Abstract
A solid polymer electrolyte is fabricated using polyethylene oxide (PEO), lithium bis(Trifluromethanesulfonyl)imide (LiTFSI), and montmorillonite (MMT), with the aim of improving lithium ion conductivity, and the resulting solid polymer electrolyte is used for all solid-state lithium/sulfur batteries. The effect of temperature and nanoclay content on the conductivity of the resulting solid polymer electrolyte is investigated. The optimized electrolyte containing 10 wt% MMT exhibits ionic conductivity of 3.22 × 10−4 S cm−1 at 60 °C, a value that meets the operation requirements of an all solid-state sulfur cell. At 60 °C, all solid-state Li/S batteries using PEO/MMT solid polymer electrolyte display a good cycling performance, delivering 998 mAh g−1 initial discharge capacities and retaining a reversible specific discharge capacity of 634 mAh g−1 after 100 cycles at 0.1 C rate. At a higher rate of 0.5 C, the solid-state batteries still could deliver an acceptable specific discharge capacity of 643 mAh g−1 at 60 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulfur is a very attractive candidate as a cathode material for rechargeable lithium battery, due to its high theoretical capacity of 1,672 mAh g−1 and high energy density of 2,600 Wh Kg−1 [1–3]. In addition, sulfur has the advantages of nature abundance, low cost, and low toxicity. Nevertheless, the insulating nature of sulfur and solubility of the polysulfides intermediate products in liquid electrolytes represent the biggest challenges for the full realization of the Li/S battery [4, 5]. Various attempts have been made to address these issues, including the optimization of electrolytes and the development of novel composite cathodes [6–13]. Among the various strategies, the development of an all solid Li/S battery is promising since the solid electrolyte can act as a physical barrier to control the dissolution of the polysulfide anions from the cathode and to prevent the attack of the anode by those same anions [13]. Due to the advantages of no electrolyte leakage, high energy density, flexible geometry, and safety solid polymer electrolytes (SPE) have been widely investigated [14–19]. Up to date, several works have been developed in the field of all solid-state Li/S battery using solid polymer electrolyte [20–23]. Polyethylene oxide (PEO) was mainly adopted as the solid polymer electrolyte of Li/S battery. However, the cell still exhibited a low reversible capacity and poor cycle life, due to the low conductivity of solid polymer electrolytes. Shin et al. reported the Li/S cells with (PEO)10LiCF3SO3-TinO2n-1 solid polymer electrolyte have an initial discharge capacity of between 1,400 and 1,600 mAh g−1 with current rate of 100 mA g−1 at 90 °C [20]. Zhu et al. reported that an all solid-state Li/S battery operated at 75 °C with (PEO)20(LiCF3SO2)2 N-γLiAlO2 as electrolyte exhibited an average capacity of 290 mAh g−1 during 50 cycles [21].
Recently, a number of related investigations have described the use of polymer/nanoclay composites in SPE [16–18]. As a layered host, nanoclays can provide a large interfacial contact area, which improves the solubility of lithium salts, due to higher dielectric property. To the best of our knowledge, there is no report on the introduction of PEO/nanoclay solid polymer electrolyte in Li/S battery. Herein, we report on the simple preparation of a solid polymer electrolyte based on poly(ethylene-oxide)/nanoclay composite for all solid-state lithium/sulfur batteries.
Experimental
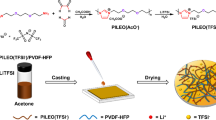
The PEO/MMT polymer electrolyte preparation is schematically presented in Fig. 1. LiTFSI (Lithium Bis(Trifluromethanesulfonyl)Imide, Sigma-Aldrich) and polyethylene oxide (PEO MW = 1 × 106 g mol−1, Sigma-Aldrich) were firstly dissolved in acetonitrile (99.8 %, Fisher Scientific) at the EO/Li molar ratio of 20:1 at 60 °C for 12 h, followed by addition of octadecylamine modified montmorillonite nanoclay (MMT, Sigma-Aldrich) particles at the ratio of 5 wt% of the total PEO20LiTFSI. After ultrasonication and stirring, the homogeneous solution was cast on a Teflon™ dish and dried in a vacuum oven at 60 °C until all solvent had evaporated. The thin PEO/MMT film was vacuum dried at 120 °C for 2 h to ensure total removal of moisture.
