Abstract
To enhance the electrochemical performances of LiMn2O4 at elevated temperature, we proposed a sol–gel method to synthesize LiNi1/3Co1/3Mn1/3O2-modified LiMn2O4. The physical and electrochemical performances of pristine and LiNi1/3Co1/3Mn1/3O4-coated LiMn2O4 cathode materials were investigated by X-ray diffraction, scanning electron microscopy, transmission electron microscopy, X-ray photoelectron spectroscopy, and electrochemical measurements, respectively. The results indicated that about 5–6-nm-thick layer of LiNi1/3Co1/3Mn1/3O2 formed on the surface of the LiMn2O4 powders. The modified LiMn2O4 exhibited excellent storage performance at 45, 55, and 65 °C compared to the pristine one, which was attributed to the suppression of electrolyte decomposition and the reduction of Mn dissolution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the advantages of being abundant, nontoxic, and inexpensive, spinel lithium manganese oxide (LiMn2O4) is a promising candidate for layered cathode materials such as LiCoO2 [1, 2]. Especially, the good stability of LiMn2O4 may ensure its large-scale usage in batteries for electric vehicle or energy storage [3]. However, LiMn2O4 shows obvious capacity fade when cycling at high temperature (50–60 °C) [4–6]. It was reported that the capacity fading mechanism at high temperature was related to the Jahn–Teller distortion and dissolution of Mn2+ ions [7, 8]. Mn dissolution is induced by HF acid, which is generated by secondary chemical reactions from temperature-enhanced electrolyte decomposition.
In order to solve this problem, earlier studies have been focused on the chemical modification of LiMn2O4 by a partial substitution of Mn with some metal ions to obtain LiM x Mn2 − x O4 (M = Co, Mg, Cr, Ni, Fe, Al, Ti, and Zn) [9–12]. These results indicated that the substitution of Mn with metal ions significantly improved the cycle performance of LiMn2O4. However, the partial substitutions decrease the capacity of LiMn2O4. Another effective way is surface coating on LiMn2O4 by an oxide with high thermal and structural stability. ZrO2, SiO2, Al2O3, and MgO [13–16] have been used to coat LiMn2O4 by some chemical processes. Nevertheless, the aforementioned oxides do not have de-intercalation and intercalation of Li ions, which will result in a decrease in initial capacity. Additionally, proper lithium ion conductivity is a fundamental parameter used to choose the coating materials. LiNi1/3Co1/3Mn1/3O2 has de-intercalation and intercalation of Li ions, which may suppress the dissolution of Mn. Therefore, it is expected that the modified LiMn2O4 will show an excellent cycle performance at elevated temperature. In this study, we proposed an approach to synthesize LiNi1/3Co1/3Mn1/3O2 on the surface of spinel LiMn2O4. The effects of the LiNi1/3Co1/3Mn1/3O2 layer on the morphology and electrochemical performances of LiMn2O4 cathode materials were examined in detail.
Experimental
LiMn2O4 powder was purchased from Hebei Strong-Power Li-ion Battery Technology Co., Ltd. (D98, China). To coat LiMn2O4 with LiNi1/3Co1/3Mn1/3O2, LiCH3COO · 2H2O (1.057 g), Mn(CH3COO)2 · 4H2O (0.85 g), Ni(CH3COO)2 · 4H2O (0.86 g), and Co(CH3COO)2 · 4H2O(0.86 g) with a stoichiometric ratio of 3:1:1:1 were dissolved in distilled water to form a clear solution. An aqueous solution of ethylene glycol and citric acid as a chelating agent was added to the mixtures. pH value at 7.0–7.5 was achieved using ammonium hydroxide. Then the LiMn2O4 powders (50 g) were slowly added to the sol and vigorously stirred at 85 °C for 8 h. The sol turned into viscous transparent gel when the water evaporated. After drying and sieving, the powder was sintered in air at 400 °C for 5 h and 750 °C for 3 h to obtain LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4. For a comparison, pristine LiMn2O4 was also heat-treated in the same condition.
