Abstract
α-Fe2O3 and Fe3O4 hollow nanospheres of size 30 ± 2 nm were synthesized by using polymeric micelles as a soft template for the first time. The hollow nanospheres were thoroughly characterized by transmission electron microscope, superconducting quantum interference device, X-ray diffraction, Fourier transform infrared spectroscopy, thermogravimetry and differential thermal analysis, cyclic voltammogram, and nitrogen sorption analyses. The α-Fe2O3 and Fe3O4 hollow nanospheres were used as anode materials in lithium-ion rechargeable batteries to investigate their electrochemical properties. The hollow particle-based electrodes exhibit high capacity, stable cycling performance, and good rate capability at different current densities. The α-Fe2O3 and Fe3O4 hollow nanospheres with nanosized shell domain favors fast lithium insertion/extraction processes during the repeated charge/discharges.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Developing Li-ion batteries (LIBs) with high specific capacities and high current densities as power sources for diverse applications is of great interest [1–4]. The development of next-generation LIBs is a key to the success of electric and hybrid electric vehicles, advanced electronic devices, and implantable medical devices [1, 5, 6]. Since the pioneering work of Poizot and co-workers in the year 2000 on the reversible full reduction of 3D metal oxides, the research on α-Fe2O3 electrode has gained much interest [7]. Iron oxide, one of the main components of the earth’s crust, is considered to be a potential candidate with a high charge/discharge current densities and high reversible capacity coupled with its low cost and environmental benignity. Stimulated by both the promising applications of iron oxides coupled with novel properties of nanosize, considerable efforts have been made in the synthesis of α-Fe2O3 nanoparticles, nanorods, nanowires, and nanotubes [8–14]. Investigation by Larcher et al. and Morales et al. on Fe2O3 nanoparticles and by Chen et al. and Zhang et al. on carbon-coated Fe3O4 nanospindles has clearly proved that the morphology of nanosized particles plays a significant role in the reactivity of the electrode materials toward lithium [15–17]. In addition, it has been proved that the electrochemical reaction of Li with Fe2O3 is thermodynamically feasible, and up to 6 mol of Li can be intercalated to give a maximum theoretical capacity of 1,007 mAh g−1 [17]. Similarly, Fe3O4 can accommodate up to 8 mol of Li making them promising anode materials for high-performance lithium-ion batteries.
Recently, polymeric micelles with core–shell–corona structure have increasingly used for the fabrication of hollow nanospheres [18]. The micelle of poly(styrene-b-2-vinylpyridine-b-ethylene oxide) (PS−PVP−PEO), consisting of a PS core, a PVP shell, and a PEO corona was used as a soft template in the fabrication of hollow silica nanospheres in our laboratory. Herein, we report on the fabrication of hollow α-Fe2O3 and Fe3O4 nanospheres with micelles of poly(styrene-b-acrylic acid-b-ethylene oxide) (PS–PAA–PEO) using FeCl3·6H2O as iron precursor. The importance of this strategy is that the PS core functions as template for hollow void, PAA shell domain containing anionic −COO− ions serves as effective reaction center for metal ions, and PEO corona stabilizes the particles from aggregation. In the present report, the precursor metal cations (Fe3+) are first precipitated on the anionic PAA block with COO− groups in the form of hydroxides, and this phase is subsequently transformed to porous α-Fe2O3 hollow nanospheres by calcinations in air atmosphere. The α-Fe2O3 hollow spheres further converted to magnetite hollow nanospheres by controlled reduction with a mixture of H2 and N2 at 350 °C. To the best of our knowledge, this is the first report for the synthesis of magnetic hollow nanospheres of size about 30 nm using soft template strategy. Both α-Fe2O3 and Fe3O4 nanospheres were further investigated as anode materials for their electrochemical lithium intercalation/deintercalation behaviors in rechargeable LIBs.