The crystalline phases of the sample were determined by X-ray diffraction (XRD, D8 Discover, Bruker) equipped with Cu Kα (λ = 1.54 Å) radiation.
The ionic conductivity was determined by electrochemical impedance spectroscopy (EIS) over the frequency range from 0.1 Hz to 1 MHz (VMP3 Potentiostat/Galvanostat, Biologic Instruments). Solid polymer electrolyte membranes were cut into 8 mm diameter disks and sandwiched between two stainless steel blocking electrodes. Li+ transference number (T+) of PEO/MMT solid polymer electrolyte is measured based on the following symmetric cell with the solid polymer electrolyte membrane between planar lithium foils: Li|PEO-LiTFSI-MMT|Li, using the combined ac impedence/dc polarization method at 60 °C [14, 15]. The equation is \( {T}^{+}=\frac{I_s\left(\varDelta V-{I}_o{R}_o\right)}{I_o\left(\varDelta V-{I}_s{R}_s\right)} \), where I is the dc current, and R o and R s are the resistance of the passivation film formed onto the metallic lithium electrodes during the measurement [16]. The electrochemical stability of PEO/MMT solid polymer electrolyte was investigated by linear sweep voltammetry of the cell with solid polymer electrolyte sandwiched between lithium metal and stainless steel electrodes. The voltage is swept from 1 V vs. Li+/Li towards the anodic values with a scan rate of 0.1 mV s−1 (VMP3, Biologic).
The active material was synthesized by heating a mixture of polyacrylonitrile (PAN), sulfur, and Mg0.6Ni0.4O as described previously [11]. The composite cathode comprised of active material S/PAN/Mg0.6Ni0.4O, acetylene black conductive agent (AB), PEO binder, and LiTFSI at a mass ratio of 55:25:15:5, dispersed in 1-methyl-2-pyrrolidinone (NMP, Sigma-Aldrich, ≥99.5 % purity). The resultant slurry was spread onto a circular piece of nickel foam (MTI, ≥99 % purity) with 1 cm in diameter. After drying in a vacuum oven for 12 h at 60 °C, the cathode was pressed at 8 MPa in order to achieve good contact between the active material and nickel foam. The thickness of electrode film is about 120 μm, and sulfur loading in each electrode is about 1.2 mg cm−2.
The electrochemical performance of Li/S batteries was investigated using coin-type cells (CR2032) composed of lithium metal anode and sulfur composite cathode separated by prepared PEO/MMT composite polymer electrolyte. The coin cells were assembled in a MBraun glove box filled with argon (99.9995 % purity). Cyclic voltammetry (CV) was performed by means of a potentiostat/galvanostat (VMP3, Biologic) at 60 °C between 1 and 3 V vs. Li+/Li at a scanning rate of 0.1 mV s−1. The cells were tested at 60 °C in terms of charge/discharge galvanostatic cycling on multi-channel battery testers (BT-2000, Arbin Instruments), setting the cut off voltages between 1 and 3 V vs. Li+/Li. Applied currents and specific capacities were calculated on the basis of the weight of S in the cathode.
Results and discussion
XRD is a powerful tool to identify intercalated structures through Bragg’s relation: λ = 2dsinθ, where λ corresponds to the wave length of the X-ray radiation used in the diffraction experiment, d corresponds to the interlayer spacing between diffraction lattice planes, and θ is the measured diffraction Angle [16].
Figure 2 shows the XRD patterns of pure MMT and PEO/MMT composites with 10 wt% MMT. The MMT exhibited a strong (001) reflection at 2θ = 5.0°, and the interlayer spacing is about 1.76 nm. For the PEO/MMT composite, based on the reflection at 2θ = 4.5°, the interlayer spacing is calculated as 1.96 nm. The 0.2 nm increase in thickness of interlayer spacing results from the PEO intercalation inside the nanoclay galleries [17].
XRD patterns of pure PEO and PEO/MMT composites with 10 wt% MMT are presented in Fig. 3. The characteristic diffraction peaks of the PEO crystalline are apparent between 2θ = 15 and 30°. The characteristic bands of the PEO/MMT composite are consistent with that of the pure PEO but with reduced intensity, which indicate the decreased crystallinity. Therefore, the incorporation of nanoclay into polymeric materials increases the amorphous content in the composite, favoring to the conductivity of PEO/MMT polymer electrolyte.
