Abstract
Nanosized LiNi0.5Mn1.5O4 spinels with the uniform average particle size of about 80–100 nm were synthesized by a high-oxidation-state manganese sol–gel method. It indicates that the resulting nanosized LiNi0.5Mn1.5O4 materials not only have a phase-pure cubic spinel structure without any impurity but also have a good dispersion even without any physical treatment. Besides, the cell based upon the resulting nanosized LiNi0.5Mn1.5O4 materials shows good cycle stability and ratio performance. It suggests that the novel method would be helpful for the synthesis and application of LiNi0.5Mn1.5O4 cathode.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the highest gravimetric and volumetric energy densities, lithium-ion batteries have become one of the most important commercially produced rechargeable batteries. Spinel LiMn2O4 has appeared as an important electrode material in lithium-ion batteries because it is low-cost and environmentally benign during the past decade. Though the use of spinel as positive electrode was reported in 1983 [1], the importance of the spinel compound has been realized only after the commercial Li battery using LiMn2O4 as active material [2]. One of the important issues regarding the use of spinels as cathode materials is their large capacity loss observed during storage and cycling at elevated temperatures [3]. Self-discharge above 55 °C and electrolyte decomposition at high voltages catalyzed by the electrode surface drastically affect cathode performance [4]. Also, capacity fade upon cycling depends largely on Mn dissolution through the following reaction due to the presence of trace acids, especially HF: 2Mn3+ → Mn4+ + Mn2+ (with Mn2+ ions going into the solution) [5, 6].
Coupled with developments of electric vehicles (EVs) and hybrid electric vehicles (HEVs), there is an increased demand for lithium-ion batteries with better performance for higher energy and voltage in recent years. The development of 5-V cathode materials that can provide stable cycling performance at high voltage is of great importance for power battery applications. Recently, a Ni-doped spinel compound of LiMn2O4, formulated LiNi0.5Mn1.5O4, has been reported as a promising candidate for lithium-ion batteries. First, LiNi0.5Mn1.5O4 spinel shows not only a high specific capacity but also an excellent cycling stability [7, 8]. The Mn4+ ions in LiNi0.5Mn1.5O4 have restrained the Mn dissolution into the electrolyte effectively, compared to LiMn2O4 in which 50 % of Mn element exists as Mn3+ ions leading to a huge Mn dissolution [2]. Second, LiNi0.5Mn1.5O4 shows a high-rate capability offered by the three-dimensional Li+ ion diffusion paths in the spinel lattice [2]. Third, LiNi0.5Mn1.5O4 has received special interest as a 5-V cathode material due to its dominant plateau at around 4.8 V [7, 8]. So, lithium-ion batteries with LiNi0.5Mn1.5O4-based cathode electrodes are expected to provide high power/energy densities for EVs, HEVs, and the other large energy storage systems.
At present, LiNi0.5Mn1.5O4 materials are usually synthesized by a calcination technology. The lack of oxygen to the LiNi0.5Mn1.5O4 material will typically occur at high temperatures during this process, which is detrimental to the formation of Mn4+ ions. It has been reported that LiNi0.5Mn1.5O4 would disproportionate to a spinel with a smaller NiO and LiyNi1−yO compounds, which leads to a decrease in the nickel content in the spinel phase and a consequent introduction of some Mn3+ ions caused by the lack of oxygen. The presence of Mn3+ ions in LiNi0.5Mn1.5O4 causes the development of a 4-V plateau and a decrease of 5-V capacities [7], and it further causes the Mn dissolution, the structure distortion, and the capacity fade upon cycling [9]. An approach to addressing this problem is that the precursor should stay at about 450 °C for 3 ~ 8 h to get high-oxidation-state manganese for reducing the negative effect of the lack of oxygen at high temperatures in the industrial production process. This synthetic method is complicated and time-consuming in practical production. It implies that a high-oxidation-state manganese compound as raw material precursor, which is good for the simplification of low-temperature oxidation process, would have a positive effect on the synthesis of LiNi0.5Mn1.5O4 material. Many high-oxidation-state manganese compounds such as trimanganese tetroxide (Mn3O4) and manganese dioxide (MnO2) have been used as a precursor of raw material, and the performance of the resulting LiNi0.5Mn1.5O4 has been improved [10], but the compound of whether Mn3O4 or MnO2 is a solid phase leads to problems in achieving mixing uniformity of particles.