Structure and morphology characterization
X-ray diffraction (XRD) patterns were recorded on a DX-2700 diffractometer (Siemens D-5000, Mac Science MXP 18) equipped with Cu Kα radiation of λ = 0.154145 nm. The diffraction patterns were recorded between scattering angles of 15° and 80° at a step of 4°/min. The morphology was studied using a scanning electron microscope (S4700, Hitachi) and transmission electron microscope (JEOL-1200EX). X-ray photoelectron spectroscopy (Kratos AXIS Ultra DLD) was employed to probe the surface for Mn valence states. After cycling, the batteries were disassembled in a glove box and the electrodes and membrane were washed with EC/DMC for several times. The cathode was used to examine the changes in structure by XRD, and the obtained solution was diluted to a suitable concentration to detect the content of Mn element. Inductively coupled plasma atomic emission spectrometry analysis was conducted on an IRIS Intrepid П XSP inductively coupled plasma emission spectrometer (THERMO).
Electrochemical and thermal characteristics
To obtain working electrodes, 85 wt% active materials, 9 wt% acetylene black, and 6 wt% polyvinylidene fluoride were homogeneously mixed in N-methyl-pyrroline. Then the resulting slurry was spread on an aluminum foil and thoroughly dried. The electrodes were punched in the form of 14-mm-diameter disks, and the typical active material mass loading was about 6 mg/cm2. The electrolyte was 1 M LiPF6 dissolved in a mixture of ethylene carbonate and dimethylene carbonate with the volume ratio of 1:1. The anode of the battery is Li electrode. The assembly process was conducted in an argon-filled glove box with the content of H2O and O2 less than 1 ppm.
Before electrochemical tests, the batteries were aged for 24 h to ensure good soakage. The cells were charged and discharged on a battery tester (CT-3008 W, NEWARE) between 3.3 and 4.35 V at the rate of 2C at elevated temperatures in a dry oven (A201113, Shanghai). Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) investigations were performed on an electrochemical workstation (PGSTAT302N, Autolab) at 25 ± 2 °C. The CV curves were recorded between 3.3 and 4.35 V at a scan rate of 0.1 mV s−1. The EIS measurements were performed over a frequency range from 10 kHz to 0.1 Hz.
Results and discussion
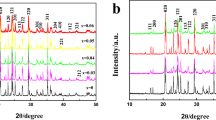
Figure 1 shows the XRD patterns of pristine and LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4. The peaks of both samples could be indexed to a cubic spinel structure with the space group Fd3m. There is no substantial difference between XRD patterns for pristine and modified LiMn2O4. The crystal lattice parameters, which were calculated by using the software Jade, are 8.245 and 8.246 Å for the pristine and LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4, respectively, indicating that the bulk structure of LiMn2O4 was unchanged after surface modification. The characteristic peaks corresponding to LiNi1/3Co1/3Mn1/3O2 are not observed because of low content (about 2.0 wt%).
Scanning electron microscopy reveals that the pristine and modified samples present a uniform particle distribution, ranging from 2 to 7 μm. The pristine spinel crystals are smooth with well-defined facets, as observed in Fig. 2a. It can be seen that the morphology and particle diameter of the LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4 powders in Fig. 2b are similar to the pristine sample. No LiNi1/3Co1/3Mn1/3O2 agglomerations and obscured facets of spinel LiMn2O4 are observed.
The morphology of the modified sample is further analyzed by TEM measurement. As shown in Fig. 2c, about 5–6-nm-thick layer of LiNi1/3Co1/3Mn1/3O2 is uniformly formed on the surface of the LiMn2O4. The coating layer is clearly distinguishable from the crystalline LiMn2O4. To further identify the homogeneity of the coating layer, the element distribution is determined by energy-dispersive X-ray spectroscopy (EDS) mapping, which is displayed in Fig. 3. The dense accumulation of Mn element is attributed to the host material of LiMn2O4, and there is no significant agglomeration of Ni and Co. These results indicate that LiNi1/3Co1/3Mn1/3O2 is homogeneously dispersed on the surface of the LiMn2O4 particles.
