Abstract
Gel polymer electrolytes were prepared using agar polymer host, NH4I, and I2 salts. The sample of agar paste with 1.0 M of NH4I and 0.2 μM of I2 exhibits the highest conductivity and lowest viscosity values at room temperature of (2.64 ± 0.19) × 10−3 S cm−1 and 1.17 ± 0.29 Pa s, respectively. All of the gel polymer electrolytes display Arrhenian behavior, and the optimum agar paste gave the lowest activation energy of 0.25 eV. It also had a good physical appearance compared with the other samples. This gel polymer electrolyte had a good potential and was applicable to a role as electrolyte in ITO-ZnO (N719 dye)/agar paste + 1.0 M NH4I + 0.2 μM I2/Au-Pd-ITO dye-sensitized solar cell.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The dye-sensitized solar cell (DSSC) was developed by Honda and Fujishima [1] by introducing a nanostructured semiconductor, dye sensitization, and a reduction and oxidation (redox) electrolyte. Grätzel and co-workers [2, 3] used a nanoporous structure with a large-surface-area titanium oxide (TiO2) thin-film electrode, a novel Ru bipyridyl complex as the dye, and an iodide/triiodide (I−/I3 −) couple as the redox electrolyte for DSSC fabrication. Zinc oxide (ZnO) is another attractive semiconductor material that has been used extensively for DSSC applications. One work showing excellent performance of a DSSC using ZnO as a working electrode was presented by Saito and Fujihara [4].
The use of liquid electrolyte is considered one of the techniques to improve the contact between the working electrode, the electrolyte, and the counter electrode. The existence of an optimal contact surface for the (I−/I3 −) redox electrolyte gives the best DSSC performance [5]. However, liquid electrolytes have several drawbacks, such as leakage, volatilization of the liquid, possible desorption and photo degradation of the attached dyes, and corrosion of the counter electrode. These shortcomings hinder their practical application and limit the long-term performance of DSSCs [6, 7]. A quasi-solid-state DSSC was developed by using a gelator to replace liquid electrolytes to solve the leakage problem. Gelation can be accomplished by adding gelator into the electrolyte [8]. In addition, the development of gel polymer electrolyte (GPE) could be used to overcome this problem. Kubo et al. [7] developed the quasi-solid-state DSSC by using gelator. This DSSC performance with GPE was comparable with DSSC using liquid electrolyte studied by Keis et al. [9].
Recently, biopolymer materials, such as chitosan, agarose, and carrageenan, have been used extensively as DSSC electrolytes [10–14]. Agar and carrageenan are other biopolymers that can possibly be used in the fabrication of DSSCs. Agar and carrageenan are in a class of marine algae biopolymers extracted from red seaweed polysaccharides and are hydrophilic in nature [15], while konnyaku (konjac glucomannan gel) is a polysaccharide that is elastic and insoluble in water [16]. Konnyaku has been used to interact synergistically with other hydrocolloids such as carrageenan [17, 18]. These three kinds of polysaccharides are usually blended together and are known commercially as agar-agar. Figure 1 illustrates the structure of agar and carrageenan. The previous study on biopolymer materials has been revealed that conductivity values of agarose and κ-carrageenan electrolyte for the PEC were 4.36 and 2.82 × 10−3 S cm−1, respectively. These conductivity values are compared to the conductivity value of liquid electrolyte (4.40 × 10−3 S cm−1) for the DSSC application [13].
In general, the enhancement of the DSSC performance also depends on counter cations of iodides salts in electrolyte, such as Li+, Na+, K+, and R4N+ [19–21]. These counter cations owe the different ionic conductivities from the electrolyte or adsorption on the oxide semiconductor surface. Thus, the counter cations lead to a shift in the conduction band level of the oxide semiconductor electrode [22]. The DSSC electrolyte also needs I−/I3 − redox ions to reduce the oxidized dye and functions as the charge carrier mediator in the regenerative DSSC. Hence, both inorganic and organic iodide salts have significant influence on the performance of the DSSC [6].
Ammonium iodide (NH4I) is presented as one of the alkali iodide besides LiI/I2 [20, 21]. NH4I has potential to be used as salt in polymer electrolyte together with I2, particularly for the DSSC electrolyte. The complex of polyethylene oxide (PEO) with NH4I salt film demonstrated a good ionic conductivity within a range of ~10−5 S cm−1 [23]. In fact, PEO–chitosan complex with NH4I + I2 has been employed as electrolyte for the DSSC. Based on these previous studies, the ionic conductivity of the polymer electrolyte attained was 8.47 × 10−4 S cm−1 [10, 14, 24].