The sol–gel method is recognized as an effective production technique in homogeneously mixing all reagents at an atomic or molecular level, extremely allowing precise control of the stoichiometric amount and final particle size [11, 12]. However, it would be hard to find or prepare an appropriate solution with a high oxidation state of manganese ions because it has got to meet the requirements as follows: (a) having any other metal ions to avoid impurities, (b) being without strong oxidizing and corrosive properties, and (c) in possession of easy preparation method and sufficient stability. Thus, the search for an alternative and novel method for the preparation of an appropriate solution with a high oxidation state of manganese ions is necessary to synthesize LiNi0.5Mn1.5O4 materials with good performances.
Besides, it is well known that larger particles with longer Li+ diffusion distance are hampered for high rate capability and cyclability [2, 11]. The nanosized positive materials can bring both high surface area and short diffusion distance during the charge–discharge process to improve the cycling performance and rate capability [2].
In this paper, a novel and simple method was used to prepare an appropriate solution with a high oxidation state of manganese ions. And based on the solution, nanosized LiNi0.5Mn1.5O4 spinels with the average particle size of about 80–100 nm were synthesized by a high-oxidation-state manganese sol–gel method. By that novel and simple method, the drawbacks which are the lack of oxygen to LiNi0.5Mn1.5O4 materials and the difficulty in homogeneously mixing all reagents at an atomic or molecular level would be solved simultaneously. Moreover, to present thorough discussions on the positive role of the novel and simple synthesis method, the nanoplate-shaped LiNi0.5Mn1.5O4 materials would also be synthesized by a co-precipitation method as a frame of reference.
Experimental
Nanosized granular LiNi0.5Mn1.5O4 spinel synthesized by a high-oxidation-state manganese sol–gel method
The chemicals used were of analytical grade. Both 3.6764 g (15 mmol) manganese acetate (Mn(Ac)2 · 4H2O) and 0.6895 g (10 mmol) lithium nitrate (LiNO3) were dissolved in a solvent of 100 mL ethanol. After the addition of 5 mL of NH3 · H2O (25–28 wt%), the air was blown into the homogeneous solution continuously. And some time later, a homogeneous black solution with a high oxidation state of manganese ions would be obtained. Then, the resulting black solution was heated to 80 °C under normal stirring conditions. After about 20 mins, the excess ammonia was driven away, and an ethanol solution within 1.4541 g (5 mmol) of nickel nitrate (Ni(NO3)2 · 6H2O) was added into the black solution. The evaporation stage at 80 °C was continued until the formation of a black gelatinous precursor. The resulting precursor was dried at 90 °C for 5 h and then heated at 750 °C for 15 h in the air. Finally, the product of nanoscale granular LiNi0.5Mn1.5O4 materials would be obtained.
In order to study the main technical properties of the resulting gelatinous precursor, a small part of the resulting precursor was redissolved into 40 mL of ethanol. After the slow addition of about 100 mL of water, a black precipitate would gradually generate. The resulting precipitate was centrifuged and dried for analysis.
Nanoplate-shaped LiNi0.5Mn1.5O4 spinel synthesized by a coprecipitation method as a frame of reference
Under well-stirred conditions, the solution of Na2CO3 was added slowly into the solution consisting of starting materials of MnCl2 · 4H2O and NiCl2 · 6H2O in deionized water with a mole ratio of 4.1:3:1 to precipitate the precursor of Ni0.5Mn1.5 (CO3)2 · xH2O powders. Followed by a preheating treatment at 480 °C for 6 h in the air, NiMnO3 · Mn2O3 powders were obtained to improve the oxidation state of manganese ions for reducing the negative effect of the lack of oxygen at high temperatures [13]. Li2CO3 and the oxide were uniformly mixed in a molar ratio of Li/Ni/Mn = 1.0:0.5:1.5 and then calcined at 850 °C for 12 h in the air. Finally, the products were recalcined at 600 °C for 6 h in the air to decrease the oxygen deficiency and get the product of the LiNi0.5Mn1.5O4 materials.