The oxidation state of manganese ions at the surface was determined from X-ray photoelectron spectroscopy (XPS) data by the curve fitting of Mn 2p spectral peaks. The experimental peak shape for Mn 2p3/2 was modeled by employing multiple-splitting patterns derived for Mn3+ and Mn4+ at binding energies of 641.6 and 642.8 eV from the standard compounds Mn2O3 and MnO2. Figure 4 shows the fit of the models to the experimental spectra for pristine LiMn2O4 and LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4, respectively. The surface of the pristine LiMn2O4 sample consists of almost equal amounts of Mn4+ and Mn3+ in Fig. 4a. By contrast, LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4 exhibited a Mn4+:Mn3+ ratio of 62.4:37.6 as shown in Fig. 4b. The difference in Mn4+:Mn3+ ratio on the surface is due to the formation of LiNi1/3Co1/3Mn1/3O2 because it has a higher valence state of manganese ions. For a further comparison, the Ni 2p and Co 2p spectra of the samples are also studied, which are shown in Fig. 4c, d. For the pristine LiMn2O4 sample, there are no Ni 2p and Co 2p peaks. For the LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4 sample, the Ni 2p region shows a Ni 2p3/2 main peak at 855.7 eV with a satellite peak at 862.1 eV, and the Co2p region shows a Co2p3/2 main peak at 780.4 eV with a satellite peak at 796.8 eV. Combined with the difference in Mn 2p spectra, it is concluded that Ni2+ and Co3+ have deposited on the surface of LiMn2O4. This result is in good agreement with the observation in TEM and EDS element mapping.
The structure of pristine LiMn2O4 and LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4 cathodes after cycling (55 °C) was examined. The results are given in Fig. 5. It can be seen that, compared with those of the pristine LiMn2O4 cathode, the diffraction peaks of the cycled LiMn2O4 cathode are obviously widened and the peak intensity declined. In addition, some extra peaks appear in the XRD pattern of the LiMn2O4 cathode after cycling, which should be assigned to Li2Mn2O4. Usually, tetrahedral Li2Mn2O4 can be generated at the final discharge stage of LiMn2O4 because of more Mn3+ and a more significant Jahn–Teller effect. On the other hand, for the LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4 cathode, the diffraction peak width changed insignificantly before and after cycling. Compared with the fresh LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4 cathode, in the XRD pattern of the cycled LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4, the peak intensity declines slightly, which may be ascribed to the LiNi1/3Co1/3Mn1/3O2 on the surface of LiMn2O4.
Figure 6 shows the galvanostatic charge–discharge curves at different temperatures of (a) pristine and (b) LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4 in a drying oven. The specific capacity increases with increased temperature, due to the higher lithium ion diffusion and lower resistance. Figure 6b shows two discharge plateaus, indicating that the LiNi1/3Co1/3Mn1/3O2 surface layer does not change the discharge mechanism of LiMn2O4 which orderly intercalates lithium ions in the tetrahedral (Fig. 6a) sites at 4.1 V and disorderly intercalates lithium ions at 3.9 V and which substantially maintains the intercalation feature of the LiMn2O4 substrate [17]; the two plateaus indicate the LiNi1/3Co1/3Mn1/3O2 surface layer rather than Ni-doped LiMn2O4 because LiMn2O4 with a Ni-doped spinel surface showed two ambiguously resolved discharging plateaus [18]. Meanwhile, LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4 shows a higher discharge capacity compared to the pristine sample. The reason may be that LiNi1/3Co1/3Mn1/3O2 has higher capacity than LiMn2O4 with equivalent quality at this voltage range.
