Abstract
Lithium ion batteries have become attractive for portable devices due to their higher energy density compared to other systems. With a growing interest to develop rechargeable batteries for electric vehicles, lithium iron phosphate (LiFePO4) is considered to replace the currently used LiCoO2 cathodes in lithium ion cells. LiFePO4 is a technically important cathode material for new-generation power lithium ion battery applications because of its abundance in raw materials, environmental friendliness, perfect cycling performance, and safety characteristics. However, the commercial use of LiFePO4 cathode material has been hindered to date by their low electronic conductivity. This review highlights the recent progress in improving and understanding the electrochemical performance like the rate ability and cycling performance of LiFePO4 cathode. This review sums up some important researches related to LiFePO4 cathode material, including doping and coating on surface. Doping elements with coating conductive film is an effective way to improve its rate ability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
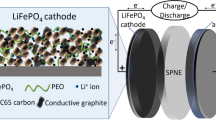
One of the greatest challenges at present is how to make use of renewable energies and the replacement of petroleum with electric propulsion as a worldwide imperative. Among the various available storage technologies, the lithium ion battery, which has conquered the portable electronic market, has become the prime candidate to power the next generation of electric vehicles (EVs) and plug-in hybrid electric vehicles (PHEVs) [1]. With regard to large-size applications of lithium ion battery such as in EVs and HEVs, in contrast, lower-cost and safe cathode materials are required. As the demand for powerful and large Li ion batteries grows, LiFePO4 has received much attention as a positive electrode (cathode) material because of its stability, low cost, and environmental friendliness. Figure 1 shows the charge and discharge diagram of LiFePO4/graphite battery [2, 3]. LiFePO4 initially includes one Li+ ion per formula unit that can be extracted and transferred to the anode in the first charge process, compensating for the oxidation from Fe2+ to Fe3+ as shown in Fig. 2 [4–6]. The theoretical capacity based on this one electron reaction is 170 mAh g−1, but the poor conductivity, resulting from the low lithium ion diffusion rate and low electronic conductivity in the LiFePO4 phase, has posed a bottleneck for commercial applications. Ever since it was introduced by Radhi et al. [5], many efforts have been made to enhance its low conductance. Improvements in conductivity have been achieved in two ways. One was to dope with certain elements in Li, Fe, or O sites to improve the intrinsic conductivity and promote the redox potential. Surface modification of the LiFePO4 is another effective way to improve the conductance and reduce the side reactions. This review sums up some important researches related to LiFePO4 cathode material, including doping and coating on surface. The latest research progresses which improve the electrochemical properties of LiFePO4 such as rate capability and cyclic performance are presented.
a Structure transformation of orthorhombic LiFePO4 and trigonal quartz-like FePO4 during charge and discharge obtained from [6]. b Cyclic voltammograms of LiFePO4 at a scan rate of 0.1 mV s−1 between 2.5 and 4.3 V (vs. Li/Li+) obtained from our experimental data
LiFePO4 doped by ions
In order to improve the conductance of LiFePO4, much effort has been paid. Coating by carbon is an efficient way to increase the electrochemical performance of these materials [7–9]. Unfortunately, the carbon coating method obviously helps nothing in the lattice electronic conductivity or chemical diffusion coefficient of lithium within the crystal [10]. Substitution of a small quantity of Li+, Fe2+, or O2− by other ions greatly improves the kinetics of materials in terms of capacity delivery, cycle life, and rate capability. The first-principles investigation also reveals that LiFePO4 is most affected by F ion doping at O site with the narrowest band gap, followed by Mn ion doping at Fe site and Na ion doping at Li site, indicating that appropriate ion doping in LiFePO4 could improve its electronic conductivity [11].
