Abstract
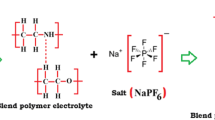
Polyethylene oxide–polymethyl methacrylate (PEO–PMMA) polymer blend electrolyte system complexed with silver salt having different ethylene carbonate (EC) concentrations was prepared using solution cast technique. Complex formation and change in structural and microstructural properties have been studied by X-ray diffraction, Fourier transform infrared, and scanning electron microscopy analysis. The thermal properties of polymer films have been examined by the differential scanning calorimetry technique. Addition of plasticizer is observed to lower melting temperature. Electrical response of polymer films has been measured as a function of EC concentration and temperature using complex impedance spectroscopy. Complex impedance data are used to analyze the conductivity, permittivity, and modulus formalism to understand the conduction mechanism. The temperature dependence of electrical conductivity of polymer electrolytes shows a sudden rise at the melting temperature of PEO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Optimization of the electrochemical properties of solid polymer electrolytes is an area of intense interest. Many groups have attempted over the years to enhance the electrochemical properties of polymer electrolytes with the addition of plasticizer, dispersal of nanofillers, addition of ionic liquid, blending of polymers, etc. [1–8]. Each of these modified systems has its own strengths and weaknesses. For instance, polymer blending provides a strategic route to improve the performance of the electrolyte over each individual polymer. In addition to this, it also allows realization of novel materials which possess enhanced performance over the parent polymer. Consequently, in the last decades, many polymer blends, e.g., PVdF–PEMA, PEO–PMMA, PVC–PMMA, PMMA–PVdF (where PVdF—polyvinylidene fluoride, PEMA—polyethyl methacrylate, PEO—polyethylene oxide, PMMA—polymethyl methacrylate, PVC—polyvinyl chloride), etc., were developed [8–12]. Out of the so far reported polymer blends, PEO–PMMA is one of the most studied polymer blends, and among the various studies carried out in this system, considered significant are (1) Li-ion conduction in PEO–PMMA polymer blends [8], (2) dynamics of PEO in PEO–PMMA polymer blends [13], and (3) application of PEO–PMMA polymer electrolyte as fuel cell [14]. Blending of PEO with PMMA inhibits the crystallization of PEO [12]. As a result of this, an enhancement in conductivity is expected because of the increased amorphous regions of the polymer host as conduction is generally taking place in the amorphous regions of PEO.
It is well known that plasticization of polymers is an important approach to increase the ionic conductivity of electrolytes [2–5]. Plasticizers are small organic molecules which embed themselves between the polymer chains and increase the spacing and free volume resulting in improvement of the conductivity of polymer electrolytes by dissociating ion aggregates [3, 4]. Basically, plasticizers penetrate easily in the host polymer matrix and cause an interaction between polymer chains. This, in turn, reduces the cohesive forces between polymer chains and increases the segmental chain mobility. In spite of the reported work on PEO–PMMA polymer blends [8, 12–15], nevertheless, the structural and electrical properties of the silver ion conducting PEO–PMMA polymer system have not been undertaken earlier and have attracted attention to study the effect of the plasticizer, ethylene carbonate (EC), in this system.
Experimental description
Starting materials
Commercially available chemicals of PEO (Mw = 3 × 105, Alfa Aesar), PMMA (Mw = 3.5 × 105, Alfa Aesar), silver nitrate (AgNO3), and EC (Aldrich) were employed as starting materials. The compositions of the polymer films are given in Table 1. All the samples were prepared by the solution cast method.
Sample preparation
PEO and PMMA were dissolved in acetonitrile separately and stirred by using a magnetic stirrer. The stirring of the PMMA solution was carried out at 323 K for 24 h to dissolve PMMA in acetonitrile. PEO and AgNO3 were mixed and stirred for 4–5 h at room temperature. Both solutions were mixed along with the desired amount of plasticizer. The obtained mixture was again stirred at room temperature for another 10 h for homogenous mixing. Finally, the solution was poured into a Teflon Petri dish, and the solvent was left to evaporate slowly at ambient temperature. The resulting films were kept in an oven at 313 K for 2 days to ensure the removal of solvent traces. The dried films were peeled off from the Petri dish and then were stored in dark desiccators to prevent any contamination from moisture or effect from light.