Cell preparation
The resulting cathode materials were mixed with carbon black and poly(vinylidene fluoride) at a weight ratio of 84:8:8 in N-methyl pyrrolidinone to prepare slurry. The slurry was coated onto a stainless steel current collector and dried at 100 °C for 2 h in a vacuum oven to prepare the cathode electrode.
Experimental cells were assembled in an argon atmosphere glove box using a lithium sheet as the anode material, the above-prepared electrode as the cathode material, the most typical 5-V electrolyte system for 1.0 mol/L LiPF6-ethylene carbonate/dimethyl carbonate (1:1, by volume) as the electrolyte material, and a Celgard (2400) porous polypropylene as the separator material.
Measurements Crystal
structure of the black precipitate obtained by the hydrolysis of the precursor and the resulting cathode materials were analyzed by a X-ray diffraction (XRD, Rigaku, D/Max-2400) measurement with Cu-Kα radiation (40 kV, 150 mA, step size = 0.02°/s). The morphologies of the cathode materials were observed on a scanning electronic microscopy (SEM, Hitachi, S-4800). Electrochemical property tests of cells were carried out on a Land cell tester CT2001A (Wuhan, China) in the voltage range of 3.5–5 V at room temperature.
Results and discussion
The main technical properties of the resulting precursor
To study the main technical properties of the resulting gelatinous precursor, a small part of the resulting precursor has been redissolved into 40 mL of ethanol. The resulting black solution system is homogeneous and stable in the air. But this system would turn into a heterogeneous mixture with the addition of enough water. The production of precipitate implies that the solution before hydrolysis may consist of manganese complex which is unstable in water. The precipitate obtained by the hydrolysis was studied by the XRD measurement after the centrifugal separation and the drying operation, as shown in Fig. 1. The low and broad diffraction peaks were detected at 2θ = 18° (002) and 60° (224) index to β-MnOOH (JCPDS no. 18–0804), and the other two broad diffraction peaks were detected at 2θ = 37° (211) and 65.7° (002) index to α-MnO2 (JCPDS no. 44–0141) [14]. It indicates that both the precursor and precipitate contain some high-oxidation-state manganese compounds, which is well consistent with our speculation.
XRD patterns of the LiNi0.5Mn1.5O4 materials
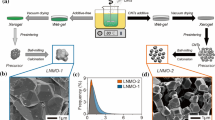
Figure 2 shows the XRD patterns of the LiNi0.5Mn1.5O4 materials [15], prepared by the high-oxidation-state manganese sol–gel method (Fig. 2(a)) and the coprecipitation method (Fig. 2(b)), respectively. It is obvious that the former has a phase-pure cubic spinel structure without any impurity, and the latter has some impurities as NiO (2θ = 37.5°, 43.8°, and 63.8°) and LiyNi1−yO (2θ = 44°) [16–18]. It indicates that LiNi0.5Mn1.5O4 materials may contribute a disproportionate spinel with a smaller NiO and LiyNi1−yO compounds at high temperature as 850 °C, though it has been recalcined at 600 °C for 6 h. But an elevated temperature is necessary for the reaction between NiMnO3 · Mn2O3 and Li2CO3 materials, due to the fact that NiMnO3 · Mn2O3 and Li2CO3 materials are mixed far from an atomic or molecular level. The presence of NiO and LiyNi1−yO compounds will lead to a decrease in the nickel content in the spinel phase of LiNi0.5Mn1.5O4 and a consequent introduction of some Mn3+ ions caused by the lack of oxygen which has negative effects on the electrochemical performance of LiNi0.5Mn1.5O4 cells. In comparison with that, our precursor could be used directly to prepare single-phase LiNi0.5Mn1.5O4 materials at low temperature with a simple method without the preheating and the annealing treatments.