Doping in the Li site
Chung et al. [12] have reported that the controlled cation doping with metal ions supervalent to Li+ increased the electronic conductivity of LiFePO4 by a factor of ~108. Although there is much controversy in this report, there is no dispute that doping can increase the conductivity of LiFePO4 in the Li site. The first-principles calculations show that the electronic conductive properties and ionic transport feature of LiFePO4 can be improved by Na doping in the Li site and favorable for high-rate performance [13]. Yin et al. [14] reported the single-phase Li1−x Na x FePO4/C (x = 0, 0.01, 0.03, 0.05) samples synthesized by in situ polymerization restriction–carbon thermal reduction method. The doped Na ion does not destroy the lattice structure of LiFePO4, and the discharge capability and cycle performance are improved by an appropriate amount of Na doping as plotted in Fig. 3a. This can be attributed to its smaller charge transfer resistance than that of LiFePO4/C. Yang et al. [15] reported the stoichiometric Cu-doped lithium iron phosphates synthesized via improved co-precipitation, followed by sintering at high temperature for crystallization. The particle size of the Li0.98Cu0.01FePO4 with pure single phase was drastically fine with 100–200 nm, and the reversible capacity, cycle number, and charge–discharge characteristics exhibited better than those of LiFePO4 as shown in Fig. 3b. Ying et al. [16] reported the spherical olivine Li0.97Cr0.01FePO4/C powders synthesized by carbothermal reduction process, and the spherical olivine Li0.97Cr0.01FePO4/C powder has a higher tap density (1.8 g cm−3) than that of the non-spherical LiFePO4 powders and then shows excellent cycling performance as given in Fig. 3c. Li et al. [17] reported submicron-sized Ti-doped LiFePO4 cathode materials synthesized by a reformative co-precipitation and normal-temperature reduction method. The result shows that higher Ti ions’ doping levels are conducive to the electrochemical performance of LiFePO4, and the sample doped with 3 at.% Ti shows the most impressive cycling performance among all samples, even after 100 cycles at 1-C rate (see Fig. 3d). Zhang et al. [18] reported the Nd-doped LiFePO4/C cathode synthesized by a novel solid-state reaction method at 750 °C without using inert gas. The results indicate that Nd3+ and carbon modification do not affect the structure, and the particle size is around 200 nm. The Li0.99Nd0.01FePO4/C powder exhibited a higher discharge capacity than that of pure LiFePO4/C at different rates as shown in Fig. 3e. Zhang et al. [19] reported the Li0.99Mo0.01FePO4/C cathode materials prepared by an easy solution method followed by heat treatment at various temperatures. The results indicate that doping does not affect the olivine structure but considerably improves its capacity delivery and cycling performance as shown in Fig. 3f. It can be ascribed to the enhancement of the electronic conductivity by ion doping and carbon coating. In addition, it has been reported that the controlled cation doping with Mg2+ [20], La3+[21], and Y3+[22] ions to Li+ also increased the electronic conductivity of LiFePO4 and then showed a higher electrochemical performance than that of pure LiFePO4.
Effect of metal ions doping in the Li vacancy on the morphology and performance of the synthesized LiFePO4 powders as deduced from XRD, SEM, and cycling performance measurements. a Li1−x Na x FePO4/C (x = 0, 0.01, 0.03, 0.05), b Li0.98Cu0.01FePO4, c Li0.97Cr0.01FePO4/C, d Li1−x Ti x FePO4 (x = 0, 0.01, 0.03, 0.05), e Li0.99Nd0.01FePO4/C, and f Li0.99Mo0.01FePO4/C from [14–19]
Doping in the Fe site
According to the report mentioned above, many researchers proposed that the dopants occupied the Li site due to their small ionic radii. Similarly, Fe site doping by small amounts of Na, Cu, Zn, Mg, Ni, Al, Co, Cr, Mn, Ru, Ti, or V also resulted in improved electrochemical properties mainly by enhancing the electronic conductivity. In addition, it has been reported that Fe site doping weakens the Li–O interaction, resulting in high ionic mobility and diffusion coefficiency [23–25]. First-principle calculation shows that doping at Fe site with alkali metal ions facilitates the diffusion of Li+ ions along the 1D pathway, which can increase both the electronic and ionic conductivity [26]. Wang et al. [27] reported Na+ and Cl− co-doped LiFePO4/C composites prepared via a simple solid-state reaction. The specific capacities of Na+/Cl−-doped LiFePO4/C material are 157 mAh g−1 at 0.2 C, 115 mAh g − 1 at 10 C, and 98 mAh g−1 at 20 C, respectively, as shown in Fig. 4a. The improvement can be ascribed to the enhanced electronic conductivity and electrode kinetics due to the micro-structural modification promoted by co-doping.