Characterization
The X-ray diffraction (XRD) measurement was carried out using monochromatic Cu-Kα radiation (=1.5418 Å) in an X-ray diffractometer (Brucker NSZ, model D8) in the range of 10–70° at a scan rate of 2°/min. The surface morphology of the plasticized polymer complexes was investigated using a scanning electron microscope (SEM) (JOEL JSM-6380LV) at 20 kV. The polymer films were gold-coated in vacuum by an electron beam gold palladium source (80 % Au, 20 % Pd) by JEOL coater (Model JFC-1600) to make them conducting and mounted onto circular aluminum stubs by using double-sided sticky tapes.
Differential scanning calorimetry (DSC) of the prepared polymer films was carried out using SII EXSTAR-6000 equipment in the range from room temperature to 363 K with a heating rate of 10 K/min in nitrogen atmosphere. The polymer samples of about 2–3 mg were encapsulated in aluminum pans. Fourier transform infrared (FT-IR) spectra of the prepared samples were recorded in the wave number range of 4,000–400 cm−1 using single beam FT-IR 4100 JASCO model by directly mounting in the sample holder in a transmission mode.
Electrical measurements
Complex impedance spectroscopy was employed for electrical measurements using the impedance gain/phase analyzer (SOLARTRON 1260) interfaced to a computer in the frequency range of 100 Hz to 1 MHz at various temperatures ranging between 303 and 373 K. For the impedance measurements, the polymer electrolyte films were sandwiched between two silver electrodes of 1 cm radius under spring pressure in a temperature-controlled furnace. The obtained impedance plots were fitted using Zview2 program (developed by Solartron Analytical).
Results and discussion
XRD
In the present study, the XRD patterns of the plasticized polymer films are recorded along with pure PEO and PMMA to correlate the structure of polymer complexes. Pure PMMA exhibits a broad hump (inset of Fig. 1a) while the characteristic crystalline peaks of pure PEO are observed at 19.3° and 23.7° (Fig. 1a). In the prepared polymer films, the intensity of PEO peaks is observed to be less, and in addition to this, two new peaks at 11° and 13° are observed only in EC-5 wt% and EC-10 wt% polymer films. Less intensity of XRD diffraction peaks of PEO in polymer films indicates the presence of a semi-crystalline phase of PEO and reduction in degree of crystallinity. These observations may be due to the plasticization effect of EC; however, the blending effect of PEO–PMMA cannot be ignored. With the increase in EC concentration, the relative intensity of X-ray peaks is observed to decrease which is an indication of reduction of crystalline phase in polymer complexes. However, for EC-7 wt%, the intensity of XRD peaks is observed to be higher than other polymer films. The absence of AgNO3 peaks [16] and the above discussion suggest complexation of AgNO3 in the polymer matrix as well as interactions between the plasticizer and polymers.
SEM
SEM spectroscopy provides a powerful tool for monitoring surface morphology. Figure 2a–c shows the SEM micrographs of the plasticized polymer complexes with different plasticizer concentrations. Figure 2a represents a SEM micrograph of the EC-5 wt% polymer blend which shows some smooth areas, indicating the homogeneity among various constituents of the polymer matrix. Increasing EC concentration as plasticizer (Fig. 2b), a substantial change in the morphological feature (spherulite texture) of the composite starts appearing. The boundary between the spherulites is an indication of the existence of an amorphous phase. At EC-10 wt%, with decrease in size, the spherulites become distinctive which in turn increases the boundary regions. At EC-15 wt%, the polymer blend film exhibits good distribution of amorphous and crystalline regions inside the polymer complex (Fig. 2c).