Because there has been no systematic research on DSSCs based on ZnO working electrodes and agar complexes with NH4I and I2 (I−/I3 −) redox electrolytes, the aim of this study was to examine the properties of DSSCs using GPE and ZnO as the working electrode. The GPEs were based on agar, ammonium iodide (NH4I), and iodine (I2) as the redox electrolyte. The agar paste GPE with the highest conductivity was then used to fabricate an ITO-ZnO (N719 dye)/GPE/Au-Pd-ITO DSSC, and its performance was measured.
Experimental
Agar paste + NH4I + I2: preparation and characterization
GPE was prepared by mixing 1.0 g of agar powder (BITES, Jim-Willie, Singapore) with 20 ml glycerol (Merck). The mixture was boiled at 80 °C for 15 min. The gel was left at 25 °C for 15 min for the gelation and cooling process. Next, the GPE preparation and characterization processes were split into two parts. For the first part, agar paste was mashed briskly. Various concentrations of NH4I (Sigma-Aldrich) salt from 0.2 to 2.0 M were added to the agar paste. Electrochemical impedance spectroscopy was used to determine the conductivity. A sample of agar paste was placed in the spacer between stainless steel (SS) block electrodes inside the Teflon cell. This cell was connected to an Autolab PGSTAT 30 frequency response analyzer (Eco Chemie B.V.) with a frequency range of 0.1 Hz to 1.0 MHz and an amplitude of 10 mV.
For the second part, the most conductive agar paste with NH4I and good physical properties was then doped with various concentrations of iodine solution (I2, Aldrich) from 0.1 to 0.4 μM to complete the GPE preparation process. The viscosity (η) was determined using a Haake Viscotester VT550. The viscosity values were calculated from the graph of η as a function of time (t). Then, the variation of the viscosity and conductivity of the agar paste GPE at room temperature was plotted.
The conductivity of the GPE at elevated temperatures was measured in a temperature range from 25 to 120 °C. The physical appearances of the agar paste GPE were investigated with a stereo zoom microscope with magnification of ×4.0.
Dye-sensitized solar cell: device fabrication and characterization
Conducting indium tin oxide (ITO) glass with a sheet resistance of 8 Ω cm−2 was used. The ZnO powder was prepared earlier by the sol–gel method [25]. ZnO powder (0.5 g) was ground with acetic acid (0.035 M) (CH3COOH, Merck) and a few drops of Triton X-100 emulsification agent (C14H22 (C2H4O)N, Sigma-Aldrich). The prepared ZnO paste was then spread on the ITO glass with an active area of 1 cm2. Then, sintering was carried out at 400 °C for 30 min in a furnace. The ZnO working electrode was left to cool prior to being dipped in 0.5 mM of dye, cis-bis(isothiocyanato) bis(2,2′-bipyridy1-4,4′-dicarboxylato)-ruthenium (II) bis-tetrabutylammonium (N-719) (Aldrich), for 1 h.
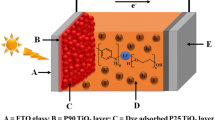
For the counter electrode, the conductive side of the ITO glass was coated with gold–palladium (Au-Pd) in the setup. Next, the agar paste GPE with the highest conductivity was placed in the space between the working electrode and counter electrode as shown in Fig. 2.
The performance of the ITO-ZnO (N719 dye)/GPE/Au-Pd-ITO cell was characterized by measuring the current density-voltage (J-V) under illumination and dark condition by connecting the cell to Autolab PGSTAT 30 GPES software (Eco Chemie, B.V.). The illumination test was carried out using a halogen lamp by Philips (120 V; 50 W) with a light intensity of 100 mW cm−2. The open circuit voltage (V OC), short circuit current density (J SC), maximum voltage (V max), and maximum current density (J max) were calculated based on the X–Y-axis intercepts and the square area under the J-V curves.