Cycling performance of metal ion-doped LiFePO4 cathode materials. a Na+, Cl− co-doped LiFePO4 from [27]; b 0, 1.5, 2.5, and 5 % ZnO-doped LiFePO4 at a discharge current intensity of 20 mA cm−2 from [30]; c Ni-doped LiFePO4/C at 0.5-C rate from [31]; d Cycling performance of LiAl x Fe1−3x/2PO4/C at 5-C rate (a) x = 0.01, (b) x = 0.02, (c) x = 0.04, (d) x = 0.06, (e) x = 0.12, (f) x = 0 from [38]; e rate performances of Co-doped LiFePO4/C from [40]; f rate performances of LiFePO4, LiFePO4/C, and LiFe0.97Cr0.03PO4/C discharged at various C rates from [42]
Chang et al. [28] reported Cu-doped LiFe1−x Cu x PO4/C (x = 0, 0.01, 0.015, 0.02, 0.025) cathode materials with a high tap density synthesized by a solid-state reaction in an inert atmosphere. LiFe0.98Cu0.02PO4/C exhibits excellent charge/discharge capacities of about 150 mAhg−1 (297 mAh cm−3) at a rate of 0.1 C and more than 127.3 mAh g−1 (252.1 mAh cm−3) at a rate of 2 C.
Liu et al. [29] first reported the spherical zinc-doped LiZn0.01Fe0.99PO4 synthesized by the solid-state route. Zn doping favors the formation of the crystal structure, expands the lattice volume, and then provides more space for lithium ion intercalation/de-intercalation. Shenouda et al. [30] reported the ZnO-doped LiFePO4 by a solid-state route. The 2.5-% ZnO-doped LiFePO4 demonstrates higher conductivity than the 1.5-% ZnO and 5-% ZnO-doped LiFePO4 or the un-doped sample. The 2.5-% ZnO-doped LiFePO4 shows excellent cycling performance at a discharge current intensity of 20 mA cm−2. The initial specific discharge capacity is about 177 mAhg−1, and the capacity reaches 167 mAhg−1 after 150 cycles as shown in Fig. 4b.
Zhang et al. [31] reported the spherical Ni-doped LiFePO4/C synthesized by the conventional solid-state reaction method. The EIS results reveal that Ni doping can decrease the resistance of LiFePO4/C composite electrode drastically and improve its reversibility. Hence, Ni-doped spherical LiFePO4/C composite exhibits better electrochemical performances compared to an un-doped one as shown in Fig. 4c. Lu et al. [32] reported the LiFe1−x Ni x PO4/C (x = 0, 0.02, 0.04, and 0.06) composites prepared by a solid-state reaction. LiFe0.98Ni0.02PO4/C delivers the highest initial discharge capacity (121 mAh g−1) and the best cycling performance (108 mAh g−1 in the 50th cycle) at the 2-C rate (2.5–4.2 V) among all samples because nickel doping enhances the P–O bond, stabilizes the structure, and then decreases the charge transfer resistance.
Several authors reported that Mn2+-doped LiFe1−x Mn x PO4 solid solution could significantly improve the kinetic properties of LiFePO4 in the region of 0 ≤ x ≤ 0.75 [33–35]. It can be concluded that Mn doping may be an effective way to enhance the electrochemical performances of LiFePO4. Li et al. [36] reported the LiFe1−x Mn x PO4/C (x = 0, 0.05, 0.1) prepared by chelation-assisted mechanochemical activation method using C2H2O4 as the chelating reagent. The results indicate that Mn2+ doping can effectively enhance the electrochemical performance especially at high charge/discharge rate. However, Chen et al. [37] reported that the substituted metal Mn2+ does not work completely at a higher discharge rate due to the poor electrical conductivity and a serious Jahn–Teller effect by in situ metal K-edge absorption analysis.