DSC
The DSC plots of plasticized polymer blends show a sharp endothermic peak indicating transition from a semi-crystalline phase to an amorphous phase, i.e., melting temperature(T m) of PEO. Table 2 lists T m, percentage of degree of crystallinity, and FWHM values at different EC concentrations in plasticized polymer complexes. The increase in EC concentration possibly reduces the internal viscosity of the polymer films; in other words, it interrupts polymer–polymer interaction by occupying the inter- and intra-chain free volume which reduces the crystalline fraction of the polymer system, leading to an enhancement in the flexibility of the polymer chains [17]. The percentage of degree of crystallinity of the polymer films has been calculated using the relation:
where ∆H 0 is the enthalpy of melting of polymer films, and ∆H (213.7 J/g) is the enthalpy of melting of 100 % crystalline PEO [18]. The FWHM value or broadness of the melting peak increases with EC concentration, which indicates an increase in amorphous phases with EC concentration. These observed variations in T m, percentage degree of crystallinity, and FWHM values suggest the complexation of EC with an increase in amorphous phase in the host polymer blend.
FT-IR
Infrared spectroscopy measures the vibrational energy levels in the region of different molecules. The IR spectra of materials vary according to their compositions and show the occurrence of the complexation and interaction between various constituents. Hirankumar et al. [16] reported characteristic peak of pure AgNO3 at 1,800 and 1,430 cm−1. The IR spectra of pure PEO, PMMA, and plasticized polymer complexes are given in panels a and b of Fig. 3 in various wave number ranges. The broad band observed in the wave number range of 2,950–2,700 cm−1 (Fig. 3b) in pure PEO is attributed to the symmetric and asymmetric C–H stretching modes of the CH2 group [19]. In pure PEO, scissoring, wagging, twisting, and rocking modes of CH2 are observed at 1,461, 1,338, 1,276, and 844 cm−1, respectively, while C–O–C bending at 537 cm−1 is observed [19–22]. In pure PMMA, the peaks at 1,721, 1,433, and 1,137 cm−1 are assigned to C=O, –OCH3 deformation, and C–O stretching vibrations, respectively [11, 23–26]. The band at 986 cm−1 is attributed to the symmetrical stretching of the C–O bond in the C–O–C linkage of PMMA [26]. The frequency at 1,275 cm−1 is assigned to asymmetric CH2 transformation of PMMA.
In the present investigated system, the characteristic peak of AgNO3 at 1,800 cm−1 broadens, indicating complexation of salt with the polymer blend. In the prepared polymer films (Fig. 3b), the stretching frequency at 1,721 cm−1 corresponding to C=O of pure PMMA is broadened and shifted to 1,709–1,746 cm−1, which indicates complex formation [25]. The triplet peak of C–O–C stretching of PEO is absent and CH2 rocking vibration of pure PEO is observed to shift to 839 cm−1. The stretching vibration of the methoxy group and the wagging mode of CH2 of pure PMMA and PEO, respectively, are absent in polymer complexes. In addition to this, some small new peaks at 1,964 cm−1and in the range of 2,690–3,630 cm−1 are observed. Also, the bending mode of EC at 903 cm−1 [27] shifts to 912 cm−1 in polymer complexes indicating the interaction of salt and EC. In the FT-IR spectra, the disappearance and change in position of PEO and PMMA vibrational bands along with some new peaks confirm the complexation among various constituents of PEO–PMMA polymer electrolytes.
