [34].
The presence of anthocyanin in each dye extract was confirmed by adding a few drops of hydrochloric acid, which resulted in the formation of bright red coloration. The change in color is due to an equilibrium shift of anthocyanin toward their flavylium cation (AH+) at low pH [35]. UV-Vis measurement was also carried out and intense peaks can be observed between 500 and 550 nm confirming the presence of anthocyanin molecules, which are known to absorb light strongly between those wavelength ranges at pH 1.0 [36]. This is shown in Fig. 3.
Determinations of the initial concentration of extracted anthocyanin from different extraction solvents were calculated by the pH differential method. This is depicted in Fig. 4. It was found that DMF as extraction solvent yielded the highest anthocyanin extracts, which was then followed by DMSO, acetone, absolute ethanol, 70 % ethanol, and distilled water, respectively. Polar aprotic solvents used in this work such as DMF, DMSO, and acetone managed to extract more anthocyanins than those of polar protic solvents such as ethanol and water. This indicates that the extracted natural anthocyanin dissolves better in polar aprotic solvents, hence showing the importance of selecting the appropriate solvent for anthocyanin extraction.
The dye extracts were then individually leveled in terms of anthocyanin dye concentration by dilution in order to study the aggregation behavior of anthocyanin molecules in each extracting solvent. The size of the aggregated molecules was measured using Zetasizer (see Fig. 5).
Figure 5 shows the particle size analysis in terms of aggregation size in solution. Larger aggregation size means that the dye molecules are intensely aggregated in their respective extraction solvent. It was observed from the size analysis that the dye molecules aggregate more in absolute ethanol followed by 70 % ethanol, DMSO, distilled water, acetone, and DMF. The aggregations were caused by interaction of molecules in the solvents, and if the aggregations are intense, this would theoretically be unfavorable for DSSC.
Incident photon-to-current efficiency and current-voltage measurements
The incident photon-to-current efficiency for each tested cell was obtained and plotted as Fig. 6. Dyes in 70 % ethanol and acetone performed better in the visible light region than the dye in absolute ethanol, DMF, DMSO, and distilled water, although the dye in absolute ethanol was more active toward the violet region; however, it decreased tremendously after 510 nm wavelength. This suggests that the type of solvent does affect the absorption spectrum of the dyes as well as the bonding between the dyes and the surface of TiO2 [37]. One of the requirements for an effective dye sensitizer in DSSC is to have a broader spectral absorption activity in the visible light region [33]. In this case, it could be expected that dyes extracted in 70 % ethanol and acetone would perform better than dyes extracted in other extracting solvents.
To further evaluate the effectiveness of these extracted dyes, their performances in terms of current-voltage characteristic were obtained and hence plotted as Fig. 7. The current-voltage parameters were also tabulated in Table 1. Progressive error analysis was carried out to estimate experimental errors that arise from open circuit voltage (V oc) and short circuit voltage (I sc), with values of ±0.001 V and ±0.01 mA cm−2, respectively. The samples taken for each cell were carried out in triplicates (n = 3) and the error functions based on the cell conversion efficiency were then calculated and shown by reporting the cell performances (Table 1). It was found that DSSC fabricated using the dye extracted in 70 % ethanol showed the best performance, which obtained 0.50 ± 0.04 % in conversion efficiency, with V oc of 0.362 V and I sc of 0.559 mA cm−2 with a fill factor of 0.534. It was also found that with an increase in short circuit current, the conversion efficiency of the overall cell was increased, which, in this case, was in agreement to the study conducted by Sreekala et al. [31].
Despite the finding from the dye aggregation size analysis has shown that the dyes in absolute ethanol aggregate the most, and hence, the lowest conversion efficiency could be expected, the performance of DSSC fabricated using dye in absolute ethanol obtained a conversion efficiency of 0.27 ± 0.01 %. This performance by dye in absolute ethanol is much higher than that of DMF, DMSO, and distilled water (lowest conversion efficiency 0.09 ± 0.003 %), respectively. The performance of DSSC fabricated using dye in acetone obtained a conversion efficiency of 0.30 ± 0.04 %. This may suggest that 70 % ethanol is the preferable solvent for efficient dye adsorption to take place among all the studied solvents. These results indicate that there is no obvious correlation between the dye interaction behavior in their respective extraction solvents and the performance of the dye after adsorption onto the TiO2 layer.
Electrochemical impedance spectrophotometer
The DSSC performances of the dye extracted in different solvents were further evaluated using electrochemical impedance spectroscopy (EIS), which investigates the kinetics of electron in DSSCs under the irradiation of 100 mW cm−2 at open circuit condition. Nyquist plots and Bode plots are as shown as Fig. 8a, b, respectively, with the data obtained from DSSCs sensitized using Ixora dye in absolute ethanol, 70 % ethanol, acetone, DMF, DMSO, and distilled water.
