Abstract
Li2Fe1−x Mn x SiO4/C (x = 0, 0.1, 0.2, 0.3, 0.4, 0.5) are prepared by a vacuum solid-state reaction of SiO2, CH3COOLi·2H2O, FeC2O4·2H2O, and Mn(CH3COO)2·4H2O. The crystalline structures, morphologies, and electrochemical performances are analyzed contrastively by X-ray diffraction (XRD), scanning electron microscopy, galvanostatic charging–discharging, and electrochemical impedance spectroscopy (EIS). The XRD and EIS results prove that Mn doping may be beneficial to the battery performances of Li2FeSiO4 materials, by reducing the crystallite sizes, decreasing transfer impedance (R ct), and increasing Li-ion diffusion coefficient (D Li+). However, the galvanostatic charge–discharge results indicate that only Li2Fe0.8Mn0.2SiO4/C shows the improved performance; its initial discharge capacity can reach to 190.7 mAh g−1. All things considered, the increased impurities after Mn doping, decided by reference intensity ratio (RIR) method, seem to impose more negative effects on the Li2Fe1−x Mn x SiO4/C performances. Under this premises, the Mn-doped content is particularly important for Li2FeSiO4 materials prepared by the vacuum solid-state method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Li2FeSiO4 has such advantages as abundant natural resources, low cost, non-toxicity, environmentally friendly, safety, and high stability. Unfortunately, just like LiFePO4, Li2FeSiO4 also suffers from the inherent low electronic conductivity and the slow Li+ diffusion kinetics [1, 2]. To solve these problems, carbon doping [3–5] and cationic substitution [6] are the common methods widely used by researchers. The former is mainly conducted by the increased conductivity and decreased crystallite sizes, thus to effectively improve the electrochemical performances of cathode materials, while the latter could decrease Li+ transfer impedance and accelerate Li+ diffusion by causing lattice defects. When the added cations with radius most close to that of the substituted ions are employed, the distortion of crystal structure is slight enough to maintain crystal stability during long charge–discharge course, especially at high rates. Therefore, cationic substitution can impose more essential influences on modified materials. To date, various doping elements such as Mg [7], Al [8], Cr [9], Zn/Cu/Ni [10], V [11], and Mn [12–15] have been tried to improve the properties of Li2FeSiO4. In these studies, Mn doping attracts more attention. This is because: Mn has a close atomic radius with Fe (117 and 116.5 pm), and it has been already confirmed that Mn can be doped well into the crystal structure of Li2FeSiO4. Especially, Mn doping could purposely improve the low theoretical capacity of Li2FeSiO4 (∼166 mAh g−1), limited by the delithiated reaction from Fe2+ to Fe3+. Here, the typical Mn-doped reports are listed as follow: C. Deng et al. prepared Li2Fe1−x Mn x SiO4 (x = 0, 0.3, 0.5, 0.7, 1) nanoparticles using a citric acid assisted sol–gel method [12]. Mn doping resulted in the higher redox potentials and larger first discharge capacity than sole Li2FeSiO4 but sacrificed their cycling performances. By comparison, the highest discharge capacity (∼172 mAh g−1) was obtained when x was equal to 0.5. Similarly, B. Shao et al. synthesized Li2Fe x Mn1−x SiO4/C (0 ≤ x ≤ 0.8) nano-composite cathodes by a novel synthesis route, i.e., a combination of spray pyrolysis and wet ball milling followed by annealing, in which Li2Fe0.5Mn0.5SiO4/C has delivered the highest discharge capacity of 149 mAh g−1 and showed the best cycle ability [13]. However, for H. J. Guo's report in which a mechanical activation-solid-state method was employed, and the optimized ratio become 0.1 (Li2Fe0.9Mn0.1SiO4/C), which has an initial discharge capacity of 158.1 mAh g−1 and a capacity retention ratio of 94.3 % after 30 cycles [14]. Furthermore, the Li2Fe3/4Mn1/4SiO4/C, prepared by X. G. He et al. [15] by a modified sol–gel method, showed the best electrochemical performance, and the first discharge capacity at room temperature could reach to 201 mAh g−1. Note that, in these study, the best appropriate ratio of Mn doping seem to be confused, possibly due to the used different methods and synthetic procedures.
Here, to simulate practical production, based on a vacuum solid-state method having been employed in our previous studies [16–18], we prepared Li2Fe1−x Mn x SiO4/C composite materials with different amount of Mn doping under the same process conditions. The effects of Mn content on crystal structure, morphology, properties, and Li+ diffusion coefficient were discussed. This work could provide valuable references for actual applications of high-quality Li2FeSiO4 cathodes.