Conductivity
The ionic conductivity of the polymer films, σ, was calculated using the relation:
where t is the thickness of the film, A is the contact area of the polymer film, and R b is the bulk resistance of the polymer film. R b values were determined from the intercept of a high frequency semicircle at the x-axis. As shown in Fig. 4, conductivity initially drops as EC content increases from 5 to 7 wt%, and thereafter, it increases nonlinearly. In the polymer film with EC-7 wt%, the increase in the degree crystallinity as evident from XRD and DSC studies may be responsible for the drop in conductivity. After 7 wt% of EC concentration, the increase in conductivity with plasticizer concentration in polymer complexes may be due to a high value of dielectric constant of EC that effectively reduces the inter-ion Coulomb interactions [28], and more number of Ag+ ions contributed to the conductivity of the complexes. Lastly, the addition of plasticizer in the polymer matrix also facilitates to decrease the ion pairing which produces a great number of charge carriers for ionic transport. Furthermore, the addition of EC lowers the melting temperature and increases the amorphous phase of the polymer blend, corresponding to conductivity enhancement. Hence, the increase in conductivity plot may be due to the higher content of the plasticizer due to which more salt has been dissociated which must have favored the enhancement in conductivity. Rhoo et al. [29] have suggested that with higher plasticizer content, opening of the narrow rivulets of the plasticizer-rich phase and large free volume of relatively superior conducting phase has taken place which results in greater ionic transport.
The inset of Fig. 4 shows the temperature dependence of ionic conductivity for EC-5 wt%. Similar behavior of conductivity with different values has been observed for different concentrations. The ionic conductivity of the plasticized polymer complex increases with temperature. The inset shows a sudden and/or sharp rise in conductivity near T m. The observed sharp rise around 333 K is due to the softening of PEO polymer near T m in the polymer complexes which is reported by several workers in PEO-based polymer electrolyte systems [19, 20]. The viscosity and crystallinity of the polymer system decrease near the melting temperature, and hence, polymer chains become more flexible resulting in an increase in segmental motion leading to a sharp rise in conductivity.
Figure 5 shows the compositional variation of AC conductivity at 323 K. It shows similar result as observed in ionic conductivity variation with EC concentration, i.e., AC conductivity values are higher in the polymer films with EC-5 wt% and EC-15 wt%. Figure 6 represents the temperature dependence of AC conductivity of EC-5 wt% polymer film. AC conductivity is observed to increase with temperature as well as frequency. Below T m, AC conductivity shows two dispersion regions, namely, one in the low frequency region and the other in the high frequency region. As temperature approaches T m, dispersion in the low frequency region disappears and only the high frequency dispersion region is observed. At higher temperatures (T > T m), the AC conductivity is frequency independent in the low frequency region. In the high frequency region, AC conductivity shows a dispersion, which shifts to higher frequencies with increase in temperature as observed in Fig. 6. The frequency dispersion effects are present in all the samples. In the high frequency region, mobility of charge carriers, Ag+, is high near the relaxation times, and hence, conductivity increases with frequency. This variation of conductivity with frequency has been analyzed using the relation given by Jonscher [30]
where the symbols have their usual meanings. The above expression is known as the power law of AC behavior because the power law of AC behavior is observed in a wide range of materials and is called as “Jonscher’s universal behavior.”
Dielectric studies
The dielectric properties of the polymer electrolyte system can be characterized by frequency-dependent parameters such as complex permittivity (ε*) which is defined as [31]:
where ε′ and ε″ represent the real and imaginary parts of the dielectric constant, ω is the angular frequency, C 0 represents the vacuum capacitance of any configurations of the electrode, and Z* is the complex impedance. The dielectric spectra contain contribution from dipolar orientation as well as diffusion of charge carrier. Figure 7 and its inset show the influence of various concentrations of EC at frequency-dependent ε′ and ε″, respectively, at 323 K. The figure shows low frequency dispersion being significant and associated with the space polarization effects arising from the electrodes. In the low frequency region, dipoles follow an oscillating electric field and reorient themselves in the field direction [32], whereas at higher frequencies, the periodic reversal of field is quite high so that contribution of the dipoles may not be able to reorient themselves resulting in low or negligible contribution of the charge carriers. Therefore, the real and imaginary parts of the dielectric constant decrease with increasing frequency and reach a constant value. The compositional variation of frequency-dependent dielectric constant (both ε′ and ε″) shows a similar trend as observed from conductivity data, i.e., initially, it decreases as EC concentration increases from 5 to 7 wt%, and beyond that concentration, its values increase. This can be understood with the increased number of dipoles and hence decreased relaxation time at a higher concentration of the plasticizer.