Results and discussion
Agar paste + NH4I + I2: A Nyquist plot
Selected Nyquist plots of the agar paste with different NH4I and I2 concentrations are shown in Fig. 3. The intersection of the imaginary axis with the real axis provides the value of R b. The R b value for pure agar paste was 1,341.60 Ω (Fig. 3a). Figure 3b, c show the Nyquist plots for agar paste with 0.2–1.0 M of NH4I and 1.2–2.0 M of NH4I, respectively. The value of R b decreases proportionally with the addition of NH4I.
The Nyquist plot of the agar paste as a function of 1.0 M of NH4I and various concentrations of I2 is shown in Fig. 3d. There is no existing capacitance component. All agar pastes show only a resistive component due to the effect of adding a high I2 concentration. The R b value decreases for 0.1 and 0.2 μM of I2. However, the R b value increases for the agar paste with 0.3 and 0.4 μM of I2. Based on the Nyquist plot results, agar paste + 1.0 M of NH4I + 0.2 μM of I2 gives the lowest R b value of 75.04 Ω for the GPE system.
The trend of the agar paste Nyquist plot is close to the trend observed in a system using glycerol as an organic solvent [26]. The small curve observed at the beginning of the agar paste plot shows the capacitive behavior at higher frequency ranges. The conductive charge carriers cannot easily cross the electrodes/electrolyte and form the curve. The concentration gradients of conductive charge carriers increase and gather near the interface of SS electrodes when an electric field is applied, which impedes the further diffusion of conductive charge carriers until an equilibrium state is reached [26, 27]. Besides that, the disassociation of NH4I and I2 provided a constant amount of I− and I3 − similar with study done by Tan et al. [28]. Thus, this can form an ordinary form of GPE. The continuous decreased of bulk resistance could be noticed when more I2 diffused into the agar paste network at optimum concentration. Nevertheless, the bulk resistance increased at 0.3 and 0.4 μM of I2. This attributed by the excessive amount of I2. At these concentrations, I2 cannot diffuse into agar paste network straightforwardly.
Agar paste + NH4I + I2: conductivity and viscosity
Figure 4 shows the conductivity and viscosity values of agar paste as a function of NH4I concentrations. The conductivity of pure agar paste coded as A was (1.50 ± 0.25) × 10−4 S cm−1. The conductivity increased slightly when 0.2 M of NH4I was added to the sample. When the concentration of NH4I was increased to 0.4 M, the conductivity value of the agar paste continued to increase. The increase in conductivity remained constant when 0.6, 0.8, and 1.0 M of NH4I were added to the agar paste, as plotted in region 1. The conductivity increased slightly in region 2 compared to region 1. The conductivity values continued to increase with NH4I concentration for the agar pastes with 1.2 to 1.8 M of NH4I. The agar paste with 2.0 M of NH4I had the highest conductivity value of (4.89 ± 0.62) × 10−3 S cm−1.
In the viscosity measurement, the agar paste had the highest viscosity value at (11.43 ± 3.97) Pa s. The viscosity decreased slightly and continuously for 0.2–1.0 M of NH4I, as shown in region 1. In region 2, when the NH4I concentration increased to 1.2–1.8 M, the viscosity continued to decrease steadily. The lowest viscosity, (2.67 ± 0.29) Pa s, was obtained in samples coded as A2.0N. The values of the conductivity and viscosity of agar paste with various concentrations of NH4I are tabulated in Table 1. The agar paste with 1.0 M of NH4I (A1.0N) was chosen as the optimum composition based on the trade-off between conductivity and viscosity values for the next step.
Meanwhile, the relation between the conductivity and viscosity values of the agar paste as a function of the optimum conductivity using 1.0 M of NH4I with various concentrations of I2 is shown in Fig. 5. The trend of the conductivity curve increased slightly for A1.0N0.1I and gave the highest conductivity value of (2.64 ± 0.19) × 10−3 S cm−1 for A1.0N0.2I. However, starting with A1.0N0.3I, the conductivity value decreased. Concurrently, when 0.1 μM I2 was added to A1.0N, the viscosity value suddenly decreased. After reaching the lowest viscosity value, the viscosity values increased abruptly for A1.0N0.3I and A1.0N0.4I. The lowest viscosity was noted in A1.0N0.2I with a value of (1.17 ± 0.29) Pa s. Table 2 lists the values of the conductivity and viscosity of the optimum composition A1.0N with various I2 concentrations.