Figure 7 shows the cycling performance of electrodes with and without LiNi1/3Co1/3Mn1/3O2 coating at (a) 25 ± 2 °C, (b) 45 ± 2 °C, (c) 55 ± 2 °C, and (d) 65 ± 2 °C. After 100 cycles at room temperature, the capacity retention of the pristine sample (94.3 %) is similar to that of the modified sample (94.4 %), as shown in Fig. 7a. However, after 100 cycles at elevated temperature as shown in Fig. 7b–d, the capacity retention increases from 94.3 to 94.4 %, 88.7 to 93.5 %, 87.5 to 93.6 %, and 81.7 to 91 %, respectively. The three times repeat tests for the preparation of the modified sample are conducted, and the capacity retention is listed in Table 1. As shown in Fig. 7, the higher the temperature, the more retention rate improved. The reason might be that the side reactions at the interface of LiMn2O4 and electrolyte become more drastic at high temperature. Therefore, the protective effect of the coating layer becomes more significant. Compared with other coating materials such as Al2O3 [19], La2O3 [20], and AlPO4 [21], surface modification by the sol–gel method can improve the high-temperature cycling stability of LiMn2O4 because the oxide layer can reduce the contact area of solid and electrolyte. However, the oxide itself is inactive with lithium ions and will result in a decrease in initial capacity. Compared to the oxide coating layer, LiNi1/3Co1/3Mn1/3O2 is more stable in the electrolyte than LiMn2O4 and has higher capacity. Thus, the electrode after coating has excellent cycle performance without reducing the initial capacity.
To further verify the effects of surface coating on manganese ion dissolution, the quality of the manganese element was directly determined by using ICP-AES. Li metal anode was washed with dilute hydrochloric acid after the 100th cycle at 55 ± 2 °C. It can be seen in Table 2 that the dissolved quality of Mn2+ ions of the pristine and LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4 electrodes was 22.54 and 10.17 μg/cm2, respectively. It can be concluded that after coating by the LiNi1/3Co1/3Mn1/3O2 layer, the dissolution of the manganese ions was significantly reduced. Therefore, the LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4 electrode had better cycle stability at elevated temperature. The reason might be that the valence state of Mn in LiNi1/3Co1/3Mn1/3O2 is +4, which will suppress the Jahn–Teller effect on the surface of LiMn2O4. Another reason is that the coating material will reduce the contact area of LiMn2O4 and electrolyte, which may decrease the dissolution of Mn. The reactivity between LiNi1/3Co1/3Mn1/3O2 and electrolyte is not yet clear, which needs further research in the future.
Figure 8 shows the CV profiles of the pristine and LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4 electrodes in the 10th and 100th cycles at the scan rate of 0.1 mV s−1. The CV peaks of the LiNi1/3Co1/3Mn1/3O2-coated sample show two symmetrical couples of redox peaks at around 3.97 and 4.11 V, respectively (Fig. 8b), indicating that electrochemical insertion and extraction reactions of Li+ ions are two step processes. It is in agreement with the two plateaus in Fig. 6 and demonstrates that LiNi1/3Co1/3Mn1/3O2 coating does not change the electrochemical reaction mechanism of LiMn2O4. After 10 cycles, two narrow and separate redox peaks appear around at 3.94 and 4.09 V, as shown in Fig. 8a. However, after the 100th cycle, due to the dissolution of Mn2+ ions into the electrolyte (Jahn-Teller distortion), both anodic and cathodic peaks become much broader and lower in peak current. In contrast, the oxidation and reduction peaks related to LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4 are much steadier after 100 cycles (Fig. 8b), which indicated that modified LiMn2O4 has better reversibility and stability than the pristine LiMn2O4.