Xu et al. [38] reported the LiAl x Fe1−3x/2PO4/C (x = 0, 0.01, 0.02, 0.04, 0.06, 0.12) prepared via an easy solution method. The results indicate that Al3+ does not affect the olivine structure but considerably improves its initial capacity and cycle performance as plotted in Fig. 4d. LiAl0.01Fe0.985PO4/C shows the best electrochemical performance at a discharge rate of 5 C, and it can be ascribed to the enhancement of the electronic conductivity by Al3+ substitution and carbon coating.
Yoon et al. [39] reported that a cobalt-doped sample was found to have less covalent P–O bonds due to the increased covalence of Fe3+–O bonds via the inductive effect compared to the pristine LiFePO4. It is likely that cobalt ion doping can stabilize the covalence of P–O bonds in LiFePO4 and then improve its electrochemical performance. Yang et al. [40] reported Co-doped LiFePO4/C materials synthesized by a hydrothermal method. The Co-doped sample shows good electrochemical performance at a discharge rate of 5 C as plotted in Fig. 4e. However, Shanmukaraj et al. [41] reported that cobalt doping does not have a favorable effect on the electrochemical performance of LiFePO4 cathode materials.
Shin et al. [42] reported the Cr-doped LiFePO4/C synthesized by a mechanochemical process followed by a one-step heat treatment. LiFe0.97Cr0.03PO4/C shows the best/excellent rate performance among all samples, delivering the discharge capacity up to 120 mAh g−1 at 10-C rate as given in Fig. 4f. Chromium doping facilitates the phase transformation between triphylite and heterosite during cycling. Recently, it was reported that Ru-doped material could offer quick Li permeation in addition to high electronic conductivity [43–45]. Yang et al. [46] reported the Ru-doped LiFePO4/C cathode material synthesized by RPR (rheological phase reaction) method, and the doped Ru enhances the conductivity and diffusion coefficient of Li+ and then improves the charge–discharge performance. The RPR preparation process is as follows: The starting materials (CH3COOLi, FeC2O4·2H2O, NH4H2PO4 and polyethylene glycol) were mixed by grinding for 10 min and then added in deionized water to get a rheological body. The mixture was calcinated in a tube furnace at 350 °C for 10 h with flowing nitrogen gas, then it was sintered at 750 °C for a few hours.
In addition, the positive effect of doping by Ti4+, Zr4+ [47], and V5+ [48, 49] in the Fe site on the rate capacity and cyclic stability of LiFePO4 also has been reported in a group of studies. Wang et al. [47] reported that the Zr- or Ti-doped LiFePO4 demonstrated a stable discharge capacity of 160–165 mAh g−1, almost approaching the theoretical capacity. The good electronic conductivity and nanocrystalline could contribute to the unique performance of LiFePO4 electrodes. Sun et al. [48] reported V-doped LiFePO4/C cathode materials prepared through a carbothermal reduction route. V-doped LiFePO4/C shows a high discharge capacity of ~70 mAh g−1 at 20 C. Bilecka et al. [50] reported a microwave-assisted liquid-phase synthesis route to LiFePO4 doped with divalent (Mn, Ni, Zn), trivalent (Al), and tetravalent (Ti) metal ions in varying concentrations. The result shows that Ni- and Zn-doped LiFePO4 with nominal dopant concentrations of 7 and 2 mol%, respectively, outperformed all the other samples. They offer initial specific charge of about 168 Ah kg−1 and excellent capacity retention of 97 % after 300 full cycles. A discharge rate of 8 C still results in 152 Ah kg−1 after 50 cycles. From the discussion mentioned above, it can be concluded that it will be promising if proper cation doping can be used in LiFePO4 to further improve its electrical conductivity, favoring fast charge and discharge rate.