The conductivity of the agar paste was inversely proportional to the viscosity when NH4I and I2 concentrations increased. This result is also in accordance with that of Bonhote et al. [29], in terms of the conductivity being inversely proportional to the viscosity based on iodide as a type of salt. The increase in the conductivity values is attributed to the increasing amount of charge carriers (H+ and I−) in the samples. Even though the main focus of this work was I− and I3 − as the main species for DSSC, the contribution of H+ to the conductivity cannot be ruled out. Nevertheless, the conductivity values in this study decreased with the addition of 0.3 and 0.4 μM of I2, which was attributed to ion-pairing phenomena. Excess I2 initially becomes I−/I3 −. A free iodide from NH4I forms a triiodide (I3 −) with the iodine according to the reduction process as follows:
The increase of I− to I3 − (higher concentration of polyiodide ions) enhances the ability of anions to hop along with H+ to the coordinate sites [30, 31]. Thus, the value of the agar paste conductivity increased. However, the charge carrier (H+ and I3 −) ion pairing occurred upon the addition of 0.3 and 0.4 μM of I2. The number of anion (I3 −) charge carriers in the agar paste decreased in proportion to the conductivity.
The continuous decrease of the viscosity values with increasing NH4I concentration in this work demonstrates that the viscosity is strongly dependent on the salt concentration. The NH4I concentration contributes to the frictional force between the constituent ions (H+ and I−) [32]. Thus, the increase in the NH4I concentration reduced the friction force of H+ and I− along the agar structure backbone. Subsequently, both of these ions can enter the agar paste, move along easily, and separate the agar structure backbone chains, reducing the viscosity value.
Furthermore, the increase of I− to I3 − in A1.0N0.1I and A1.0N0.2I reduced the viscosity values, which was similar to what happened for the agar paste + NH4I in various concentrations. The decrease in viscosity could be the reason why the conductivity of the samples increased slightly. In contrast, the viscosity values suddenly increased with increasing concentrations of I2 to 0.3 and 0.4 μM. At this point, the high concentrations of I2 increased the friction force of paired ions inside the agar paste. In addition, Renard et al. [33] found that an increase of I3 − ion pairing contributed to increased viscosity values. The conductivity values of GPE in this work were almost similar (~10−3 S cm−1) with those reported in a previous work on electrolyte based on biopolymer (~10−6 to 10−4 S cm−1) [10, 24], agarose (10−4 to 10−3 S cm−1) [20, 21], and agar and carrageenan (10−3 S cm−1) [34] for DSSCs.
Agar paste + NH4I + I2: conductivity–thermal dependence behavior
The temperature dependences for A, A1.0N, A1.0N0.1I, A1.0N0.2I, and A1.0N0.3I at a temperature range of 298 to 393 K are shown in Fig. 6. The conductivity of samples increased proportionally to the temperature and showed an Arrhenius type relation. The pure agar paste had the lowest conductivity compared with the other samples. The conductivity increased slightly in A1.0N compared with the pure agar paste. When 0.1 and 0.2 μM of I2 were added to the agar paste + 1.0 M NH4I samples, the conductivity became higher. Conversely, the conductivity decreased for A1.0N0.3I.
There was no sudden change in the value of conductivity with temperature because these complexes do not undergo any phase transitions within this temperature range. Normally, the polymer matrix is amorphous and has large amounts of free volume cages [35]. Thus, as the temperature increases, the agar paste samples can expand easily and produce free volume proportionally to the overall mobility of H+ and I− ions along the agar backbone chain [36]. Moreover, the segmental mobility assists ion transport. Therefore, the retarding effect of the ion clouds almost balances [37]. However, the increasing ion association decreased the free volume amount even at elevated temperature for A1.0N0.3I. At this stage, the lower fraction of free volume was no longer balanced as the number of anion charge carriers (I3 −) was continuously increased. As a result, the conductivity decreased with increasing I2 concentration at elevated temperatures. The conductivity values are almost similar to those reported in previous studies on GPE temperature dependence plots [35, 38, 39].