Nyquist plot determines the charge transfer resistance which relates to a recombination of excited electrons (R k) in the TiO2/dye/electrolyte interface [38], which was observed from the diameter of the semicircle. The larger the arc indicates higher R k value which corresponds to the lower probability of the excited electrons to recombine. The R k values are 126.8, 181.7, 492.5, 423.9, 773.1, and 1185 Ω for DSSCs sensitized with Ixora dye in 70 % ethanol, acetone, absolute ethanol, DMF, distilled water, and DMSO, respectively. The low recombination resistance (R k) in the TiO2/dye/electrolyte interface directly relates to the decay of V oc in DSSCs due to the high recombination process. Similar values of V oc of that of DSSC sensitized with dye in acetone and in 70 % ethanol were exhibited, which may result from their similar recombination resistance. However, higher electron density of DSSC sensitized with dye in 70 % ethanol (ɳ s = 1.90 × 1026 cm−3) than DSSC sensitized with dye in acetone (ɳ s = 1.07 × 1026 cm−3) gives rise to a higher current (I sc); hence, better cell performance was achieved. [39].
Bode plot consists of two peaks: one peak is at a lower frequency and the other one is at a higher frequency which corresponds to effective lifetime of electrons on the TiO2/dye/electrolyte interface and effective lifetime of electron on the platinum, respectively [38]. The peak frequency at the low-frequency range is inversely proportional to the effective electron lifetime of electrons in the TiO2/dye/electrolyte interface, and the values obtained were 12.6 Hz for DSSC using dye in 70 % ethanol, 7.9 Hz for DSSC using dye in DMF and distilled water, and 6.3 Hz for DSSC using dye in acetone, DMSO, and absolute ethanol. These results show that DSSCs fabricated using dye in acetone, DMSO, and absolute ethanol have higher effective electron lifetime, which is essential for efficient flow of electrons in the TiO2 matrix.
Other parameters such as effective diffusion coefficient of electron (D eff), electron transport resistance (R w), and electron density (ɳ s) in the TiO2 matrix were determined and tabulated in Table 2. In order to achieve high efficiency of DSSC, it is hence necessary to obtain high electron diffusion coefficient, high electron density, and high electron recombination resistant [40]. Importantly, our results show that DSSC using dye extracted from 70 % ethanol exhibited the highest effective D eff to a factor 10 times compared to the other tested cells. Additionally, with the lowest electron transport resistance (3.34 Ω) obtained in DSSC using dye using 70 % ethanol, this positively allowed better diffusion of electrons within the TiO2 matrix and, thus, undoubtedly enhanced the overall DSSC performance. From our experimental data, the effect of different solvents used in the extraction does affect the kinetics of electrons in the TiO2/dye/electrolyte interface, and the consistency of both findings is best indicated by low electron transport resistance and high electron density that lead to the higher conversion efficiency achieved.
Conclusions
Dye sensitizers from I. coccinea flower were extracted in six different solvents, namely, 70 % ethanol, absolute ethanol, acetone, DMF, DMSO, and distilled water. More anthocyanins were extracted when using polar aprotic solvents as the extraction solvent. When tested in DSSC, polar protic solvents exhibit better cell conversion performances than the dyes extracted in polar aprotic solvent. Interaction between dyes-solvent-TiO2 was the determining factor in this case. Overall, it was found that the dye sensitizer extracted in 70 % ethanol achieved the highest conversion efficiency (0.50 ± 0.04 %) and was opted to its highest electron diffusion coefficient and lowest transport resistance in TiO2/dye/electrolyte interface. Dye extracted in distilled water (despite being known as a universal solvent) achieved the lowest with 0.09 ± 0.003 % conversion efficiency. This study highlights the importance of selecting the right medium for extraction as well as the favorable solvent medium during dye adsorption onto TiO2 is important as it can affect the overall efficiency of the fabricated DSSC.