The dependence of the PEO/MMT solid electrolyte ionic conductivity on the content of nanoclay is depicted in Fig. 4a. The ionic conductivity was enhanced at low amounts of MMT, reaching the maximum value of 2.75 × 10−5 S cm−1 at 25 °C for the composite containing 10 wt% MMT. A decrease in conductivity was observed when the amount of MMT was increased to 15 wt%, consistent with the behavior previously reported for the PEO/LiClO4/MMT system [18]. The incorporation of nanoclays into polymeric materials increases the amorphous content of the polymer in the composite, as confirmed by XRD measurement (Fig. 3), facilitating the movement of Li+ ion due to enhanced chain segmental motion [18]. However, beyond 10 wt%, the insulating nature of MMT overcomes the favorable effects it imparts on the Li+ ion motion and reduces the overall ionic conductivity of the composite.
Figure 4b shows the logarithmic temperature dependence of the ionic conductivity for the PEO/MMT system. The linear behavior of the curves suggests that the conductivity obeys the Arrhenius equation σ = σ 0 exp(−E a /RT), where R is the gas constant, σ is the conductivity of polymer electrolyte, σ 0 is the pre-exponential factor, and T is the testing temperature in absolute scale [24]. The temperature dependence displays two linear regions with different activation energies, 42 and 12 kJ mol−1 for the lower and the higher temperature regions, respectively. Nevertheless, the conductivity of PEO/MMT polymer electrolyte reaches 3.22 × 10−4 S cm−1 at 60 °C, a value that meets the operation requirements of an all solid-state sulfur cell [20–23].
The fraction of the current carried by Li+ ions through the polymer electrolyte is fundamental for the performance of lithium batteries [19]. To understand the Li+ transport mechanism in PEO/LiTFSI/MMT polymer electrolyte, Li+ transference number studies were carried out at 60 °C. As shown in Fig. 4a, PEO/LiTFSI/MMT composite containing 10 wt% MMT is found to be optimal from a conductivity point of view for practical applications. Therefore, we tested the Li+ transference numbers of PEO/LiTFSI/10%MMT and a control sample (PEO/LiTFSI), which are 0.45 and 0.17, respectively. This increase in transference number may be attributed to the Lewis acid–base interactions: the Lewis acid sites on the anionic surface of MMT can interact with oxygen elements in PEO (Lewis base), and hence weaken the interactions between oxygen elements and Li ion [19]. As a result, more free Li ions are released, and the Li+ transference number is increased [16].
It is pertinent that the electrolyte has a wide electrochemical stability window, which determines the voltage range of its applicability [25]. Linear sweep voltammetry experiments (Fig. 5) showed that the PEO/MMT solid polymer electrolyte prepared in this work is electrochemically stable up to 4.0 V. As the potential is swept towards the anodic values, an abrupt current rise was observed at about 4.0 V, which could be attributed the electrolyte decomposition at the inert electrode interface. This value of the stability window appears to be high enough to ensure safe application of the solid polymer electrolyte in Li/S batteries, where the working voltage upper cut off is limited within 3 V vs. Li+/Li.
Figure 6a shows the cyclic voltammograms (CV) of the all solid-state Li/S cell at 60 °C. No electrochemical processes are observed in addition to those related to the Li-S reactions, implying that the PEO/MMT solid polymer electrolyte is electrochemically inactive within the studied potential region. This confirms the electrochemical stability of as prepared solid polymer electrolyte in the voltage range of its applicability. Furthermore, a pronounced reduction process is observed in the first cycle, possibly due to side reactions of formation of the solid electrolyte interface (SEI). After the initial cycle, the heights of the main peaks remain at a similar level, indicating good reversibility of the system.