Experimental
Material preparation and characterization
The α-Fe2O3 hollow nanospheres were fabricated as depicted in Scheme 1. The micelle solution was adjusted to pH 8 by using dilute NaOH solution. The required amount of micelle solution (10 mL) was stirred for a few minutes followed by addition of FeCl3·6H2O under vigorous stirring (the ratio of Fe3+/PAA was 10). Then dilute NH4OH was added slowly until the pH is reached to about 10 and the obtained brown precipitate was aged for 2 days at room temperature under static conditions. The composite particles were repeatedly washed with distilled water and ethanol and dried at 60 °C. In order to remove the polymeric template as well as to crystallize the hollow particles, the composite particles were heated to 400 °C for 3 h in a muffle furnace under air. The α-Fe2O3 hollow nanospheres obtained by the above method were further reduced to Fe3O4 hollow nanospheres using a mixture of H2 and N2 at 350 °C for 2 h. Prior to transformation of Fe2O3 to Fe3O4 hollow nanospheres, the suitable reduction temperature (350 °C for 2 h) was identified since high temperature (above 450 °C) leads to total reduction of Fe3+ to a certain extent. Wide-angle X-ray diffraction (XRD) was recorded on a Shimadzu XRD-7000 (WXRD) diffractometer. N2 adsorption/desorption analyses were carried out by using an Autosorp I apparatus. Transmission electron microscope (TEM) images were recorded on JEOL JEM-1210 (80 kV) and JEM-2100 (200 kV) electron microscopes. Fourier transform infrared (FTIR) spectra were recorded on a Jasco FT/IR 7300 spectrometer by using a KBr pellet technique. Thermogravimetry and differential thermal analysis (TG–DTA) were obtained with a MAC Science TG-DTA 2100 instrument under air. The magnetic measurement was made on Quantum Design SQUID MPMS-XL instrument, and temperature-dependant magnetization data were obtained at 5,000 Oe
Electrode preparation
For lithium insertion studies in lithium-ion batteries, the Fe2O3/Fe3O4 nanosphere (5 mg) was mixed mechanically with teflonized acetylene black (2 mg), and then the mixture was pressed on a stainless steel mesh as the current collector under a pressure of 500 kg/cm2 and dried at 160 °C for 4 h under vacuum. The electrochemical characterizations were carried out using CR-2032 coin-type cells with pure lithium (Cyprus Foote Mineral Co.) as an anode. The electrolyte used was 1 M LiPF6-EC/DMC (1:2 by volume, Ube Chemicals Co. Ltd.). The coin cell assembling was performed in a glove box filled with argon (dew point, lower than −80 °C). Cyclic voltammograms (CVs) were recorded by Hokuto denko HSV-100 (Japan) in a beaker-type cell which contains Fe2O3 or Fe3O4 working electrode, and lithium foil acts as both reference and counter electrodes. The beaker-type cell was assembled in the argon filled glove box and sealed well using Teflon seal tape to prevent air oxidation.
Results and discussion
The fabrication of Fe2O3 and Fe3O4 hollow nanospheres using PS–PAA–PEO micelles is depicted in Scheme 1. On gradual addition of FeCl3 solution to the above micelles, the anionic PAA shell block effectively binds with Fe3+ ions through electrostatic interaction as shown in the Scheme 1. The charge neutralization between –COO− and Fe3+ ions also confirmed from change in the ξ-potential from −36 to 0 mV with gradual addition of FeCl3 to the micelle solution (Fig. S1, supporting information). Thermal analyses (TG/DTA) of iron oxide/polymer composite particles (prior to calcinations) indicated that most of the templates were decomposed below 400 °C (Fig. S2, supporting information). The FTIR spectrum of composite particles (Fig. S3-A, supporting information) containing –COO–Fe3+ complex exhibits bands at 1,737 and 1,456 cm−1 corresponding to asymmetric and symmetric stretching vibration of –COO−, respectively. The FTIR spectra of α-Fe2O3 and Fe3O4 materials exhibited bands at 580, 850–1,040, and 1,600 cm−1 characteristic of Fe–O bond vibrations (Fig. S3B-C, supporting information) [19]. The absence of C–H, –C=C–, and –COOH bonds stretching vibrations of phenyl groups of the polymer backbone in the calcined sample suggests the complete removal of templates from the hollow