Experimental
According to the chemical formula of Li2Fe1−x Mn x SiO4 (x = 0, 0.1, 0.2, 0.3, 0.4, 0.5), the stoichiometric amount of analytical reagents, lithium acetate dehydrate, iron oxalate dehydrate, manganese acetate tetrahydrate, nano-silica, and 10 wt% mass of glucose monohydrate as carbon sources, were ground to fine powder together and then heated from room temperature to 700 °C for 10 h in a vacuum tube furnace keeping −0.1 MPa throughout. The final products were used as Li2Fe1−x Mn x SiO4/C materials with the designed Mn content.
The Li2Fe1−x Mn x SiO4 crystal structures were analyzed by powder X-ray diffraction (Ultima IV, Rigaku) employing Cu K α1 radiation (λ = 0.154056 nm) in the two-theta range of 10–80°. The morphologies were observed using a field emission scanning electron microscope (FESEM; JSM-7500 F, Japan JEOL). Electrochemical experiments were performed using CR2016-type coin cells. The mixtures of Li2Fe1−x Mn x SiO4/C, acetylene black, and polyvinylidene fluoride with a weight ratio of 80:10:10 were used as the cathode. The Li|LiPF6(EC + DMC)|Li2Fe1−x Mn x SiO4/C cells were fabricated in an Ar-filled glove box and measured on a 5-V/2-mA battery-testing system (Land CT2001A, China Wuhan Jinnuo Electronics Co., Ltd.). The charge–discharge cycles were carried out at room temperature with cut-off voltage of 1.5–4.5 V under a constant current density of 10 mAh g−1. The specific capacities were calculated based on the net mass of Li2Fe1−x Mn x SiO4. The AC impedance spectra were measured via an electrochemical workstation (CHI 604D, Shanghai CH Instruments, Inc.) in the frequency range from 0.1 Hz to 100 kHz with perturbation amplitude of 5 mV.
Results and discussion
XRD
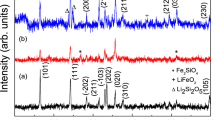
Figure 1 compares X-ray diffraction (XRD) patterns of Li2Fe1−x Mn x SiO4/C with different amount of Mn doping. The main characteristic peaks of all samples are similar and can be assigned to Li2FeSiO4 phase. All the samples have an orthorhombic structure (space group Pmn21) and similar lattice parameters. This result is consistent with the other reports [12, 14]. Furthermore, the semiquantitative XRD analysis with reference intensity ratio (RIR) method was used to roughly evaluate the samples' components. The obtained results, along with crystallite sizes calculated by Scherrer formula (D = Kλ/(B 0.5cosθ, where D denotes crystallite size, K is Scherrer constant, λ is X-ray wavelength, B 0.5 is the peak width at half height and θ is diffraction angle) were shown in Table 1. Compared with original Li2FeSiO4, Mn doping results in the reduced crystallite sizes. Moreover, the proportions of active materials in the Mn-doped samples obviously decrease along with the significant increased MnO and Li2SiO3 impurities, except for Li2Fe0.8Mn0.2SiO4 sample, whose main phase content is of 83.6 %, close to that of Li2FeSiO4 synthesized by the same method. This result proves that the manganese salt may actively participate in the thermal reaction, and to some extent, increase the complexity of solid-phase reaction.
SEM
Figure 2 shows the scanning electron microscopy (SEM) photographs of Li2Fe1−x Mn x SiO4/C with different amount of doped Mn. All the samples are made up of some irregular-shaped particles in various sizes, and the significant effect of Mn doping on the morphologies of final products are hardly ever observed. But it is obvious to notice that the particle sizes shown in Fig. 2 are relatively larger than crystallite sizes calculated by XRD data (∼40 μm) because our observations from SEM photographs are the aggregates of single crystalline grains. This difference usually means serious aggregates of primary particles in these samples, which can be mainly attributed to the employed solid-state method.
Electrochemical performance
Figure 3 shows the typical charge–discharge curves of Li2Fe1−x Mn x SiO4/Li coin cells at room temperature. By comparison, three key features should be noted.
First, with the increased Mn content, the charge plateau at 3.5 V shortens and that above 4.0 V increases gradually. That is to say that Mn doping leads to a higher proportion for the plateau-like charge curve above 4.0 V. This phenomenon can be presented as evidence that the doped Mn can be involved in the delithiated reaction during charging. However, the first charge curves of all tested samples are still higher than the subsequent cycles, demonstrating that Mn doping does not prevent from the structural rearrangements of Li2FeSiO4 mainly occurred in the first several cycles [2, 17, 19].
Second, compared with Li2FeSiO4/C material, only Li2Fe0.8Mn0.2SiO4/C shows higher specific capacity in all Mn-doped samples, and its initial discharge capacity can reach to 190.7 mAh g−1. For this result, the negative influence of more impurities (see “XRD”) can play a more important role.