SEM images
The SEM images of granular LiNi0.5Mn1.5O4 materials prepared by two different methods are shown in Fig. 3. Although we did not pay any physical treatment to disperse the powders before the SEM measurements, a good dispersion is manifested for the sample prepared by the high-oxidation-state manganese sol–gel method. Under a high magnification ratio, it is obvious that the sample prepared by the high-oxidation-state manganese sol–gel method is made up of many three-dimensional nanoscale particles, with the average particle size of about 80–100 nm (Fig. 3a–c). But the image of nanoplate-shaped LiNi0.5Mn1.5O4 materials prepared by the co-precipitation method is very different from this. As shown in Fig. 3d–f, the sample prepared by the coprecipitation method is made up of many fine crystalline nanoplate shapes with the average grain size of about 50-nm-thick basal planes and about 300-nm dimensions. However, many nanoplate shapes have regularly gathered together to form a micron-scale spherical LiNi0.5Mn1.5O4 particle, which would decrease the specific surface area sharply. The outcome of a smaller specific surface area will lead to a decrease of the interface of electrode material and electrolyte, which will consequently do harm to the diffusion of Li+ ions to reduce cycling performance and rate capability [2, 19].
Electrochemical properties of the LiNi0.5Mn1.5O4/Li half cells
Figure 4 shows the cycle performance of LiNi0.5Mn1.5O4/Li cells with 0.5C of discharge rate at room temperature. It is obvious that the capacity of the cell with coprecipitation synthesizing LiNi0.5Mn1.5O4 decreases extremely fast, which initially delivers 123 mAh g−1 and only retains 89.4 mAh g−1 (72.7 %) at the 250th cycle. The cell with the high-oxidation-state manganese sol–gel synthesizing LiNi0.5Mn1.5O4 showed good cycle performance, which initially delivers 128 mAh g−1 and retains 108 mAh g−1 (84.4 %) at the 250th cycle, and benefited from the small polarization resistance, short diffusion distance, and good ionic conductivity of the cathode materials with three-dimensional nanoscale structure [2].
The first charge–discharge curves of LiNi0.5Mn1.5O4-based cells were shown in Fig. 5. Each of the cells exhibits obvious plateau at about 4.7 V vs. Li/Li+, which is attributed to the Ni2+/Ni4+ redox couple [20]. However, the one with the high-oxidation-state manganese sol–gel synthesizing LiNi0.5Mn1.5O4 shows a higher 5-V capacity than the other. The former delivers 83 % of the total discharge capacity, while the latter only delivers 70 % above 4.6 V. Besides, though the Mn3+ ion quantity could weaken by the improved synthesis method, it failed to completely eliminate the presence of Mn3+ ions due to the fact that a small 4.1-V plateau is still consequently introduced [20–22].
Mean voltage, the voltage at half time of the cells’ running, reflects how long the cells can run under normal work voltage. As shown in Fig. 6, it is obvious that the cell with the high-oxidation-state manganese sol–gel synthesizing LiNi0.5Mn1.5O4 has higher mean voltage (about 4.62 V at the first cycle) than that of the cell with coprecipitation synthesizing LiNi0.5Mn1.5O4 (about 4.55 V at the first cycle). The reason may be due to the fact that the electrode polarization resistance of the cell with the high-oxidation-state manganese sol–gel synthesizing LiNi0.5Mn1.5O4 is lower than that of the cell with coprecipitation synthesizing LiNi0.5Mn1.5O4. More than that, the presence of a few Mn3+ ions as well as Ni3+ ions usually leads to a 4.0-V platform at the discharge curve, which would also consequently lead to take a drop in the mean voltage.
The discharge capacities of LiNi0.5Mn1.5O4/Li cells with different discharge rates at room temperature are shown in Fig. 7. With the increase of the discharge rate, the discharge capacity decreases owing to the increased current which results in a quick increase of polarization resistance and decrease of the voltage. However, in our experiment, the cutoff voltage was still set at 3.5 V, at which the discharge is not completed owing to inadequate utilization of the active ingredients. Benefiting from the nanoscale materials which could bring both high surface area and short diffusion distance during the charge–discharge process, the rate capability of every cell is improved [10]. Moreover, the cell with the nanoscale LiNi0.5Mn1.5O4 which is prepared by the high-oxidation-state manganese sol–gel method shows better current discharge capability retentions. As shown in Fig. 7, the nanoscale granular LiNi0.5Mn1.5O4 spinels exhibits discharge capacities of 131 and 118 mAh g−1 at 0.2C and 2C rates, respectively, whereas the nanoplate-shaped spinel cathode prepared by a coprecipitation method exhibits 126 and 96 mAh g−1 at those respective rates, for the similar reasons discussed above.