Doping in the O site
Besides cation doping, there are some researches relative to the substitution of the small amount of Cl− and F− for O2− anion. Cl doping has been found to be effective in enhancing the electrochemical performance of cathodes such as LiNi0.7Co0.3O2 [51]. It also has been reported that oxygen substitution with anion such as F− is effective to acquire high-rate capability and cycle stability for layered structure LiNiO2 [52], LiNi1/3Co1/3Mn1/3O2 [53] cathode materials, and spinel LiMn2O4 [54, 55] and LiMn1.5Ni0.5O4 [56, 57] material. Sun et al. [58] reported Cl-doped LiFePO4/C cathode materials synthesized through a carbothermal reduction route, and it presented a high discharge capacity of ~90 mAh g−1 at a rate of 20 C at room temperature as given in Fig. 5a. EIS and CV indicate that the improved Li+ diffusion capability is attributed to the microstructure modification of LiFePO4 via Cl doping. Yang et al. [59] also reported the electrochemical performances of Cl-doped LiFePO4/C at 15 C at a high Cl doping level, which presented a capacity of ~90 mAhg−1. Lu et al. [60] reported the F-doped LiFePO4/C nanoparticles synthesized via a low-temperature hydrothermal reaction followed by high-temperature treatment. The F-doped sample shows increased initial discharge voltage at various C rates as shown in Fig. 5b because F doping can improve the electrical conductivity of these cathode materials, and the discharge capacities at different rates are 167.3 (0.1 C), 145.2 (1 C), 132.0 (2 C), 120.4 (5 C), 101.3 (10 C), and 90.5 (15 C) mAh g−1, respectively. Liao et al. [61] reported the effects of fluorine substitution on the electrochemical properties of LiFePO4/C cathode materials. They also found that F substitution can improve the rate capability of LiFePO4/C materials, and the LiFe(PO4)0.9 F0.3/C sample shows the best high-rate performance among all samples.
LiFePO4 coated by carbon
Carbon coating has been known to be effective not only in enhancing the electrical conductivity of metal oxides but also in increasing their absorbing ability against organic molecules. In addition, a coated carbon layer would protect the metal oxides from chemical corrosion [62–78]. Hence, carbon coating is one of the most important techniques used to improve the specific capacity, rate performance, and cycling life of LiFePO4. Carbon coating also reduces the particle size of LiFePO4 by inhibiting particle growth [63–65, 78] and suppresses the oxidation of Fe2+ to Fe3+ during sintering act as a reducing agent [65]. However, the analysis of the experimental data suggests that carbon coating has a more significant effect on the rate performance than particle size reduction and doping. The particles of about 300 nm exhibit good rate capability that is comparable to those of the nanosized particles [76]. Huang et al. [8] also reported that both particle size minimization and intimate carbon contact are needed to optimize the rate capability of this material. Cho et al. [66] reported that the carbon coating thickness has a more significant effect on the capacity as given in Fig. 6. Their results indicate that the electrochemical properties of LiFePO4 are correlated to the amount of carbon and its coating thickness and uniformity, and some amounts of graphite-like carbon in the disordered carbon structure can enhance the electronic conductivity of the carbon deposit. The type and morphology of the carbon deposits depend on the source of the carbon. Graphitic carbon generally provides higher conductivity and thus higher rate capacities at large discharge rates, so carbons with large sp2/sp3 ratios are generally preferred [77].
Discharge capacity versus cycle number for various LiFePO4 electrodes at 0.2-C rate from [66]
LiFePO4 particles with other coated conductive films, such as Ag [78–80], Ag + C [81], CuO + C [82], SiO2 [83], TiO2 [84], ZrO2 [85], CeO2 [86],NiP [87], PPy/PEG conductive layer [88, 89], and Fe2P [90], also improve their electrochemical performance. The synthesis methods and the electrochemical performance of LiFePO4 coated by non-carbon compounds are shown in Table 1. The improvement of cycling performance and discharge capacity for LiFePO4 coated by Ag [78–80], Ag + C [81], CuO[82], PPy/PEG [88], and Fe2P [90] is due to the increase of electronic conductivity and then results in a very significant increase of electro-active zones. The SiO2 coating increases the order of lithium ion intercalating the outer lattice of the particle and then improves capacity retention significantly [83]. The TiO2 coating itself is not stable and partially dissolved upon cycling, causing redeposition of Ti at the C anode. Ti deposit can remarkably improve the electrochemical performance at high charge–discharge rate of LiFePO4/Li cell due to the more active Ti [84]. The ZrO2 coating can remarkably improve the high-rate performance due to the amelioration of the electrochemical dynamics on the LiFePO4 electrode/electrolyte interface resulting from the effects of the ZrO2 nanolayer coating [85]. The CeO2 coating decreases the contact resistance and the charge transfer resistance and then improves the electrochemical performance [86]. NiP coating can sustain the structure stability and conductivity of LiFePO4 upon cycling because NiP coating has a good metallic mechanical property [87]. The coated results indicate that the surface treatment should be an effective way to improve the comprehensive properties of the cathode materials for lithium ion batteries.