Electrical modulus formalism is useful to study the relaxation process in materials [31]. The electrical relaxation process is due to distribution of relaxation times which may be due to inhomogeneity, conducting domain, mixture of phases, etc. The real, M′, and imaginary, M″, parts of the electrical modulus are given by:
Frequency-dependent M″ and M′ plots of various concentrations of EC in PEO–PMMA–AgNO3 polymer films are shown in Fig. 8 and its inset, respectively. M′ and M″ show an increasing trend with frequency at a particular temperature indicating the bulk effect. The lower or negligible values of M′ and M″ at lower frequencies indicate that the electrode polarization phenomenon makes a small contribution to the electrical modulus. Below T m, M′ plot (inset of Fig. 9) shows two dispersion regions which turn into a single one as temperature approaches T m. Similarly, below T m, the frequency-dependent M″ spectra (Fig. 9) show two peak maxima, and as temperature approaches T m, the first peak in the low frequency region disappears and the single relaxation peak in the mid-frequency region remains. These double relaxation features at temperatures T < T m are also in agreement with AC conductivity behavior. This behavior can be explained as at higher temperatures, i.e., T > T m, the semi-crystalline regions of PEO melt; in other words, the system becomes amorphous which results in single dispersion in conductivity or single relaxation in modulus plots. A detailed study regarding this can be found in our earlier report published elsewhere [15]. At T > T m, the frequency of M″max is found to shift systematically towards the higher frequency side with increase in temperature. Similar features in the modulus spectra below and above the T m of PEO in mixed electrolytes of MEEP–PEO doped with NaSCN are observed by Subramony and Kulkarni [31]. Scaling of the modulus spectra [33] has been carried out in order to understand the occurrence of dynamical processes in different concentrations at a particular temperature (Fig. 10). The scaled plot of the modulus does not show a single superposable master curve which indicates that the relaxation processes depend on the EC concentration, i.e., compositional dependent.
Relaxation processes occurring inside the ionic materials can be visualized and distinguished by impedance, electric modulus, and dielectric permittivity [34]. The frequency-dependent imaginary part of complex impedance, Z″, modulus, M″, and dielectric permittivity, ε″, for EC-5 wt% polymer electrolyte are plotted (Fig. 11a–c). The absence or presence of a peak in the imaginary impedance versus the frequency plot can be correlated to space charge effects and nonlocalized conductivity. The M″ plot is very useful when the relaxation behavior is presumed to be due to the motion of ions as in modulus analysis, and the electrode effects occurring at lower frequencies are suppressed. Also, the maxima of Z″ and M″ do not occur at the same frequency, where the Z″ spectrum broadened on the low frequency side while the M″ spectrum broadened on the high frequency side, indicating distribution of relaxation times, i.e., non-Debye nature of polymer films. Dielectric relaxation is a result of the reorientation process of dipoles in the polymer chains, which show a peak in the ε″ spectra. For electrolytes with higher ion concentration, the movement of ions from one site to another perturbs the electric potential of the surroundings. In the imaginary part of the modulus spectra, a relaxation peak is observed (for the conductivity processes), whereas no peak is seen in the dielectric spectra, suggesting that ionic and polymer segmental motion is strongly coupled and hence manifesting as a single peak in the M″ spectra with no corresponding feature in the dielectric spectra.
a Frequency-dependent plot of Z″ and M″ for PEO–PMMA–AgNO3–EC (5 wt%) plasticized polymer electrolyte at 323 K. b Frequency-dependent plot of ε″ and M″ for PEO–PMMA–AgNO3–EC (5 wt%) plasticized polymer electrolyte at 323 K. c Frequency-dependent plot of ε″ and Z″ for PEO–PMMA–AgNO3–EC (5 wt%) plasticized polymer electrolyte at 323 K
Conclusions
The structural and electrical conductivity behavior of PEO–PMMA–AgNO3–EC polymer films has been studied. SEM analysis shows a change in surface morphology with EC concentration. Complexation of the polymer complexes has been established by XRD, FT-IR, and DSC analysis. Conductivity isotherm shows an enhancement in conductivity with EC concentration minima at 7 wt%. The temperature dependence of ionic conductivity shows a sharp rise as temperature approaches T m, indicating a liquid-like ion transport in a solid polymer matrix above T m. AC conductivity of polymer films increases with temperature and frequency. AC conductivity and modulus spectra show two dispersion regions which turn into a single one as temperature approaches T m. The scaling of the imaginary part of the modulus spectra indicates compositional dependence of the relaxation process.
