Abstract
The carbon microtubules core structure LiFePO4 is synthesized using a cotton fiber template-assisted method. The crystalline structure and morphology of the product is characterized by X-ray diffraction and field emission scanning electron microscopy. The charge–discharge kinetics of the LiFePO4 electrode is investigated using cyclic voltammetry and electrochemical impedance spectroscopy. The result shows that the well-crystallized carbon microtubules core structure LiFePO4 is successfully synthesized. The as-synthesized material exhibits a high initial discharge capacity of 167 mAh g−1 at 0.2 C rate. The material also shows good high-rate discharge performance and cycling stability, about 127 mAh g−1 and 94.7 % capacity retention after 100 cycles even at 5.0 C rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the pioneering work of Padhi and coworkers [1] in 1997, the olivine structure lithium iron phosphate (LiFePO4) has been recognized as a promising cathode material for lithium-ion batteries. Compared with other cathode materials, LiFePO4 exhibits the advantages of high theory capacity (~170 mAh g−1), excellent thermal stability, no toxicity, low cost, and high reversibility, and so on. However, its intrinsic low electronic conductivity (~10−9 S cm−1) and low Li-ions diffusion coefficient (~10−14 cm2 s−1) limit the rate capability and then its application. Many efforts have been tried to solve these problems, such as coating some conductive materials on the surface of the particles, doping some cations or anions into the structure, and minimizing the particle size, etc. [2–6].
LiFePO4 usually be prepared by using sol–gel, hydrothermal route, carbon thermal reduction, and rheological method [7–11], and some groups also have been synthesized by using various morphology cathode materials through a template-assisted method, and some very interesting results are obtained [12–14].
Cotton fiber, a kind of natural fiber, is mainly composed of cellulose. The cotton fiber is very suitable for template because it, without having being removed after chemistry reaction, is very cheap and environmentally friendly. In fact, cotton fiber has been used as a template to synthesize metal oxide microtubules such as biomorphic SnO2 microtubules [15] and TiO2 hollow fibers [16]. Therefore, the cheap cotton fiber was chosen as the template to synthesize the carbon microtubules core structure LiFePO4 in the experiment. The structure, morphology, and electrochemical performances are studied in detail.
Experiments
Preparation of the carbon microtubules core structure LiFePO4
The carbon microtubules core structure LiFePO4 was synthesized by using a template-assisted method (cotton fiber as the template). All the reagents used in the experiment were in analytical purity and used without further purification. FePO4·2H2O and Li2CO3 were chosen as starting materials to prepare the precursor and oxalic acid as both complexant and reductant. The stoichiometric amount of FePO4·2H2O, Li2CO3, and oxalic acid were dissolved in an appropriate quantity of distilled water with continuous stirring at 90 °C, until a light green clear sol was obtained. Then, an appropriate amount of cotton fiber was added into the green sol and stirred continually at 90 °C for 10 h. After washing and filtering, the precursor was sintered at 350 °C for 5 h and at 700 °C for 10 h, respectively, under N2 atmosphere to prevent the formation of Fe3+ impurities.
For comparison, we also prepared LiFePO4/C using a conventional sol–gel method with the same reagents used above, and the sintering process was also the same as above.
To evaluate the carbon content in the prepared composites, a simple method was used as described in articles [17, 18]. The sample was heated to 700 °C at a rate of 5 °C/min for 6 h in air to burn off the residual carbon. At the same time, the LiFePO4 will be oxidized to Li3Fe2(PO4)3 and Fe2O3 (the weight will be increased by 5.1 %). So, the amount of carbon content can be calculated as follows:
where c is the carbon content, and m and m′ are the weight of the sample before and after heat treatment. Based on Eq. (1), we can calculate the carbon content of the prepared carbon microtubules core structure LiFePO4 to be about 13.2 %.
Characterization of the carbon microtubules core structure LiFePO4
The crystalline phase was analyzed by X-ray diffraction (XRD, RINT-2500V, Rigaku Co.) with Cu Kα radiation (λ = 1.5418 Å) in the range of 15° ≤ 2θ ≤ 75° with a scanning rate of 2° per min. The particle morphologies of the prepared powders were determined by field emission scanning electron microscopy (FESEM, Supra 55VP).