a Initial CV profiles of all solid-state Li/S cell at 60 °C; the measurement is conducted at a scan rate of 0.1 mV s−1 in the voltage range of 1.0 to 3.0 V vs. Li+/Li; b Charge/discharge profiles (at 0.1 C) of all solid-state Li/S cell at 60 °C; c Cycle performance (at 0.1 C) of all solid-state Li/S cell at 60 °C; d Rate capability of all solid-state Li/S cell at 60 °C
In order to investigate the electrochemical performance of the all solid-state Li/S cells, galvanostatic charge/discharge cycling tests were carried out at 60 °C. The system delivers a specific capacity of 998 mAh g−1 in the first discharge at 0.1 C, and a reversible capacity of 591 mAh g−1 is obtained in the second cycle (Fig. 6b). The voltage profiles for the second and third cycles show similar behavior, namely, the discharge curves do not present the typical two plateaus related to the step-wise reactions between lithium and polysulfides. This is consistent with the CV results discussed above. The cycling performance of the cells with PEO/MMT solid polymer electrolyte was also carried out at 60 °C as shown in Fig. 6c. An initial increase of capacity is observed for the first 20 cycles at 0.1 C, suggesting a gradual activation of the solid polymer electrolyte electrochemical properties during the charge/discharge processes. We speculate that this is due to the slow penetration and transport of the lithium ion through the cathode and solid polymer electrolyte. After 100 cycles, the system still maintains a reversible specific discharge capacity of 634 mAh g−1, equivalent to 63.5 % capacity retention from the initial discharge capacity.
The kinetic behavior of the solid-state Li/S cell was further evaluated, and the rate capability of the cells was examined at 60 °C. The results for different C rates, namely 0.1, 0.2, and 0.5 C are shown in Fig. 6d. The capacity decreases gradually with the increase in the discharge charge current. Initial capacities of 905 and 643 mAh g−1 are obtained at rates of 0.2 C and 0.5 C, respectively, showing the acceptable rate capability of the cells with PEO/MMT solid polymer electrolyte.
Conclusions
A poly(ethylene-oxide)/montmorilonite composite was prepared and evaluated as solid electrolyte for the lithium/sulfur batteries. A five-fold increase in ionic conductivity is observed by addition of 10 wt% MMT. All solid-state Li/S batteries containing PEO/MMT solid polymer electrolyte displays good cycling performance, retaining a reversible specific discharge capacity of 634 mAh g−1 after 100 cycles at 0.1 C rate. In the rate capability test, initial capacities of 905 and 643 mAh g−1 are obtained at rates of 0.2 and 0.5 C, respectively, showing the acceptable rate capability of the cells with PEO/MMT solid polymer electrolyte.
References
Armand M, Tarascon JM (2008) Building better batteries. Nature 451:652–657
Zhang Y, Bakenov Z, Zhao Y, Konarov A, Doan TNL, Sun KEK, Yermukhambetova A, Chen P (2013) Effect of nanosized Mg0.6Ni0.4O prepared by self-propagating high temperature synthesis on sulfur cathode performance in Li/S batteries. Powder Technol 235:248–255
Zhao Y, Zhang Y, Gosselink D, Doan TNL, Sadhu M, Cheang HJ, Chen P (2012) Polymer electrolytes for lithium/sulfur batteries. Membranes 2:553–564
Evers S, Nazar LF (2013) New approaches for high energy density lithium-sulfur battery cathodes. Acc Chem Res 46:1135–1143
Zhang Y, Zhao Y, Sun KEK, Chen P (2011) Development in lithium/sulfur secondary batteries. Open Mater Sci J 5:215–221
Zhang SS (2012) Role of LiNO3 in rechargeable lithium/sulfur battery. Electrochim Acta 70:344–348
Zhang Y, Bakenov Z, Zhao Y, Konarov A, Wang Q, Chen P (2013) Three-dimensional carbon fiber as current collector for lithium/sulfur batteries. Ionics. doi:10.1007/s11581-013-1042-7