References
Pradhan DK, Choudhary RNP, Samantaray BK, Karan NK, Katiyar RS (2007) Effect of plasticizer on structural and electrical properties of polymer nanocomposite electrolytes. Int J Electrochem Sci 2:861–871
Pradhan DK, Samantaray BK, Choudhary RNP, Karan NK, Thomas R, Katiyar RS (2011) Effect of plasticizer on structural and electrical properties of nanocomposite solid polymer electrolytes. Ionics 17:127–134
Kumar M, Sekhon SS (2002) Role of plasticizer’s dielectric constant on conductivity modification of PEO–NH4F polymer electrolytes. Eur Polym J 38:1297–1304
Kumar M, Sekhon SS (2002) Ionic conductance behaviour of plasticized polymer electrolytes containing different plasticizers. Ionics 8:223
Johan MR, Shy OH, Ibrahim S, Yassin SMM, Hui TY (2011) Effects of Al2O3 nanofiller and EC plasticizer on the ionic conductivity enhancement of solid PEO–LiCF3SO3 solid polymer electrolyte. Solid State Ionics 196:41–47
Pandey GP, Agarwal RC, Hashmi S (2011) Performance studies on composite gel polymer electrolytes for rechargeable magnesium battery applications. J Phys Chem Solids 72:1408–1413
Kumar Y, Hashmi S, Pandey GP (2011) Ionic liquid mediated magnesium ion conduction in poly(ethylene oxide) based polymer electrolytes. Electrochim Acta 56:3864–3873
Osman Z, Ansor NM, Chew KW, Kamarulzaman N (2005) Infrared and conductivity studies on blends of PMMA/PEO based polymer electrolytes. Ionics 11:431–435
Sivakumar M, Subadevi R, Rajendran S, Wu HC, Wu NL (2007) Compositional effect of PVdF–PEMA blend gel polymer electrolytes for lithium polymer batteries. Eur Polym J 43:4466–4473
Ramesh S, Leen KH, Kumatha K, Arof AK (2007) FTIR studies of PVC/PMMA blend based polymer electrolytes. Spectrochim Acta A 66:1237–1242
Rajendran S, Kannan R, Mahendran O (2001) An electrochemical investigation on PMMA/PVdF blend-based polymer electrolytes. Mater Lett 49:172–179
Radhakrishnan S, Venkatachalapathy PD (1996) Effect of casting solvent on the crystallization in PEO/PMMA blends. Polymer 37:3749–3752
Sakai VG, Maranas JK, Peral I, Copley JRD (2008) Dynamics of PEO in blends with PMMA: study of the effect of blend composition via quasi-elastic neutron scattering. Macromolecules 41:3701–3710
Przyluski J, Dabrowska A, Stys S, Wieczorek W (1993) Ambient temperature proton polymeric electrolytes based on poly(ethylene oxide)–poly(methyl methacrylate) blends. Solid State Ionics 60:141–146
Sharma P, Kanchan DK, Gondaliya N, Pant M, Jayswal MS (2012) Conductivity relaxation in Ag + ion conducting PEO–PMMA–PEG polymer blends. Ionics. doi:10.1007/s11581-012-0738-4
Hirankumar G, Selvasekarapandian S, Bhuvaneswari MS, Baskaran R, Vijayakumar M (2006) Ag+ ion transport studies in a polyvinyl alcohol-based polymer electrolyte system. J Solid State Electrochem 10:193–197
Platzer NAJ (1965) Plasticization and plasticizer processes. Advances in Chemistry Series, vol 48. The American Chemical Society, Washington. doi:10.1021/ba-1965-0048, ISBN13: 9780841200494e ISBN: 9780841222281