The relationship of the variation of the activation energy and conductivity at room temperature is shown in Fig. 7. The activation energy of pure agar paste, A1.0N, A1.0N0.1I, A1.0N0.2I, and A1.0N0.3I at room temperature was calculated from the linear temperature dependence plot in Fig. 6. The highest activation energy value was 0.30 eV for pure agar paste. The activation energy value decreased slightly for A1.0N and A1.0N0.1I. The lowest activation energy, found for A1.0N0.2I, was 0.25 eV. However, when the I2 concentration increased to 0.3 μM in A1.0N0.3I, the activation energy value increased. The variations of the activation energy values were inversely proportional to the conductivity. The results of the activation energy values for agar paste samples with different I2 concentrations are summarized in Table 3.
The variation of activation energy values was influenced by the different concentrations of I2. The conductivity of agar paste + 1.0 M NH4I + different concentrations of I2 decreased slightly after reaching a maximum conductivity, which is attributed to the increase of the redox couple species (I3 −) at high I2 concentrations. Therefore, the conductivity values increased when the I2 concentration increased to 0.1 and 0.2 μM. Concurrently, the activation energies needed for the H+ and I3 − ions to hop together on the coordinating site of the agar were lower compared with those of the pure agar paste and A1.0N. Nonetheless, conductivity decreased, and the activation energy value increased for A1.0N0.3I, which is attributed to the ion pairing in agar paste. Thus, the hopping mechanism of H+ and I3 − ions occurred with a high activation energy value at the agar coordinating site.
Agar paste + NH4I + I2: physical properties
The images of agar powder transformed to agar paste when glycerol and various concentrations of NH4I were added are shown in Fig. 8. Figure 8a shows that agar powder has a flaky physical appearance with an average size of about 42 μm. Pure agar paste (Fig. 8b) and A0.2N (Fig. 8c) are the bulky pastes obtained after the addition of glycerol and 0.2 M of NH4I, respectively. The agar paste changed to a clear transparent paste when the NH4I concentrations were increased to 1.0 M (A1.0N), as shown in Fig. 8d. However, when the concentrations of NH4I were increased from 1.4 to 2.0 M (Fig. 8e, f), NH4I was not mixed homogeneously with the agar paste due to the “cage” phenomenon. Hence, at these concentrations, the sample showed brownish spots due to the problem of ions being encapsulated within the agar paste.
The images of agar paste + 1.0 M of NH4I with different concentrations of I2 are shown in Fig. 9. In Fig. 9a, b, it can be observed that the images of A1.0N0.1I and A1.0N0.2I show fewer brownish spots. At this concentration of I2, I2 (solution) could dissolve the brownish spots of NH4I. The formations of brownish spots shed some light on the conductivity and viscosity results. Normally, the salts (NH4I and I2) dissolve into gel electrolyte (agar paste). The good dissolution of salts was shown by the formation of a transparent paste.
However, in samples with high I2 concentration (A1.0N0.3I and A1.0N0.4I), the formation of brownish spots started again (Fig. 9c, d). This second formation of brownish spots was mostly due to the association of I2. Herein, when the salt reached the maximum dissociation to free charge carriers, the free charge carriers became associated again. The formation of subsequent brownish spots in this work is a physical evidence to support the reassociation of charge carriers (ion pairing). At this high concentration of I2 and ion pairing phenomena, the salt cannot mix well with the agar paste. Hence, the brownish spots appeared clearly on agar paste surface. These results are also parallel with the conductivity and viscosity results. Based on the physical properties, A1.0N0.2I shows better physical properties, conductivity, and viscosity compared with the other samples. Hence, A1.0N0.2I was chosen as the GPE for DSSC fabrication.
ITO-ZnO (N719 dye)/A1.0N0.2I/Au-Pd-ITO: current density-voltage
Figure 10 shows the current density-voltage curve characteristics of the DSSCs based on ITO-ZnO (N719 dye)/A1.0N0.2I/Au-Pd-ITO under an illumination of 100 mW cm−2. Table 4 listed the J-V curve properties of PEC based ITO-ZnO (N719 dye)/A1.0N0.2I/Au-Pd-ITO under illumination of 100 mW cm−2. Equations 2–5 represent the mechanism of ITO-ZnO (N719 dye)/A1.0N0.2I/Au-Pd-ITO. This mechanism is proposed based on the photophysical process and combined reduction and oxidation reaction in TiO2 DSSCs discovered by Calogero and Marco [40].