Conclusions
The development of LiNi0.5Mn1.5O4 cathode materials that can provide stable cycling performance at high voltage is of great importance for power battery applications. In this paper, nanosized LiNi0.5Mn1.5O4 spinels were synthesized by a high-oxidation-state manganese sol–gel method. By that novel and simple method, both the lack of oxygen to the LiNi0.5Mn1.5O4 material and the difficulty in homogeneously mixing all reagents at an atomic or molecular level would be solved simultaneously. The resulting nanosized LiNi0.5Mn1.5O4 materials not only have a phase-pure cubic spinel structure without any impurity but also have a good dispersion with the uniform average particle size of about 80–100 nm, even without any physical treatment. Besides, cells based upon the resulting nanosized LiNi0.5Mn1.5O4 materials show good cycle stabilities and ratio performances. It suggests that this novel method would be helpful for the synthesis and application of LiNi0.5Mn1.5O4 cathode.
References
MM Thackeray, WIF David, PG Bruce, JB Goodenough (1983) Mater. Res. Bull. 18:461
R Pitchai, V Thavasi, S G Mhaisalkar, S Ramakrishna (2011) J. Mater. Chem. 21:11040
A Blyr, C Sigala, G Amatucci, D Guyomard, Y Chabre, JM Tarascon (1998) J. Electrochem. Soc. 145:194
Deng D, Kim MG, Lee JY, Cho J (2009) Energy Environ. Sci 2:818
D Aurbach, Y Gofer (1991) J. Electrochem. Soc. 138:3529
D Aurbach, A Zaban, A Schechter, Y Eineli, E Zinigrad, B Markovsky (1995) J. Electrochem. Soc. 142:2873
Q Zhong, A Bonakdarpour, M Zhang, Y Gao, JR Dahn (1997) J. Electrochem. Soc. 144:205
K Amine, H Tukamoto, H Yasuda, Y Fujita (1996) J. Electrochem. Soc. 143:1607
J Arrebola, A Caballero, L Hernán, J Morales, ER Castellón, JR Barrado (2007) J. Electrochem. Soc. 154:178
M Jo, YK Lee, KM Kim, J Cho (2010) J. Electrochem. Soc. 157:841
Liu ZQ, Wang WL, Liu XM, Wu MC, Li D, Zeng Z (2004) J Solid State Chem 177:1585
Guo HJ, Li XH, Wang ZX, Peng WJ, Cao X, Li HF (2009) J Power Sources 189:95
Gao J, Li JJ, Jiang CY, Wan CR (2010) J Electrochem Soc 157:899
Xu MW, Kong LB, Zhou WJ, Li HL (2007) J. Phys. Chem. C, 111: 19141
Zhao GY, Lin YB, Zhou T, Lin Y, Huang YD, Huang ZG (2012) J Power Sources 215:63
Kuthanapillil, M Shaju, PG Bruce (2008) Dalton Trans. 40:5471
Zhong QM, Bonakdarpour A, Zhang MJ (1997) J Electrochem Soc 144:205
Sha O, Wang SL, Qiao Z, Yuan W, Tan ZY, Xu Q, Su YJ (2012) Mater Lett 89:251
Aragón MJ, Lavela P, León B, Pérez-Vicente C, Tirado JL, Vidal-Abarca C (2010) J. Solid State. Electrochem 14:1749
Q Zhong, A Bonakdarpour, M Zhang, Y Gao, JR Dahn (1997) J. Electrochem. Soc. 205:144
Y Idemoto, H Narai, N Koura (2003) J. Power Sources 125:119
Xu B, Qian D, Wang ZY, YS Meng (2012) Mater. Sci. Eng., R 73:51
Acknowledgments
This work was supported by the Science and Technology Planning Project of Gansu Province (no. 110RJYA056), the Fundamental Research Funds for Universities of Gansu Province (no. 1105ZTC136), and the Natural Science Foundation of China (no. 20961004 and no. 51002164).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, XL., Li, YL., Li, SY. et al. Nanosized LiNi0.5Mn1.5O4 spinels synthesized by a high-oxidation-state manganese sol–gel method. Ionics 19, 1489–1494 (2013). https://doi.org/10.1007/s11581-013-0897-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-013-0897-y