Zhao Y, Zhang Y, Bakenov Z, Chen P (2013) Electrochemical performance of lithium gel polymer battery with nanostructured sulfur/carbon composite cathode. Solid State Ionics 234:40–45
Zhang Y, Zhao Y, Bakenov Z, Babaa MR, Konarov A, Ding C, Chen P (2013) Effect of graphene on sulfur/polyacrylonitrile nanocomposite cathode in high performance lithium/sulfur batteries. J Electrochem Soc 160:A1194–A1198
Zhang Y, Bakenov Z, Zhao Y, Konarov A, Doan TNL, Malik M, Paron T, Chen P (2012) One-step synthesis of branched sulfur/polypyrrole nanocomposite cathode for lithium rechargeable batteries. J Power Sources 208:1–8
Zhang Y, Zhao Y, Yermukhambetova A, Bakenov Z, Chen P (2013) Ternary sulfur/polyacrylonitrile/Mg0.6Ni0.4O composite cathode for high performance lithium/sulfur batteries. J Mater Chem A 1:295–301
Zhang Y, Zhao Y, Konarov A, Gosselink D, Soboleski HG, Chen P (2013) A novel nano-sulfur/polypyrrole/graphene nanocomposite cathode with a dual-layered structure for lithium rechargeable batteries. J Power Sources 241:517–521
Hassoun J, Sun YK, Scrosati B (2011) Rechargeable lithium sulfide electrode for a polymer tin/sulfur lithium-ion battery. J Power Sources 196:343–348
Abraham KM, Jiang Z, Carroll B (1997) Highly conductive PEO-like polymer electrolytes. Chem Mater 9:1978–1988
Tao RY, Zhao Y, Fujinami T (2007) Lithium borate-PEO polymer electrolytes characterized with high lithium ion transference numbers. Mater Sci Eng B 137:69–73
Kim S, Park SJ (2007) Preparation and ion-conducting behaviors of poly(ethylene oxide)-composite electrolytes containing lithium montmorillonite. Solid State Ionics 178:973–979
Burgaz E (2011) Poly(ethylene-oxide)/clay/silica nanocomposites: Morphology and thermomechanical properties. Polymer 52:5118–5126
Kim S, Hwang EJ, Jung Y, Han M, Park S (2008) Ionic conductivity of polymeric nanocomposite electrolytes based on poly(ethylene oxide) and organo-clay materials. Colloid Surf A-Physicochem Eng Asp 313–314:216–219
Angulakshmi N, Nahm KS, Nair JR, Gerbaldi C, Bongiovanni R, Penazzi N, Stephan AM (2013) Cycling profile of MgAl2O4-incorporated composite electrolytes composed of PEO and LiPF6 for lithium polymer batteries. Electrochim Acta 90:179–185
Shin JH, Kim KW, Ahn HJ, Ahn JH (2002) Electrochemical properties and interfacial stability of (PEO)10LiCF3SO3-TinO2n-1 composite polymer electrolytes for lithium/sulfur battery. Mat Sci Eng B-Solid 95:148–156
Zhu XJ, Wen ZY, Gu ZH, Lin ZX (2005) Electrochemical characterization and performance improvement of lithium/sulfur polymer batteries. J Power Sources 139:269–273
Jeon BH, Yeon JH, Kim KM, Chung IJ (2002) Preparation and electrochemical properties of lithium-sulfur polymer batteries. J Power Sources 109:89–97
Yu X, Xie J, Yang J, Wang K (2004) All solid-state rechargeable lithium cells based on nano-sulfur composite cathodes. J Power Sources 132:181–186
Xiao Q, Wang X, Li W, Li Z, Zhang T, Zhang H (2009) Macroporous polymer electrolytes based on PVDF/PEO-b-PMMA block copolymer blends for rechargeable lithium ion battery. J Membr Sci 334:117–122
Zhang Y, Zhao Y, Bakenov Z, Gosselink D, Chen P (2014) Poly(vinylidene fluoride-co-hexafluoropropylene)/poly(methylmethacrylate)/nanoclay composite gel polymer electrolyte for lithium/sulfur batteries. J Solid State Electrochem 18:1111–1116
Acknowledgments
This research was financially supported by Positec, Natural Sciences and Engineering Research Council of Canada (NSERC), Canadian Foundation for Innovation (CFI), and the Canada Research Chairs (CRC). One of the authors (YZ) thanks the China Scholarship Council for Study Abroad Scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Zhao, Y., Gosselink, D. et al. Synthesis of poly(ethylene-oxide)/nanoclay solid polymer electrolyte for all solid-state lithium/sulfur battery. Ionics 21, 381–385 (2015). https://doi.org/10.1007/s11581-014-1176-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1176-2