References
Hemmatzadeh R, Mohammadi A (2013) Improving optical absorptivity of natural dyes for fabrication of efficient dye-sensitized solar cells. J Theor Appl Phys 7:1–7
Anandan S (2007) Recent improvements and arising challenges in dye-sensitized solar cells. Sol Energy Mater Sol Cells 90(91):843–846
Chu Y, Meisen P (2011) Review and comparison of different solar energy technologies. Global Energy Network Institute (GENI) 1–56. http://www.geni.org/globalenergy/research/review-and-comparison-of-solar-technologies/Review-and-Comparison-of-Different-Solar-Technologies.pdf. Accessed 22 Oct 2014
Nazeeruddin MK, Baranoff E, Grätzel M (2011) Dye-sensitized solar cells: a brief overview. Sol Energy 85:1172–1178
Gokilamani N, Muthukumarasamy N, Thambidurai M, Ranjitha A, Velauthapillai D, Senthil TS, Balasundaraprabhu R (2013) Dye-sensitized solar cells with natural dyes extracted from rose petals. J Mater Sci Mater Electron 24:3394–3402
Polo AS, Iha NYM (2006) Blue sensitizers for solar cells: natural dyes from Calafate and Jaboticaba. Sol Energy Mater Sol Cells 90:1936–1944
Basheer B, Mathew D, George BK, Reghunadhan Nair CP (2014) An overview on the spectrum of sensitizers: the heart of dye sensitized solar cells. Sol Energy 108:479–507
Fang X, Li Y, Zhang S, Bai L, Yuan N, Ding J (2014) The dye adsorption optimization of ZnO nanorod-based dye-sensitized solar cells. Sol Energy 105:14–19
Lund T, Nguyen PT, Tran HM, Pechy P, Zakeeruddin SM, Grätzel M (2014) Thermal stability of the DSC ruthenium dye C106 in robust electrolytes. Sol Energy 110:96–104
Narayan MR (2012) Review: dye sensitized solar cells based on natural photosensitizers. Renew Sust Energ Rev 16:208–215
Wongcharee K, Meeyoo V, Chavadej S (2007) Dye-sensitized solar cell using natural dyes extracted from rosella and blue pea flowers. Sol Energy Mater Sol Cells 91:566–571
Abdel-Latif MS, El-Agez TM, Taya SA, Batniji AY, El-Ghamri HS (2013) Plant seeds-based dye-sensitized solar cells. Mater Sci Appl 4:516–520
Ahmadian R (2011) Estimating the impact of dye concentration on the photoelectrochemical performance of anthocyanin-sensitized solar cells: a power law model. J Photonics Energy 1:011123
Lee JK, Yang M (2011) Progress in light harvesting and charge injection of dye-sensitized solar cells. Mater Sci Eng B 176:1142–1160
Ekanayake P, Kooh MRR, Kumara NTRN, Lim A, Petra MI, Voo NY, Lim CM (2013) Combined experimental and DFT–TDDFT study of photo-active constituents of Canarium odontophyllum for DSSC application. Chem Phys Lett 585:121–127
Dumbrava A, Enache I, Oprea CI, Georgescu A, Girtu MA (2012) Toward a more efficient utilisation of betalains as pigments for dye sensitized solar cells. Dig J Nanomater Biostruct 7:339–351
Park SK, Han YS (2014) Efficient dye-sensitized solar cells with surface-modified photoelectrodes. Sol Energy 110:260–267
Chiba Y, Islam A, Watanabe Y, Komiya R, Koide N, Han L (2006) Dye-sensitized solar cells with conversion efficiency of 11.1 %. Jpn J Appl Phys 45:638–640
Kim HJ, Bin YT, Karthick SN, Hemalatha KV, Raj CJ, Venkatesan S, Park S, Vijayakumar G (2013) Natural dye extracted from Rhododendron species flowers as a photosensitizer in dye sensitized solar cell. Int J Electrochem Sci 8:6734–6743
Shanmugam V, Manoharan S, Anandan S, Murugan R (2013) Performance of dye-sensitized solar cells fabricated with extracts from fruits of ivy gourd and flowers of red frangipani as sensitizers. Spectrochim Acta A Mol Biomol Spectrosc 104:35–40
Prima EC, Yuliarto B, Suendo V (2014) Suyatman, Improving photochemical properties of Ipomea pescaprae, Imperata cylindrica (L.) Beauv, and Paspalum conjugatum Berg as photosensitizers for dye sensitized solar cells. J Mater Sci Mater Electron 25:4603–4611
Zhou H, Wu L, Gao Y, Ma T (2011) Dye-sensitized solar cells using 20 natural dyes as sensitizers. J Photochem Photobiol A Chem 219:188–194
Szostak R, De Souza ECF, Antunes SRM, Borges CPF, De Andrade AVC, Rodrigues PRP, Antunes AC (2015) Anthocyanin from Vitis labrusca grape used as sensitizer in DSSC solar cells. J Mater Sci Mater Electron 26:2257–2262