Cell assembly and electrochemical tests
Electrochemical performances of the prepared LiFePO4 were investigated using CR2025 coin cell. The cathode was fabricated from a mixture of the as-synthesized powders, acetylene black, and polyvinylidene fluoride with a weight ratio of 80:15:5, and the electrolyte was 1 M LiPF6 in a mixture of ethyl carbonate, diethyl carbonate, and dimethyl carbonate (volume ratio 1:1:1). The slurry was then cast on an aluminum foil and dried at 120 °C overnight under a vacuum. The cells (CR2025) were assembled in an argon-filled glove box using a lithium metal foil as the counter electrode and microporous polypropylene sheet (Celgard 2400, Celgard, Inc., USA) as the separator. The cells were charged and discharged between 2.3 and 4.3 V on a charge/discharge apparatus (BTS-51, Neware, China). Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were conducted by using the CHI650 electrochemical working station.
Results and discussion
Material characterizations
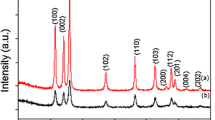
Figure 1 shows the XRD pattern of the carbon microtubules core structure LiFePO4 (sample) which was prepared by using a template-assisted method. The strong and narrow diffraction peaks of the sample indicate that the orthorhombic olivine structure LiFePO4 (JCPDS 83–2092) is successfully synthesized [19] in the experiment. All the diffraction peaks can be indexed on the basis of the orthorhombic olivine-type structure with the Pnma space group. In addition, there are no any other peaks such as carbon diffraction peaks found, indicating that the cotton fiber did not destroy the lattice of LiFePO4, and the generated carbon may exist in an amorphous state.
Since the morphology and particle sizes of LiFePO4 have a great influence on their electrochemical performance, FESEM and TEM analyses of the prepared carbon microtubules core structure LiFePO4 were carried out. The FESEM and TEM images are shown in Fig. 2. A carbon microtubules core structure is clearly shown in Fig. 2a, b. The diameter of the carbon microtubule is about 6 μm, the wall thickness is about 0.8 μm, and the LiFePO4 particles (about 50~100 nm) are distributed on the surface of the carbon microtubules. Such a structure is favorable for Li+ ions diffusion and electrons transport during the charge and discharge process. Because the electrolyte is able to penetrate into the carbon microtubules, reduce the diffusion distance of Li+ ions, and increase the contact area between cathode material and electrolyte, these are all the benefits for Li+ ions diffusion. At the same time, the carbon microtubules can provide a channel for electrons transport, decreasing the cell resistance and improving the electron conductivity of the cathode material, as shown in Fig. 3. The electrons will transport along the wall of the carbon microtubules easily, and the Li+ ions will migrate directly into the electrolyte in charge process.
Electrochemical properties
The initial charge–discharge curves of the as-synthesized LiFePO4 and the contrast sample between 2.3 and 4.3 V at 0.2 C (1.0 C = 150 mA/g) rate are shown in Fig. 4 (the obtained charge or discharge capacity is calculated based on LiFePO4 not including the amorphous carbon). The two samples are all shown as a long and flat voltage plateau at 3.397~3.459 and 3.396~3.464 V (according to the initial discharge capacity of 167 and 156 mAh g−1, respectively), which is the main characteristic of the two-phase reaction of LiFePO4. The polarization potential of the as-synthesized samples and contrast sample is 62 and 68 mV, respectively (insert of Fig. 4), indicating that the kinetics of the as-synthesized samples is indeed improved. Especially, the polarization potential of the as-synthesized samples is only 62 mV, which is smaller than the previous reports [20, 21], indicating a higher reaction kinetics of the as-synthesized LiFePO4 and better electrochemical reversibility.
Figure 5 shows the discharge curves and the rate performance of the as-synthesized LiFePO4 at 0.2, 0.5, 1.0, 2.0, and 5.0 C rate under room temperature. It was seen that with the current density increasing gradually, the discharge capacity of the LiFePO4 also decreases. The discharge capacities are 167, 156, 150, 144, and 136 mAh g−1 at 0.2, 0.5, 1.0, 2.0, and 5.0 C rate, respectively. At the same time, when recovering the former testing rate (1.0 C rate in the experiment), the discharge capacity which almost has no any decrease indicates the good cyclic reversibility of the electrode. In addition, as the discharge current increases, the discharge capacity gradually decreases, but the electrode still maintains a long and flat voltage plateau as shown in Fig. 5. This phenomenon is directly related to the short Li+ ions diffusion distance and high electrons conductivity during charge–discharge progress and then the good cyclic reversibility.