Third, the Mn-doped cathodes (except for x = 0.1 sample) show significant capacity loss after 50 cycles. Taking Li2Fe0.8Mn0.2SiO4/C as an example, its discharge capacity at 50th cycle is down to 60 mAh g−1, and the retention is only 31.5 % as compared to that at the first discharge. Many researchers attributed it to the crystal instability, mainly caused by Jahn–Teller effect of Mn3+ [12–15]. Thus, Mn doping can improve specific capacity but in most cases at the expense of cycling performance.
EIS
Figure 4 shows typical Nyquist impedance spectra of Li2Fe1−x Mn x SiO4/Li coin cells after 2 cycles. The measured spectra all exhibit a semicircle in high frequency and a straight line in the low frequency. The high-frequency semicircle is related to the contact impedance between the electrode material and the electrolyte in the charge transfer process. The low-frequency straight line is associated with Warburg impedance of lithium ion diffusion in the electrode material. Furthermore, in the equivalent circuit shown at the top right corner in Fig. 4, R b represents the bulk resistance; R ct refers to the charge transfer resistance on the interface of electrode and electrolyte; Z w represents the Warburg impedance caused by lithium ion diffusion in solid-phase electrode; CPE is a constant phase element, informing the degree of deviation of impedance behavior from capacity behavior; C int represents the capacity reactance resulted from lithium ion accumulation and consumption in active materials.
From Table 2, we can see that the R b values of all the tested samples are lightly different. It is acceptable for this due to the electrolyte usage amount, electrode piece position, sealing force, etc. during the process of manual assembly of coin cells. Note that compared with that of Li2FeSiO4/C, the R ct of the Mn-doped materials decrease obviously, indicating that the Mn doping can lower the kinetic barriers of Li+ transfer, accelerate the delithiation procedure, increase the electrochemical reaction kinetics, and improve the electrochemical performances of Li2Fe1−x Mn x SiO4/C. In addition, the decreased C int values can be explained that the more effective Li+ transfer can buffer the charge carriers' accumulation and consumption in the local active materials.
The Li-ion diffusion coefficient (D Li+) in active electrode can be calculated from the low-frequency plots according to the following equation [20–22]:
where R is the gas constant, T is the absolute temperature, A is the surface area of the electrode, n is the number of electrons per molecule during oxidation, F is the Faraday constant, C is the concentration of lithium ion, and σ is the Warburg factor which is relative with Z′:
where B is a constant and ω is the frequency in low-frequency region. Therefore, σ can be decided by the slope of ω −0.5–Z′ curve, as shown in Fig. 5. The calculated σ and D Li+ are both listed in Table 2. Comparison shows that all the Mn-doped materials (except for x = 0.1 sample) have lightly increased D Li+ values, in which Li2Fe0.8Mn0.2SiO4/C sample shows a highest D Li+ value of 3.49 × 10−16 cm2 s−1. The results verify that the diffusion capability of lithium ion can be effectively improved by Mn doping.
Conclusion
Based on the above discussions, we can conclude that the Mn doping can bring some beneficial effects to improve the electrochemical performances of Li2Fe1−x Mn x SiO4/C products, such as reducing the crystallite sizes, decreasing the R ct value, lowering the kinetic barriers of Li+ transfer, increasing the D Li+ values, and so on. However, it also leads to a significant amount of impurities and serious aggregates in the final product, which is close related to the employed vacuum solid-state method. For this reason, the doped Mn content is more important to achieve the purpose of optimizing Li2FeSiO4 materials. Here, the Li2Fe0.8Mn0.2SiO4/C sample showed the highest initial discharge capacity of 190.7 mAh g−1, which could be attributed to its less impurities and higher D Li+ values. Regrettably, it still showed obvious capacity fading, whose discharge capacity at 50th cycle dropped to 60 mAh g−1 only with the capacity retention of 32.5 %. Therefore, future researches should be mainly focused on overcoming this defect, aiming at strengthening the crystal stability of the Mn-doped materials and improving their long-cycle performances.