Money BK, Hariharan K (2007) Lithium ion conduction in lithium metaphosphate based systems. Appl Phys A 88:647–652
Mohan VM, Raja V, Sharma AK, Rao VVRN (2006) Ion transport and battery discharge characteristics of polymer electrolyte based on PEO complexed with NaFeF4 salt. Ionics 12:219–226
Ramesh S, Yuen TF, Shen CJ (2008) Conductivity and FTIR studies on PEO–LiX [X: CF3SO −3 , SO 2−4 ] polymer electrolytes. Spectrochim Acta A 69:670–675
Noor SAM, Ahmad A, Talib IA, Rahman MYA (2010) Morphology, chemical interaction, and conductivity of a PEO-ENR50 based on solid polymer electrolyte. Ionics 16:161–170
Frech R, Chintapalli S, Bruce PG, Vincent CA (1999) Crystalline and amorphous phases in the poly(ethylene oxide)–LiCF3SO3 system. Macromolecules 32:808–813
Deka M, Kumar A (2010) Enhanced ionic conductivity in novel nanocomposite gel polymer electrolyte based on intercalation of PMMA into layered LiV3O8. J Solid State Electrochem 14:1649–1656
Chatterjee A (2010) Properties improvement of PMMA using nano TiO2. J Appl Polym Sci 118:2890–2897
Rajendran S, Bama VS, Prabhu MR (2010) Preparation and characterization of PVAc–PMMA-based solid polymer blend electrolytes. Ionics 16:283–287
Ramesh S, Bing KN (2012) Conductivity, mechanical and thermal studies on poly(methyl methacrylate)-based polymer electrolytes complexed with lithium tetraborate and propylene carbonate. J Mater Eng Perform 21:89–94
Kalyanasundraram S, Gopalan A, Muniyandi N, Renganathan NG, Saito Y, Kataoka H, Stephan AM, Elizabeth RN (2001) Ionic conductivity, thermal stability and FT-IR studies on plasticized PVC/PMMA blend polymer electrolytes complexed with LiAsF6 and LiPF6. Ionics 7:44–52
Johan MR, Ting LM (2011) Structural, thermal and electrical properties of nano manganese-composite polymer electrolytes. Int J Electrochem Sci 6:4737–4748
Rhoo HJ, Kim HT, Park JK, Hwang TS (1997) Ionic conduction in plasticized PVC/PMMA blend polymer electrolytes. Electrochim Acta 42:1571–1579
Jonscher AK (1977) The ‘universal’ dielectric response. Nature 267:673
Subramony JA, Kulkarni AR (1994) Conductivity relaxation in phosphazene based polymer electrolytes. Solid State Ionics 67:235–239
Ibrahim S, Yasin SMM, Nee NM, Ahmad R, Johan MR (2012) Conductivity and dielectric behaviour of PEO-based solid nanocomposite polymer electrolytes. Solid State Commun 152:426–434
Karmakar A, Ghosh A (2012) Dielectric permittivity and electric modulus of polyethylene oxide (PEO)–LiClO4 composite electrolytes. Curr Appl Phys 12:539–543
Sen S, Pramanik P, Choudhary RNP (2006) Impedance spectroscopy study of the nanocrystalline ferroelectric (PbMg)(ZrTi)O3 system. Appl Phys A 82:549–557
Acknowledgments
PS thankfully acknowledges the financial support by UGC, New Delhi, India for RFSMS fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, P., Kanchan, D.K. & Gondaliya, N. Effect of ethylene carbonate concentration on structural and electrical properties of PEO–PMMA polymer blends. Ionics 19, 777–785 (2013). https://doi.org/10.1007/s11581-012-0797-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-012-0797-6