Electrochemical impedance spectra (EIS) and equivalent circuits are shown in Fig. 9. The measurements were carried out with a fully charged state (4.35 V). An intercept in the high-frequency region of the Z rel axis indicates the ohmic resistance (R s), the combined resistance of the electrolyte, and the contacts of the cell [22]. The semicircle in the high–middle-frequency region corresponds to the charge transfer (R ct) process on the electrode interface, revealing the lithium transfer rate parameters and the capacitance of the solid electrolyte interface (SEI) [23]. The inclined line in the lower frequency region represents the Warburg impedance (Z w), which corresponds to the diffusion of Li+ in LiMn2O4 particles [24]. The plots are fitted and listed in Table 3. As shown in the table, after 10 cycles, the R s of LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4 is slightly larger than that of the pristine sample because the coating layer may slightly increase the electrolyte and contact resistance. The charge transfer resistance of both samples is approximately similar (26.8 and 22.4 Ω cm2). After 100 cycles, the change in R s is negligible. However, the R ct value of the LiNi1/3Co1/3Mn1/3O2-coated electrode (32.6 Ω cm2) is much smaller than that of the pristine electrode (78.2 Ω cm2). It attributes to the restraint of structural instability caused by the subsequent Mn dissolution and vacancy formation. This result is also in accordance with the enhanced cycling performance of LiNi1/3Co1/3Mn1/3O2-coated electrodes.
Conclusions
In summary, the surface of the LiMn2O4 sample was modified by LiNi1/3Co1/3Mn1/3O2 using a sol–gel method. TEM and XPS results confirm the existence of the LiNi1/3Co1/3Mn1/3O2 layer. A uniform and dense layer about 5–6 nm was coated on the surface of pristine LiMn2O4. The LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4 sample exhibits much better cycling stability at elevated temperature compared with the pristine sample. The CV tests indicated that the LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4 electrode has better reversibility and stability. Meanwhile, the charge transfer resistance of the LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4 was much less than that of the pristine sample after 100 cycles, which is ascribed to the better structural stability and restraint of Mn dissolution. These results demonstrated that this is an effective way to improve the high-temperature cyclic performance of spinel LiMn2O4.
References
Pitchai R, Thavasi V, Mhaisalkar SG, Ramakrishna S (2011) Nanostructured cathode materials: a key for better performance in Li-ion batteries. J Mater Chem 21(30):11040–11051
Zhao S, Bai Y, Ding LH, Wang B, Zhang WF (2013) Enhanced cycling stability and thermal stability of YPO4-coated LiMn2O4 cathode materials for lithium ion batteries. J Solid State Ionics 247:22–29
Kim WK, Han DW, Ryu WH, Lim SJ, Kwon HS (2012) Al2O3 coating on LiMn2O4 by electrostatic attraction forces and its effects on the high temperature cyclic performance. J Electrochimica Acta 71:17–21
Arumugam D, Paruthimal Kalaignan G (2011) Electrochemical characterizations of surface modified LiMn2O4 cathode materials for high temperature lithium battery applications. J Thin Solid Films 520:338–343
Li X (2012) Rui Yang etc, Enhanced electrochemical properties of nano-Li3PO4 coated on the LiMn2O4 cathode material for lithium ion battery at 55 °C. J Materials Letter 66:168–171
Kim D, Park S, Chae OB, Ryu JH, Kim YU, Yin RZ, Oh SM (2012) Re-deposition of manganese species on spinel LiMn2O4 electrode after Mn dissolution. J Electrochem Soc 159(3):A193–A197
Gummow RJ, Dekock A, Thackeray MM (1994) Improved capacity retention in rechargeable 4 V lithium/lithium manganese oxide (spinel) cells. J Solid State Ionics 69(1):59–67