nanospheres. Furthermore, thermal analysis of calcined samples revealed negligible amount of organics in the Fe2O3 hollow particles. Figure 1a, c shows the TEM images of α-Fe2O3 and Fe3O4 hollow nanospheres, respectively; the average particle size and void space diameter of hollow nanospheres were found to be 30 ± 2 and 15 ± 1 nm, respectively; the reduction of α-Fe2O3 to Fe3O4 has not affected the spherical morphology of particles. All the hollow particles are polycrystalline, and the shell domain is comprised of nanoparticles of diameter about 2–4 nm as evidenced from the high-resolution TEM images (Fig. 1b). The wall thickness estimated from TEM images was found to be 8 ± 0.5 nm. Figure 2 shows the powder XRD patterns of nanosized hollow iron oxides; the XRD profile prior to hydrogen treatment matches well with the standard α-Fe2O3 (JCPDS 33-0664) [20, 21]. After reduction, the crystalline structures could be indexed to Fe3O4 (JCPDS 75-1609). All detected sharp diffraction peaks could be indexed as Fe3O4, and the absence of peak at 2θ = 44 ° clearly confirms the absence of metallic iron (Fe0) [21]. It is important to note that controlled reduction at low temperature (at 350 °C for 2 h) avoids Fe metal formation unlike the high-temperature treatment using H2. Furthermore, the α-Fe2O3 hollow nanospheres were completely converted to Fe3O4 because the peaks unique for α-Fe2O3 (including the peaks at 24.1, 33.08, and 40.86) disappeared completely. The formation of Fe3O4 is further confirmed by the color change from brown to totally black materials.
The Brunauer–Emmett–Teller surface areas evaluated by nitrogen adsorption/desorption analyses of α-Fe2O3 and Fe3O4 hollow nanospheres were found to be 106 and 93 m2 g−1, respectively. Magnetic characterization was performed using a superconducting quantum interference device (SQUID). The temperature-dependant magnetization at 5,000 Oe for Fe3O4 hollow particles is shown in the supporting information (Fig. S4). The hollow Fe3O4 nanospheres are superparamagnetic at room temperature. The hollow particles exhibited a magnetic moment of 22.3 emu/g which is comparable to values obtained by Sun et al. for Fe3O4 hollow nanospheres of size about 20–25 nm [21]. Furthermore, the decrease in magnetic susceptibility with decreasing temperature indicates the hollow nanospheres become antiferromagnetic at low temperature, and the antiferromagnetism increases with decreasing temperature up to 2 K. The Néel temperature (T N), the critical temperature for onset of antiferromagnetism, is observed at about 200 K characteristics of magnetite particles [22].
Figure 3a, b shows discharge–charge curves in the voltage window of 0.005−3.0 V (vs. Li) at a rate of 0.25 C up to 50 cycles, and for clarity, only selected cycles are shown in the voltage versus capacity profiles. During the first discharge, both α-Fe2O3 and Fe3O4 exhibit voltage plateaus at about 1.1 and 0.8 V (vs. Li) resulting from the lithium reaction with iron oxide nanospheres [14, 17]. The voltage plateau at 1.1 continues until a capacity of about 220 ± 20 mAh g−1 is reached for both hametite and magnetite which is ascribed to Li insertion into the shell domain of hollow nanospheres, whereas the voltage plateau at 0.8 V continuing up to 950 mAh g−1 reflects the reduction of Fe3+ to Fe0 metal clusters [16]. The charge curve showed a sloping plateau at 1.95 V due to reverse oxidation reaction of Fe0 → Fe3+. The total first-discharge capacity is 1,430 ± 10 mAh g−1 which corresponds to 8.6 mol of Li/mol of Fe2O3, whereas Fe3O4 exhibited slightly higher discharge capacity 1,495 mAh g−1 (9.0 mol Li/mol of Fe3O4) at similar experimental conditions. The first-discharge profiles of both hametite and magnetites qualitatively resemble that observed by Larcher et al. and Morales et al. on Fe2O3 nanoparticulate and Chen et al. on Fe2O3 nanotubes [14–17]. It is noteworthy to mention that in the complete reduction of Fe3+ → Fe0, one would expect a maximum uptake of 6 Li/Fe2O3 or 8 Li/Fe3O4 (1,005 mAh g−1), and the excess capacity must originate from electrolyte decomposition in the low-potential region and subsequent formation of solid electrolyte interphase on the hollow nanospheres [23, 24].