In addition, the cycle performance of the as-synthesized sample was also measured. The cycling curves at 5.0 C rate under room temperature are shown in Fig. 6. The discharge capacities are 127 mAh g−1 after 100 cycles. The discharge capacity retentions are about 94.7 %. It shows good cycling stability, and the electrode shows only a capacity fading of 0.05 % per cycle. It is also attributed to the short Li+ ions diffusion distance and high electrons conductivity in the carbon microtubules core structure LiFePO4, and then the low irreversible capacity loss, to good cycling stability even at a high rate.
CV measurements
CV is a well-suited technique to evaluate the cathode performance and electrode kinetics. Figure 7 shows the CV profiles of the as-synthesized LiFePO4 electrode between 2.3 and 4.3 V at different scanning rates (0.1, 0.2, 0.5, and 1.0 mV s−1, respectively). A couple of redox peaks is observed at 3.328 and 3.535 V in the CV curve obtained at the scanning rate of 0.1 mV s−1. These peaks correspond to the extraction and insertion of lithium ions in the electrode. As the scanning rate is raised, the oxidation peak current shifts to a higher and the reduction peak current shifts to lower, indicating the increased kinetic polarization and increased internal resistance [22]. Because the peak current I p is proportional to the square root of the scan rate v 1/2 (as shown in Fig. 8), so I p can be expressed by the classical Randles–Sevchik equation:
where n is the number of electrons per species reaction, A is the interface between the electrolyte and the active material, C is the concentration of Li+ ions, D is the diffusion coefficient of Li+ ions, and v is the potential scan rate. Based on Eq. (2) and the scan rate, peak current, the apparent diffusion coefficient of Li+ ions can be calculated to be at an order of 10−11 cm2 S−1 for both charging and discharging electrodes. The obtained Li+ ions diffusion coefficient by CV curves is three orders of magnitude larger than the pure LiFePO4 (~10−14 cm2 S−1). However, the value of lithium-ion diffusion coefficient D obtained by CV is not accurate enough in such a two-phase reaction system, and the value of D may be overestimated, because the liquid electrolyte could penetrate into the carbon microtubules core structure LiFePO4 during charge and discharge process [23–25] as discussed above.
EIS measurements
To further understand the kinetics process of the electrode materials, EIS measurements were carried out in the two-electrode coin cell CR2025. The Nyquist plot of the as-synthesized LiFePO4 sample was presented in Fig. 9. The semicircle at high-to-medium frequency is attributed to the charge transfer resistance (R ct), and the inclined line at low frequency represents Li+ ions diffusion resistance in electrode bulk, namely, the Warburg impedance. The numerical value of diameter of the semicircle (at high-to-medium frequency) on the real axis is approximately equal to R ct [26]. As shown in Fig. 9, the as-synthesized LiFePO4 electrode exhibits the R ct as small as about 15 Ω, indicating the low electrochemical polarization, fast electrode/electrolyte interface reaction, and lithium diffusion processes. The low electrochemical resistances are attributed to the special structure of the as-synthesized LiFePO4 material, which is favorable for Li+ ions diffusion and electrons transport.
Conclusion
The carbon microtubules core structure LiFePO4 was synthesized by using a cotton fiber template-assisted method. XRD and FESEM analysis indicate that the well-crystallized carbon microtubules core structure LiFePO4 was successfully prepared. The charge–discharge tests show that the prepared composite has good electrochemical performances as follows:
-
1.
High discharge capacity (the initial discharge capacity is 167 mAh g−1 at 0.2 C rate)
-
2.
Good rate performance (167, 156, 150, 144, and 136 mAh g−1 at 0.2, 0.5, 1.0, 2.0, and 5.0 C rate, respectively)
-
3.