Tripathi M, Upadhyay R, Pandey A, Dubey PK (2013) Natural dye-based photoelectrode for improvement of solar cell performance. Ionics 19:1179–1183
Aduloju KA, Shitta MB, Justus S (2011) Effect of extracting solvents on the stability and performances of dye-sensitized solar cell prepared using extract from Lawsonia inermis. Fundam J Mod Phys 1:261–268
Kim HJ, Kim DJ, Karthick SN, Hemalatha KV, Raj CJ, Ok S, Choe Y (2013) Curcumin dye extracted from Curcuma longa L. used as sensitizers for efficient dye-sensitized solar cells. Int J Electrochem Sci 8:8320–8328
Abdou EM, Hafez HS, Bakir E, Abdel-Mottaleb MSA (2013) Photostability of low cost dye-sensitized solar cells based on natural and synthetic dyes. Spectrochim Acta A Mol Biomol Spectrosc 115:202–207
Warkoyo EAS (2011) The solvent effectiveness on extraction process of seaweed pigment. Makara Teknologi 15:5–8
Singh LK, Karlo T, Pandey A (2013) Performance of fruit extract of Melastoma malabathricum L. as sensitizer in DSSCs. Spectrochim Acta A Mol Biomol Spectrosc 118:938–943
Otaka H, Kira M, Yano K, Ito S, Mitekura H, Kawata T, Matsui F (2004) Multi-colored dye-sensitized solar cells. J Photochem Photobiol A Chem 164:67–73
Sreekala CO, Jinchu I, Sreelatha KS, Janu Y, Prasad N, Kumar M, Sadh AK, Roy MS (2012) Influence of solvents and surface treatment on photovoltaic response of DSSC based on natural curcumin dye. IEEE J Photovolt 2:312–319
Mubiningtyas DI (2009) Isolation and identification of anthocyanin-shelled red flowers (Ixora coccinea). In: Chemistry, Gadjah Mada University. http://etd.ugm.ac.id/index.php?mod=penelitian_detail&sub=PenelitianDetail&act=view&typ=html&buku_id=42271&obyek_id=4. Accessed 12 Feb 2014
Lim A, Kumara NTRN, Tan AL, Mirza AH, Chandrakanthi RLN, Petra MI, Ming LC, Senadeera GKR, Ekanayake P (2015) Potential natural sensitizers extracted from the skin of Canarium odontophyllum fruits for dye-sensitized solar cells. Spectrochim Acta A Mol Biomol Spectrosc 138:596–602
Hong YX, Zong SZ, Jie L, Tan LY, Xue CY, Shang G, Xiao W, Shun WY, Lin ZM (2013) Efficient natural dye-sensitized solar cells based on spin-coated TiO2 anode materials. Chin Phys Lett 30:118801
Trouillas P, Di Meo F, Gierschner J, Linares M, Sancho-García JC, Otyepka M (2015) Optical properties of wine pigments: theoretical guidelines with new methodological perspectives. Tetrahedron 71:3079
Lee J, Durst RW, Wrolstad RE (2005) Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int 88:1269–1278
Al-Alwani MAM, Mohamad AB, Kadhum AAH, Ludin NA (2015) Effect of solvents on the extraction of natural pigments and adsorption onto TiO2 for dye-sensitized solar cell applications. Spectrochim Acta A Mol Biomol Spectrosc 138:130–137
Guliania R, Jainb A, Kapoora A (2012) Exact analytical analysis of dye-sensitized solar cell: improved method and comparative study. Open Renew Energy J 5:49–60
Nath NCD et al (2013) Electrochemical approach to enhance the open-circuit voltage (Voc) of dye-sensitized solar cells (DSSCs). Electrochim Acta 109:39–45
Adachi M, Sakamoto M, Jiu J, Ogata Y, Isoda S (2006) Determination of parameters of electron transport in dye-sensitized solar cells using electrochemical impedance spectroscopy. J Phys Chem B 110:13872–13880
Acknowledgments
The authors thank Prof. J.M.R. Sarath Bandara and Dr. Linda Biaw Leng Lim, Universiti Brunei Darussalam (UBD) for the assistance extended through fruitful discussion and support in this study. The Brunei Research Council (BRC) of Science and Technology Research Grant (S&T 17) is acknowledged for financial support.
Conflict of interest
The manuscript is approved by all of the authors and host authorities, and no conflict of interest is expressed regarding the publication of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, A., Pg Damit, D.N.F.B. & Ekanayake, P. Tailoring of extraction solvent of Ixora coccinea flower to enhance charge transport properties in dye-sensitized solar cells. Ionics 21, 2897–2904 (2015). https://doi.org/10.1007/s11581-015-1489-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1489-9