The discharge capacities of the α-Fe2O3 electrode (Fig. 3a) in the 1st, 2nd, 5th, 10th, 25th, and 50th cycles are 1,435, 1,109, 895, 681, 518, and 490 mAh g−1, respectively. The corresponding charge capacity values are 998, 943, 811, 650, 503, and 485 mAh g−1 for the 1st, 2nd, 5th, 10th, 25th, and 50th cycles, respectively. The coulombic efficiency of first cycle was found to be 69.5 %. The corresponding capacity versus cycle number plot is shown in Fig. 4a. As can be seen, both discharge and charge capacities decrease up to 15 cycles and thereafter the capacity stabilizes. After 50 cycles with 100 % depth of discharging and charging at a rate of 0.25 C, the electrode capacity decreased to 485 mAh g−1. The capacity retention of Fe2O3 from 15th to 50th cycle is 84.2 %. Similar charge/discharge cycling performance was also observed on α-Fe2O3 nanotube electrodes and nanoflakes embedded on copper foil [17, 25]. Furthermore, it is relevant to mention that the capacity of commercial α-Fe2O3 faded within few cycles to 250 mAh g−1 and gradually decreased to about 130 mAh g−1 in less than 50 cycles [26]. However, the performance of α-Fe2O3 hollow nanospheres in the present study is more than three times higher than that of commercial Fe2O3 and porous α-Fe2O3 nanotubes in the absence of any additives [27].
The magnetite (Fe3O4) hollow nanospheres, however, exhibit enhanced charge/discharge capacities (Fig. 3b) compared to α-Fe2O3. The discharge capacity values (Fe3O4) at 0.25 C rate in the voltage window of 0.005–0.3 V (vs. Li) for 1st, 2nd, 5th, 10th, 25th, and 50th cycles are 1,498, 1,139, 1,041, 890, 694, and 690 mAh g−1, respectively. The corresponding charge capacity values of Fe3O4 are 1,060, 1,044, 990, 867, 695, and 688 mAh g−1 for the 1st, 2nd, 5th, 10th, 25th, and 50th cycles, respectively. The coulombic efficiency of first cycle was found to be 71 %, marginally higher than the α-Fe2O3 hollow nanospheres. The cycling performance of Fe3O4 (Fig. 4b) also shows similar behavior as that of α-Fe2O3 and the discharge capacities decrease up to 15 cycles possibly due to the formation of stable electrolyte film, and complete coverage of all hollow nanoparticles may require several charge/discharge cycles and similar observation was also noticed by other groups [14–17]. The observed discharge capacity after 50 cycles (690 mAh g−1) for Fe3O4 hollow nanospheres is considerably higher than the carbon/Fe3O4 nanospindles (600 mAh g−1) composite electrodes, where the Fe3O4 nanoparticles were mixed with carbon matrix [25]. However, it is worth to note that carbon/Fe3O4 electrode delivers 600 mAh g−1 after 80 cycles. On comparison with commercial Fe3O4 nanoparticles, which fades quickly and as a result provides low capacity (152 mAh g−1, after 50 cycles) [26], the Fe3O4 hollow nanospheres exhibit 4.5 times higher discharge capacity than the commercial Fe3O4. The capacity fading may be partly due to volume change during repeated charge/discharges similar to Sn- and Si-based anodes [28]. The difference in electrochemical performance of α-Fe2O3 and Fe3O4 with nearly similar size 30 ± 2 nm is attributed to the intrinsic electronic conductivities of latter which contribute to higher discharge/charge capacities and superior performance than the α-Fe2O3 [29]. This fact is further confirmed from the enhanced performance of Fe3O4/single-walled carbon nanotubes (SWNT) due to high electronic conductivity of these composite electrodes containing SWNT [30]. Therefore, one can expect still improved performance of Fe3O4 hollow nanospheres when mixed with appropriate carbon additives. At this juncture, we are investigating the performance of synthesized Fe2O3 and Fe3O4 with different carbon additives. We believe this may enhance the initial coulombic efficiency and cyclability.