Good high rate cycling stability (retained about 94.7 % of its initial capacity after 100 cycles even at 5.0 C rate)
These performances attribute to the special structure, which is favorable for Li+ ions diffusion (shortening the diffusion distance of Li + ions) and electrons transport (providing the channel for electrons transport). The results demonstrated that a cotton fiber template-assisted method is a promising approach to prepare high-performance LiFePO4 composite for lithium-ion batteries.
References
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) J Electrochem Soc 144:1188–1194
Shin HC, Nam KW, Chang WY (2011) Electrochim Acta 56:1182–1189
Zhang W, Hu Y, Tao X, Huang H, Gan YP, Wang CT (2010) J Phys Chem Solids 71:1196–1200
Zhang Q, Wang SM, Zhou ZF, Ma GL, Jiang WW, Guo XS, Zhao S (2011) Solid State Ionics 191:40–44
Liu H, Feng Y, Wang Z, Wang K, Xie JG (2008) Powder Technol 184:313–317
Zhao L, Hu YS, Li H, Wang ZX, Chen LQ (2011) Adv Mater 23:1385–1388
Liu YY, Cao CB, Li J (2010) Electrochim Acta 55:3921–3926
Gaberscek M, Dominko R, Bele M, Remskar M, Hanzel D, Jamnik J (2005) Solid State Ionics 176:1801–1805
Liang GC, Wang L, Ou X, Zhao X, Xu SZ (2008) J Power Sources 184:538–542
Wang LN, Zhang ZG, Zhang KL (2007) J Power Sources 167:200–205
Zhong M, Zhou ZT (2010) Solid State Ionics 181:1607–1610
Liu XH, Wang JQ, Zhang JY, Yang SR (2006) Chin J Chem Phys 19:530–534
Sides CR, Croce F, Young VY, Martin CR, Scrosati B (2005) Electrochem Solid-State Lett 8:A484–A487
Yang ST, Yue HY, Yin YH, Yang JX, Yang WG (2006) Electrochim Acta 51:4971–4976
Sun B, Fan TX, Xu JQ, Zhang D (2005) Mater Lett 59:2325–2328
Su BT, Wang K, Hu CL, Mu HM, Dong N, Tong YC, Bai J (2008) Chem Regents 30:112–114
Beninati S, Damen L, Mastragostino M (2008) J Power Sources 180:875–879
Belharouak I, Johnson C, Amine K (2005) Electrochem Commun 7:983–988
Yao JW, Wu F, Qiu XP, Li N, Su YF (2011) Electrochim Acta 56:5587–5592
Ge YC, Yan XD, Liu J, Zhang XF, Wang JW, He XG, Wang RS, Xie HM (2010) Electrochim Acta 55:5886–5890
Wang XC, Huang YD, Jia DZ, Guo ZP, Miao M (2012) Preparation and characterization of high-rate and long-cycle LiFePO4/C nanocomposite as cathode material for lithium-ion battery. J Solid State Chem 16(1):17–24. doi:10.1007/s10008-010-1269-4
Lee KL, Jung JY, Lee SW, Moon HS, Park JW (2004) J Power Sources 130:241–246
Fey GTK, Huang KP, Kao HM, Li WH (2011) J Power Sources 196:2810–2818
Xie J, Imanishi N, Zhang T, Hirano A, Takeda Y, Yamamoto O (2009) Electrochim Acta 54:4631–4637
Tang K, Yu XQ, Sun JP, Li H, Huang XJ (2011) Electrochim Acta 56:4869–4875
Lu F, Zhou YC, Liu J, Pan Y (2011) Electrochim Acta 56:8833–8838
Acknowledgments
This work was support by the Science and Technology program of Urumqi, China (grant no. K111410005), the “West Light” Talents Cultivation Program of Chinese Academy of Sciences (grant no. XBBS200919), and the Main Direction Program of the Knowledge Innovation Program of Chinese Academy of Sciences (grant no. KGCX2-YW-359).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wumair, T., Dou, J., Zhang, L. et al. Synthesis of carbon microtubules core structure LiFePO4 via a template-assisted method. Ionics 19, 1855–1860 (2013). https://doi.org/10.1007/s11581-013-0916-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-013-0916-z