Conclusions
LiFePO4 has been considered as one of the primary battery materials for EV, HEV, and PHEV applications due to its flat voltage profile, low material cost, abundant material supply, and better environmental compatibility compared to other cathode materials. The improvement of LiFePO4 cathode materials is a big challenge in order to fulfill the requirements of future energy storage. From the discussion earlier, one of the best methods to improve the power performance of LiFePO4 is to improve its electronic conductivity by doping and coating. Coating with carbon or other conductive films can help to modify the total electronic conductivity of LiFePO4 composites, while doping with cations and anions may improve the intrinsic electronic conductivity. It is sure that doping and coating will play, more and more, an important role in improving the electrochemical performance of LiFePO4. Hence, the doped and coated LiFePO4 is one of the promising cathode materials for next-generation power lithium ion batteries since it shows excellent performance such as good safety, cyclability, and rate capability. It can be concluded that the doped and coated LiFePO4 may be the trend of development of power lithium ion battery cathode material in the long term.
References
Tarascon J-M, Recham N, Armand M, Chotard J-N, Barpanda P, Walker W, Dupont L (2010) Chem Mater 22:724–739
Yan J, Zhang J, Su Y-C, Zhang X-G, Xia B-J (2010) Electrochim Acta 55:1785–1794
Goodenough JB, Kim Y (2011) J Power Sources 196:6688–6694
Dragana J, Dragan U (2009) J Power Sources 190:538–544
Radhi K, Nanjundaswamy KS, Goodenough JB (1997) J Electrochem Soc 144:1188
Whittingham MS (2004) Chem Rev 104:4271–4301
Chen Z, Dahn JR (2002) J Electrochem Soc 149:A1184
Huang H, Yin SC, Nazar LF (2001) Electrochem Solid-State Lett 4:A170
Liu AF, Hu ZH, Wen ZB, Lei L, An J (2010) Ionics 16:311–316
Xie H, Zhou ZT (2006) Electrochim Acta 51:2063
Xu J, Chen G (2010) Physica B 405:803–807
Chung SY, Bloking JT, Chiang YM (2002) Nat Mater 1:123–128
Ouyang CY, Wang DY, Shi SQ, Wang ZX, Li H, Huang XJ, Chen LQ (2006) Chin Phys Lett 23:61
Yin X, Huang K, Liu S, Wang H (2010) J Power Sources 195:4308–4312
Yang R, Song X, Zhao M, Wang F (2009) J Alloys Compd 468:365–369
Ying J, Lei M, Jiang C, Wan C, He X, Li J, Wang L, Ren J (2006) J Power Sources 158:543–549
Li L, Li X, Wang Z, Wu L, Zheng J, Guo H (2009) J Phys Chem Solids 70:238–242
Zhang Q, Wang S, Zhou Z, Ma G, Jiang W, Guo X, Zhao S (2011) Solid State Ionics 191:40–44
Zhang M, Jiao L-F, Yuan H-T, Wang Y-M, Guo J, Zhao M, Wang W, Zhou X-D (2006) Solid State Ionics 177:3309–3314
Roberts MR, Vitins G, Owen JR (2008) J Power Sources 179:754–762
Luo S, Tian Y, Li H, Shi K, Tang Z, Zhang Z (2010) J Rare Earths 28:439–442
Tian Y, Kang X, Liu L, Xu C, Qu T (2008) J Rare Earths 26:279–283
Abbate M, Lala SM, Montoro LA, Rosolenb JM (2005) Electrochem Solid-State Lett 8:A288
Wang D, Li H, Shi S, Huang X, Chen L (2005) Electrochim Acta 50:2958