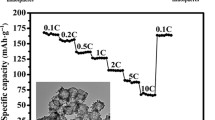
Figure 5a, b shows the rate performances of α-Fe2O3 and Fe3O4 hollow nanospheres, respectively. At a low rate of 0.15 C (Fe3O4, charge–discharge of all active materials in period of 400 min) or 0.1 C (Fe2O3), both α-Fe2O3 and Fe3O4 show about 1,100 to 1,150 mAh g−1. However, at a high rate of 5 C (discharge/charge of all active materials within 12 min), the specific capacity of α-Fe2O3 decreases to 265 mAh g−1, whereas that of Fe3O4 reduced to 410 mAh g−1 at the same rate of 5 C. Thus, consistence with other nanostructured materials with different morphologies, the iron oxide hollow particles also show higher capacity at slower rate (0.1 C) than that at faster rate (5 C). However, the α-Fe2O3 and Fe3O4 electrodes almost regain their original high capacities when the rate was again lowered to 0.3 C after being exposed to high current loads (5 C), which indicates the high stability of hollow nanosphere-based electrodes. The improved electrochemical performance of both Fe2O3 and Fe3O4 is attributed partially to the unique hollow spherical morphology. More importantly, the void space not only effectively buffers against charge storage and local volume change but also provides better electrical contact and shorter diffusion path length providing better rate capability. In addition, enhanced capacity retention, rate performance, and cycling performance of Fe3O4 over Fe2O3 hollow nanospheres are solely attributed to inherent electronic conductivities of magnetite particles. Figure 6 shows the CVs of α-Fe2O3 and Fe3O4 in the potential window 0.005–3 V (vs. Li+/Li) at a scan rate of 3 mV/min. During the first cycle, a smooth sloping curve (cathodic) up to about 0.8 V with shoulders at 1.68 and 1.1 V is indicative of insertion of Li and reduction of Fe3+ in α-Fe2O3 in the shell domain of hollow nanospheres. The appearance of a strong oxidation peak at 1.6 V suggests the oxidation of Fe0 to Fe3+ ions. The second cathodic sweep differs from the first one; the shoulders at 1.68 and 1.1 V appeared in the first scan vanished in the second cycle. The cyclic voltammogram of Fe3O4 is slightly different from Fe2O3 (Fig. 6b). In the cathodic polarization process of the first cycle, a reduction peak appeared at 0.5 V corresponding to Li insertion and reduction of Fe3+ to metallic iron. However, the oxidation scanning exhibited two peaks at 1.55 and 2.14 V indicating the oxidation of Fe0 to Fe2+ and then to Fe3+. The second cathodic sweep exhibited an additional shoulder at 1.5 V, and essentially the peak positions are unaltered with subsequent cycles.
Conclusions
In summary, we have demonstrated a simple soft template method for fabrication of α-Fe2O3 and Fe3O4 hollow nanospheres of size 30 ± 2 nm with void space of about 14–15 nm. Fe3O4 hollow particles exhibited a magnetic moment of 22.5 emu/g as estimated by SQUID. The charge/discharge cycling performance and rate performance α-Fe2O3 hollow nanospheres were comparable to that of α-Fe2O3 nanotube and nanoflakes coated on conducting copper foil. Fe3O4 hollow nanospheres exhibited higher discharge capacity after 50 cycles (690 mAh g−1) which is considerably higher than the carbon/Fe3O4 nanospindles (600 mAh g−1) composite electrodes and commercial Fe3O4. The Fe3O4 nanospheres show 4.5 times higher discharge capacity than the commercial materials, and the improved rate performance is attributed partially to the unique hollow spherical morphology coupled with hollow void space. The void space not only acts as buffer medium against charge storage and local volume change but also provides better electrical contact and shorter diffusion path length and therefore provides better rate capability.