The monolayer of dye (D) adsorbed chemically at the ZnO surface was irradiated by light (hv) and converted to the electronically excited state (D*). It is preferable for the excited state to lie a long way above the conduction band (CB) edge of the ZnO nanoparticles:
In this condition, the electron (e−) was injected into the conduction band of ZnO:
Next, to allow the reaction (Eq. 3a) to occur successfully, the electron injection process needed to compete with the deactivation reaction. Herein, the deactivation reaction is the process of reduction of D* to its origin form, D:
In order to attain a high-current generation, the oxidation of iodide [41] to triiodide (I3 −) in (Eq. 4a) must effectively compete with the charge-separated state during the recombination process (Eq. 4b):
The failure of the oxidation reaction to compete with the charge-separated state will decrease the amount of current production in DSSCs. On the other hand, the I3 − will accept electrons from the counter electrode (Au-Pd-ITO glass) to continue the reduction reaction. Initially, the counter electrode obtained electrons from the external circuit of DSSCs. Thus, I3 − was reduced to I−:
Once again, the reduction of I3 − to I− (Eq. 9a) must effectively compete with the charge-separated state during the recombination process (Eq. 9b) to avoid a decrease in current production in DSSCs:
Finally, the electrons released in the oxidation process enter the ZnO layer, and the circuit is completed. The D+ also returns to its origin form, D. This mechanism takes place continually as long as the DSSCs are exposed to the light.
The voltage produced by DSSCs in this work is based on the difference in energy levels between ZnO and the transition media (mediator) of I−/I3 −. It depends on the mediator and solvent used as well, besides the condition of the ZnO and the intensity of illumination. Agar paste GPE containing a mixture of (I−/I3 −) from NH4I and I2 serves as the charge carrier in the mediator. Meanwhile, the current produced by the cell is directly related to the amount of light absorbed by the dye. The amount of light here was fixed at 100 mW cm−2.
Based on results, the agar paste demonstrated as a potential material for the DSSC electrolyte. Although ZnO electrode sensitized N-719 was a good semiconductor material for the DSSC working electrode, but the contact between ITO-ZnO (N719 dye) and A1.0N0.2I was not good in this work, lead to the low efficiency of the DSSC.
However, the physical properties of agar paste GPE (transparent slurry gel) give an advantage that improves the DSSC photophysical and redox reaction. This is due to the high conductivity of agar paste GPE (10−3 S cm−1) in contrast with the low conductivity of solid polymer electrolytes (SPE) (10−6-10−4 S cm−1) for DSSCs. Therefore, the value of J SC is much higher compared with the other DSSC studies based on polysaccharides SPE (2–5 and 19.23 μA cm−2) [10, 24]. The J-V curve pattern is also similar to those reported in other studies on DSSCs [12, 42].
Conclusion
GPE-based agar paste + NH4I + I2 with various concentrations of redox electrolyte was prepared and characterized. Based on the GPE characterization analysis, agar paste + 1.0 M NH4I + 0.2 μM I2 (A1.0N0.2I) gave the highest conductivity (2.64 ± 0.19) × 10−3 S cm−1, lowest viscosity (1.17 ± 0.29) Pa s and lowest activation energy of 0.25 eV. This sample had a good physical appearance compared with the other samples in term of transparency and mix well with NH4I and I2. The A1.0N0.2I was then successfully fabricated as a GPE for DSSCs with the combination of ITO-ZnO (N719 dye)/A1.0N0.2I/Au-Pd-ITO.