Prosini PP, Zane D, Pasquali M (2001) Electrochim Acta 46:3517
Li H, Wang Z, Chen L, Huang X (2009) Adv Mater 21:4593
Wang Z-H, Yuan L-X, Wu M, Sun D, Huang Y-H (2011) Electrochim Acta 56:8477–8483
Chang Z-R, Lv H-J, Tang H, Yuan X-Z, Wang H (2010) J Alloys Compd 501:14–17
Liu H, Cao Q, Fu LJ, Li C, Wu YP, Wu HQ (2006) Electrochem Commun 8:1553
Shenouda AY, Liu Hua K (2009) J Alloys Compd 477:498–503
Zhang W, Hu Y, Tao X, Huang H, Gan Y, Wang C (2010) J Phys Chem Solids 71:1196–1200
Lu Y, Shi J, Guo Z, Tong Q, Huang W, Li B (2009) J Power Sources 194:786–793
Nakamura T, Sakumoto K, Okamoto M, Seki S, Kobayashi Y, Takeuchi T, Tabuchi M, Yamada Y (2007) J Power Sources 174:435–441
Yamada A, Koizumi H, Nishimura SI, Sonoyama N, Kanno R, Yonemura M, Nakamura T, Kobayashi Y (2006) Nat Mater 5:357–360
Yamada A, Kudo Y, Liu KY (2001) J Electrochem Soc 148:A747–A754
Li C, Hu N, Wang C, Kang X, Wumair T, Han Y (2011) J Alloys Compd 509:1897–1900
Chen Y-C, Chen J-M, Hsu C-H, Lee J-F, Yeh J-W, Shih HC (2009) Solid State Ionics 180:1215–1219
Xu J, Chen G, Teng Y-J, Zhang B (2008) Solid State Commun 147:414–418
Yoon W-S, Chung KY, Nam K-W, McBreen J, Wang D, Huang X, Li H, Chen L, Yang X-Q (2008) J Power Sources 183:427–430
Yang J, Bai Y, Qing C, Zhang W (2011) J Alloys Compd 509:9010–9014
Shanmukaraj D, Wang GX, Murugan R, Liu HK (2008) Mater Sci Eng B 149:93–98
Shin HC, Park SB, Jang H, Chung KY, Cho WI, Kim CS, Cho BW (2008) Electrochim Acta 53:7946–7951
Wang H, Xia H, Lai MO, Lu L (2009) Electrochem Commun 11:1539–1542
Lin C-Y, Jhan Y-R, Duh J-G (2011) J Alloys Compd 509:6965–6968
Jhan Y-R, Lin C-Y, Duh J-G (2011) Mater Lett 65:2502–2505
Wang Y, Yang Y, Hu X, Yang Y, Shao H (2009) J Alloys Compd 481:590–594
Wang GX, Needham S, Yao J, Wang JZ, Liu RS, Liu HK (2006) J Power Sources 159:282–286
Sun CS, Zhou Z, Xu ZG, Wang DG, Wei JP, Bian XK, Yan J (2009) J Power Sources 193:841–845
Yang G, Jiang C, He X, Ying J, Cai F (2012) Ionics 18:59–64
Bilecka I, Hintennach A, Rossell MD, Xie D, Novak P, Niederberger M (2011) J Mater Chem 21:5881–5890
Li XL, Kang FY, Shen WC, Bai XD (2007) Electrochim Acta 53:1761–1765
Kubo K, Fujiwara M, Yamada S, Arai S, Kanda M (1997) J Power Sources 68:553
Kim GH, Kim JH, Myung ST, Yoon CS, Sun YK (2005) J Electrochem Soc 152:A1707
Son JT, Kim HG (2005) J Power Sources 147:220–226
Wu C, Wu F, Chen L, Huang X (2002) Solid State Ionics 152–153:327–334
Oh S-W, Park S-H, Kim J-H, Bae YC, Sun Y-K (2006) J Power Sources 157:464–470
Du G, NuLi Y, Yang J, Wang J (2008) Mater Res Bull 43:3607–3613
Sun CS, Zhang Y, Zhang XJ, Zhou Z (2010) J Power Sources 195:3680–3683
Yang L, Jiao L, Miao Y, Yuan H (2009) J Solid State Electrochem 13:1541–1544
Lu F, Zhou Y, Liu J, Pan Y (2011) Electrochim Acta 56:8833–8838
Liao X-Z, He Y-S, Ma Z-F, Zhang X-M, Wang L (2007) J Power Sources 174:720–725
Yi T-F, Zhu Y-R, Zhu X-D, Shu J, Yue C-B, Zhou A-N (2009) Ionics 15:779–784