References
Dominko R (2008) Li2MSiO4 (M = Fe and/or Mn) cathode materials. J Power Sources 184:462–468
Nytén A, Abouimrane A, Armand M, Gustafsson T, Thomas JO (2005) Electrochemical performance of Li2FeSiO4 as a new Li-battery cathode material. Electrochem Commun 7:156–160
Zhang S, Deng C, Yang SY (2009) Preparation of Nano-Li2FeSiO4 as Cathode Material for Lithium-Ion Batteries. Electrochem Solid-State Lett 12:A136–A139
Muraliganth T, Stroukoff KR, Manthiram A (2010) Microwave-Solvothermal Synthesis of Nanostructured Li2MSiO4/C (M = Mn and Fe) Cathodes for Lithium-Ion Batteries. Chem Mater 22:5754–5761
Dahbi M, Urbonaite S, Gustafsson T (2012) Combustion synthesis and electrochemical performance of Li2FeSiO4/C cathode material for lithium-ion batteries. J Power Sources 205:456–462
Islam MS, Dominko R, Masquelier C, Sirisopanaporn C, Armstrong AR, Bruce PG (2011) Silicate cathodes for lithium batteries: alternatives to phosphates? J Mater Chem 21:9811–9818
Zhang S, Deng C, Fu BL, Yang SY, Ma L (2010) Doping effects of magnesium on the electrochemical performance of Li2FeSiO4 for lithium ion batteries. J Electroanal Chem 644:150–154
Lan JY, Zhao MS, Wang YZ, Qiao YQ (2011) Effent of Al3+ doping on structure and electrochemical performance of Li2FeSiO4. Chin J Inorg Chem 27:1497–1502
Zhang S, Deng C, Fu BL, Yang SY, Ma L (2010) Effects of Cr doping on the electrochemical properties of Li2FeSiO4 cathode material for lithium-ion batteries. Electrochim Acta 55:8482–8489
Deng C, Zhang S, Yang SY, Fu BL, Ma L (2011) Synthesis and characterization of Li2Fe0.97M0.03SiO4 (M = Zn2+, Cu2+, Ni2+) cathode materials for lithium ion batteries. J Power Sources 196:386–392
Hao H, Wang J, Liu J, Huang T, Yu A (2012) Synthesis, characterization and electrochemical performance of Li2FeSiO4/C cathode materials doped by vanadium at Fe/Si sites for lithium ion batteries. J Power Sources 210:397–401
Deng C, Zhang S, Yang SY (2009) Effect of Mn substitution on the structural, morphological and electrochemical behaviors of Li2Fe1−xMnxSiO4 synthesized via citric acid assisted sol-gel method. J Alloys Compd 487:L18–L23
Shao B, Abe Y, Taniguchi I (2013) Synthesis and electrochemical characterization of Li2FexMn1−xSiO4/C (0 ≤ x ≤ 0.8) nanocomposite cathode for lithium-ion batteries. Powder Technol 235:1–8
Guo HJ, Cao X, Li XQ, Li LM, Li XH, Wang ZX, Peng WJ, Li QH (2010) Optimum synthesis of Li2Fe1-xMnxSiO4/C cathode for lithium ion batteries. Electrochim Acta 55:8036–8042
He XG, Yang GL, Sun LQ, Xie HM, Wang RS (2010) Research on modification of cathode material Li2FeSiO4/C for lithium-ion battery. Chem J Chin Univ 31:2148–2152
Gao K, Dai CS, Lv J, Li SD (2012) Thermal dynamics and optimization on solid-state reaction for synthesis of Li2MnSiO4 materials. J Power Sources 211:97–102
Gao K, Zhang J, Li SD (2013) Morphology and electrical properties of Li2FeSiO4/C prepared by a vacuum solid-state reaction. Mater Chem Phys 139:550–556
Gao K, Dai CS, Lv J, Feng XM (2013) Effects of Carbon contents on morphology and electrical properties of Li2MnSiO4/C prepared by a vacuum solid-state method. Russ J Electrochem. doi:10.1134/S1023193513050066
Dominko R, Bele M, Gaberscek M, Meden A, Remskar M, Jamnik J (2006) Structure and electrochemical performance of Li2MnSiO4 and Li2FeSiO4 as potential Li-battery cathode materials. Electrochem Commun 8:217–222
Yi TF, Xie Y, Zhu YR, Zhu RS, Ye MF (2012) High rate micron-sized niobium-doped LiMn1.5Ni0.5O4 as ultra high power positive-electrode material for lithium-ion batteries. J Power Sources 211:59–65
Yang JL, Kang XC, Hu L, Gong X, He DP, Peng T, Mu SC (2013) Synthesis and electrochemical performance of Li2FeSiO4/C/carbon nanosphere composite cathode materials for lithium ion batteries. J Alloys Compd 572:158–162
Liu H, Cao Q, Fu LJ, Li C, Wu YP, Wu HQ (2006) Doping effects of zinc on LiFePO4 cathode material for lithium ion batteries. Electrochem Commun 8:1553–1557
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (NSFC) (grant no. 21003087), Research Fund for the Doctoral Program of Higher Education (grant no.20101404120001), and China Postdoctoral Science Foundation (grant no. 20110491051).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, K. Effect of Mn doping on electrochemical properties of Li2FeSiO4/C cathode materials based on a vacuum solid-state method. Ionics 20, 809–815 (2014). https://doi.org/10.1007/s11581-013-1043-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-013-1043-6