References
Honda K, Fujishima A (1972) Nature 238:37
O’Regan B, Gratzel M (1991) Nature 353:737–740
Nazeeruddin MK, Kay A, Rodicio I, Humphry-Baker R, Mueller E, Liska P, Vlachopoulos N, Graetzel M (1993) J Am Chem Soc 115:6382–6390
Saito M, Fujihara S (2008) Energy Environ Sci 1:280–283
Hara K, Horiguchi T, Kinoshita T, Sayama K, Sugihara H, Arakawa H (2000) Sol Energy Mater Sol Cells 64:115–134
Wang Y (2009) Sol Energy Mater Sol Cells 93:1167–1175
Kubo W, Murakoshi K, Kitamura T, Yoshida S, Haruki M, Hanabusa K, Shirai H, Wada Y, Yanagida S (2001) J Phys Chem B 105:12809–12815
Hara K, Arakawa H (2005) Dye-sensitized solar cells. In: Luque A, Hegedus S (eds) Handbook of photovoltaic science and engineering. Wiley, New York, pp 663–700
Keis K, Magnusson E, Lindström H, Lindquist S-E, Hagfeldt A (2002) Sol Energy Mater Sol Cells 73:51–58
Mohamad SA, Yahya R, Ibrahim ZA, Arof AK (2007) Sol Energy Mater Sol Cells 91:1194–1198
Suzuki K, Yamaguchi M, Kumagai M, Tanabe N, Yanagida S (2006) C R Chim 9:611–616
Kaneko M, Hoshi T (2003) Chem Lett 32:872–873
Kaneko M, Hoshi T, Kaburagi Y, Ueno H (2004) J Electroanal Chem 572:21–27
Mohamad SA, Ali MH, Yahya R, Ibrahim ZA, Arof AK (2007) Ionics 13:235–240
Norman FS (2006) 2nd ed., CRC Press
Teramoto A, Fuchigami M (2000) J Food Sci 65:491–497
Cairns P, Atkins EDT, Miles MJ, Morris VJ (1991) Int J Biol Macromol 13:65–68
Piculell L, Borgström J, Chronakis IS, Quist PO, Viebke C (1997) Int J Biol Macromol 21:141–153
Ciancia M, Milas M, Rinaudo M (1997) Int J Biol Macromol 20:35–41
Wang W, Guo X, Yang Y (2011) Electrochim Acta 56:7347–7351
Yang Y, Hu H, Zhou C-H, Xu S, Sebo B, Zhao X-Z (2011) J Power Sources 196:2410–2415
Hara K, Horiguchi T, Kinoshita T, Sayama K, Arakawa H (2001) Sol Energy Mater Sol Cells 70:151–161
Maurya KK, Srivastava N, Hashmi SA, Chandra S (1992) J Mater Sci 27:6357–6364
Buraidah MH, Teo LP, Majid SR, Arof AK (2010) Opt Mater 32:723–728
Alias SS, Ismail AB, Mohamad AA (2009) J Alloy Compd 499:231–237
Al-Kahlout A, Vieira D, Avellaneda C, Leite E, Aegerter M, Pawlicka A (2009) Ionics 16:13–19
Wang M, Qi L, Zhao F, Dong S (2005) J Power Sources 139:223–229
Tan WC, Alias SS, Ismail AB, Mohamad AA (2011) J Solid State Electrochem :1-10
Bonhote P, Dias A-P, Papageorgiou N, Kalyanasundaram K, Gratzel M (1996) Inorg Chem 35:1168–1178
Kalaignan GP, Kang M-S, Kang YS (2006) Solid State Ion 177:1091–1097
Vieira DF, Avellaneda CO, Pawlicka A (2007) Electrochim Acta 53:1404–1408
Salihoǧlu S, Tarı Ö, Yurtseven H (2002) Mater Chem Phys 73:339–342
Renard I, Li H, Marsan B (2003) Electrochim Acta 48:831–844
Ueno H, Endo Y, Kaburagi Y, Kaneko M (2004) J Electroanal Chem 570:95–100
Wu J, Lan Z, Wang D, Hao S, Lin J, Huang Y, Yin S, Sato T (2006) Electrochim Acta 51:4243–4249
Ramesh N, Duda JL (2001) J Membr Sci 191:13–30
Saikia D, Kumar A (2005) Eur Polym J 41:563–568
Yue Z, McEwen IJ, Cowie JMG (2003) Solid State Ion 156:155–162
Mallik H, Sarkar A (2006) J Non-Cryst Solids 352:795–800
Calogero G, Marco GD (2008) Sol Energy Mater Sol Cells 92:1341–1346
Shobukawa H, Tokuda H, Tabata S-I, Watanabe M (2004) Electrochim Acta 50:305–309
Philias JM, Marsan B (1999) Electrochim Acta 44:2915–2926
Acknowledgments
SSA would like to thank MOSTI for the NSF scholarship and USM-RU-PRGS for grant no. 8031020. AAM is grateful for the financial support for the study provided by USM Short Term grant no. 6039030.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alias, S.S., Mohamad, A.A. Effect of NH4I and I2 concentration on agar gel polymer electrolyte properties for a dye-sensitized solar cell. Ionics 19, 1185–1194 (2013). https://doi.org/10.1007/s11581-012-0840-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-012-0840-7