Jang DH, Shin YJ, Oh SM (1996) Dissolution of spinel oxides and capacity losses in 4 V Li/LixMn2O4 coils. J Electrochem Soc 143(7):2204–2211
Wu XL, Kim SB (2002) Improvement of electrochemical properties of LiNi(0.5)Mn(1.5)O(4) spinel. J Power Sources 109(1):53–57
Tarascon JM, Wang E, Shokoohi FK, Mckinnon WR, Colson S (1991) The spinel phase of LiMn2O4 as a cathode in secondary lithium cells. J Electrochem Soc 138(10):2859–2864
Hernan L, Morales J, Sanchez L, Santos J (1999) Use of Li-M-Mn-O [M = Co, Cr, Ti] spinels prepared by a sol-gel method as cathodes in high-voltage lithium batteries. J Solid State Ionics 118(3–4):179–185
Thackeray MM, Johnson CS, Kim JS, Lauzze KC, Vaughey JT, Dietz N, Abraham D, Hackney SA, Zeltner W, Anderson MA (2003) ZrO2- and Li2ZrO3-stabilized spinel and layered electrodes for lithium batteries. J Electrochem Commun 5(9):752–758
Huang B, Li X, Wang Z, Guo H, Xiong X, Wang J (2014) A novel carbamide-assistant hydrothermal process for coating Al2O3. J Alloys Compd 583:313–319
Zheng ZH, Tang ZL, Zhang ZT, Shen WC, Lin YH (2002) Surface modification of Li1.03Mn1.97O4 spinels for improved capacity retention. J Solid State Ionics 148(3–4):317–321
Gnanaraj JS, Pol VG, Gedanken A, Aurbach D (2003) Improving the high-temperature performance of LiMn2O4 spinel electrodes by coating the active mass with MgO via a sonochemical method. J Electrochem Commun 5(11):940–945
Wu F, Wang M, Su YF, Chen S, Xu B (2009) Effect of TiO2-coating on the electrochemical performances of LiCo1/3Ni1/3Mn1/3O2. J Power Sources 191(2):628–632
He XM, Li JJ, Cai Y, Wang YW, Ying JR, Jiang CY, Wan CR (2005) Preparation of co-doped spherical spinel LiMn2O4 cathode materials for Li-ion batteries. J Power Sources 150:216–222
Li X, Xu Y, Wang C (2009) Suppression of Jahn-Teller distortion of spinel LiMn2O4 cathode. J Alloys Compound 479:310
Kim WK, Dong H (2012) Al2O3 coating on LiMn2O4 by electrostatic attraction forces and its effects on the high temperature cyclic performance. J Eletrochemica Acta 71:17–21
L. F, S. W, Lu Han. Enhanced electrochemical properties of LiMn2O4 cathode material coated by 5wt.% of nano-La2O3, Journal of Materials Letters 78(2012) 116-119.
Liu D, He Z (2007) Increased cycling stability of AlPO4-coated LiMn2O4 for lithium ion batteries. J Mater Letters 61:4703–4706
Myung ST, Izumi K, Komaba S, Sun YK, Yashiro H, Kumagai N (2005) Role of alumina coating on Li-Ni-Co-Mn-O particles as positive electrode material for lithium-ion batteries. J Chem Mater 17(14):3695–3704
Jang SB, Kang SH, Amine K, Bae YC, Sun YK (2005) Synthesis and improved electrochemical performance of Al (OH)(3)-coated Li[Ni1/3Mn1/3Co1/3]O2 cathode materials at elevated temperature. J Electrochimica Acta 50(20):4168–4173
Levi MD, Gamolsky K, Aurbach D, Heider U, Oesten R (2000) On electrochemical impedance measurements of LixCo0.2Ni0.8O2 and LixNiO2 intercalation electrodes. Electrochim Acta 45(11):1781–1789
Acknowledgments
This work was supported by the National Science Foundation of China (No. 50672026). This work was also supported by the Shanghai Nanotechnology Promotion Center (No. 12ZR1448800)
Author information
Authors and Affiliations
Corresponding author
Additional information
LiNi1/3Co1/3Mn1/3O2 is a new coating material on LiMn2O4. A uniform and dense layer about 5–6 nm was formed on the surface of LiMn2O4. The LiNi1/3Co1/3Mn1/3O2 coating layer significantly improves the high-temperature cycling stability of LiMn2O4.
Rights and permissions
About this article
Cite this article
Yan, J., Liu, H., Wang, Y. et al. Enhanced high-temperature cycling stability of LiNi1/3Co1/3Mn1/3O2-coated LiMn2O4 as cathode material for lithium ion batteries. Ionics 21, 1835–1842 (2015). https://doi.org/10.1007/s11581-015-1371-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1371-9