References
Armand M, Tarascon JM (2008) Nature 451:652
Li N, Martin CR, Scrosati B (2001) J Power Sources 97–98:240
Wang H, Abe T, Maruyama S, Iriyama Y, Ogumi Z, Yoshikawa K (2005) Adv Mater 17:2857
Park M, Kang Y, Wang G, Dou S, Liu H (2008) Adv Funct Mater 18:455
Chen J, Cheng F (2009) Acc Chem Res 42:713
Jiang C, Hosono E, Zhou H (2006) NanoToday 1:28
Poizot P, Laruelle S, Grugeon S, Dupont L, Tarascon JM (2000) Nature 407:496
Xu XN, Wolfus Y, Shaulov A, Yeshurun Y, Felner I, Nowik I, Koltypin Y, Gedanken A (2002) Appl Phys Lett 91:4611
Ji J, Ohkoshi S, Hashimoto K (2004) Adv Mater 16:48
Woo K, Lee HJ, Ahn JP, Park YS (2003) Adv Mater 20:1761
Wang X, Chen X, Gao L, Zheng Z, Ji M, Tang C, Sen T, Zhang Z (2004) J Mater Chem 14:905
Fu YY, Wang RM, Xu J, Chen J, Yan Y, Narlikar AV, Zhang H (2003) Chem Phys Lett 379:373
Shen XP, Liu HJ, Pan L, Chen KM, Hong JM, Xu Z (2004) Chem Lett 33:1128
Larcher D, Masquelier C, Bonnin D, Chabre Y, Masson V, Leriche JB, Tarascon JM (2003) J Electrochem Soc 150:A133
Larcher D, Bonnin D, Rivals I, Personnaz L, Tarascon JM (2003) J Electrochem Soc 150:A1643
Morales J, Sanchez L, Martin F, Berry F, Ren X (2005) J Electrochem Soc 152:A1748
Chen J, Xu L, Li W, Gou X (2005) Adv Mater 17:582
Khanal A, Inoue Y, Yada M, Nakashima K (2007) J Am Chem Soc 129:1534
Tan H, Xue JM, Shuter B, Li X, Wang J (2010) Adv Funct Mater 20:722
Bang JH, Suslick KS (2007) J Am Chem Soc 129:2242
Peng S, Sun S (2007) Angew Chem Int Ed 46:4155
Yi DK, Selvan ST, Lee SS, Papaefthymiou GC, Kundaliya D, Ying JY (2005) J Am Chem Soc 127:4990
Xing WB, Dahn JR (1997) J Electrochem Soc 144:1195
Mukai SR, Hasegawa T, Takagi M, Tamon H (2004) Carbon 42:837
Zhang WM, Wu XL, Hu JS, Guo YG, Wan LJ (2008) Adv Funct Mater 18:3941
Tartaj P, Amarilla JM (2011) J Power Sources 196:2164
Guo YG, Hu YS, Sigle W, Maier J (2007) Adv Mater 19:2087
Aifantis KE, Huang T, Hackney SA, Sarakonsri T, Yu A (2012) J Power Sources 197:246
Taberna PL, Mitra S, Poizot P, Simon P, Tarascon JM (2006) Nat Mater 5:567
Ban C, Wu Z, Gillaspie DT, Chen L, Yan Y, Blacburn JL, Dillion AC (2010) Adv Mater 22:E145
Acknowledgments
One of the authors (KN) thanks the Japan Society for the Promotion of Science for a Grant-in-Aid for Scientific Research (20310054).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
DLS, TG/DTA, FTIR and SQUID data.
Below is the link to the electronic supplementary material.
ESM 1
(DOC 166 kb)
Rights and permissions
About this article
Cite this article
Sasidharan, M., Gunawardhana, N., Yoshio, M. et al. α-Fe2O3 and Fe3O4 hollow nanospheres as high-capacity anode materials for rechargeable Li-ion batteries. Ionics 19, 25–31 (2013). https://doi.org/10.1007/s11581-012-0716-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-012-0716-x