Sanchez MAE, Brito GES, Fantini MCA, Goya GF, Matos JR (2006) Solid State Ionics 177:497
Lin Y, Gao MX, Zhu D, Liu YF, Pan HG (2008) J Power Sources 184:444–448
Wang K, Cai R, Yuan T, Yu X, Ran R, Shao Z (2009) Electrochem Acta 54:2861
Cho Y-D, Fey GT-K, Kao H-M (2009) J Power Sources 189:256–262
Zhi X, Liang G, Wang L, Ou X, Gao L, Jie X (2010) J Alloys Compd 503:370–374
Kim K, Jeong JH, Kim I-J, Kim H-S (2007) J Power Sources 167:524–528
Pan F, Chen X, Li H, Xin X, Chang Q, Jiang K, Wang W-l (2011) Electrochem Commun 13:726–729
Doeff MM, Wilcox JD, Kostecki R, Lau G (2006) J Power Sources 163:180–184
Pei B, Wang Q, Zhang W, Yang Z, Chen M (2011) Electrochim Acta 56:5667–5672
Maccario M, Croguennec L, Weill F, Cras FL, Delmas C (2008) Solid State Ionics 179:2383–2389
Zhou W, He W, Li Z, Zhao H, Yan S (2009) J Solid State Electrochem 13:1819–1823
Chen S-Y, Gao B, Su L-H, Mi C-H, Zhang X-G (2009) J Solid State Electrochem 13:1361–1366
Li CC, Wang YH, Yang TY (2011) J Electrochem Soc 158:A828–A834
Zhang W-J (2010) J Electrochem Soc 157:A1040–A1046
Fergus JW (2010) J Power Sources 195:939–954
Zhang W-J (2011) J Power Sources 196:2962–2970
Park KS, Son JT, Chung HT, Kim SJ, Lee CH, Kang KT, Kim HG (2004) Solid State Commun 129:311–314
Chiu K-F, Chen C-L (2010) Surf Coat Tech 205:1642–1646
Mi CH, Cao YX, Zhang XG, Zhao XB, Li HL (2008) Powder Technol 181:301–306
Cui Y, Zhao X, Guo R (2010) J Alloys Compd 490:236–240
Li Y-D, Zhao S-X, Nan C-W, Li B-H (2011) J Alloys Compd 509:957–960
Chang H-H, Chang C-C, Su C-Y, Wu H-C, Yang M-H, Wu N-L (2008) J Power Sources 185:466–472
Liu H, Wang GX, Wexler D, Wang JZ, Liu HK (2008) Electrochem Commun 10:165–169
Yao J, Wu F, Qiu X, Li N, Su Y (2011) Electrochim Acta 56:5587–5592
Song G-M, Wu Y, Xu Q, Liu G (2010) J Power Sources 195:3913–3917
Fedorková A, Oriňáková R, Oriňák A, Wiemhöfer H-D, Kaniansky D, Winter M (2010) J Solid State Electrochem 14:2173–2178
Fedorková A, Nacher-Alejos A, Gómez-Romero P, Oriňáková R, Kaniansky D (2010) Electrochim Acta 55:943–947
Kim CW, Park JS, Lee KS (2006) J Power Sources 163:144–150
Kim CH, Lee MH, Jeong WT, Lee KS (2005) J Power Sources 146:534–538
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 50902001), the key project of Scientific Research Foundation sponsored by the Education Department of Anhui Province, China (No. KJ2010A045), the Foundation for Young Talents in College of Anhui Province, China (No. 2010SQRL033ZD), and the Scientific Research Foundation of Graduate School of Anhui University of Technology (No. 2011013). This work is also supported by the Program for Innovative Research Team in Anhui University of Technology.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yi, TF., Li, XY., Liu, H. et al. Recent developments in the doping and surface modification of LiFePO4 as cathode material for power lithium ion battery. Ionics 18, 529–539 (2012). https://doi.org/10.1007/s11581-012-0695-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-012-0695-y