Abstract
There are many methods available to synthesize nanomaterials and the glow discharge plasma electrolysis is a novel and a green method in this category. It is seen that most of the papers are published after 2005 and the interest in it is growing due to its applicability in the industry for preparing nanomaterials at large scale. But, only few results are available yet and most of them are on metal nanoparticle preparation, so that more studies are needed to understand the nature of growth of the nanoparticles under glow discharge in liquid and its applicability in preparing semi-conductor nanomaterials. Many have tried many methods to prepare nanoparticles by the glow discharge and a review like this is the need of the time to understand its present status that helps to modify the present situation to a better one. This review classifies all the available methods of nanomaterials synthesis in liquid by glow discharge in to three and it is discussed in detail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As there is plenty of room at the bottom [1], we have plenty of methods to reach at the bottom. At the bottom, the nanoparticles behave differently even if they are made up of the same chemistry of their bulk counterpart. Peculiar properties are observed due to their size below a characteristic length scale of a specific property since most of their atoms are at their surface. There are many methods available today to synthesize nanomaterials, and of course, glow discharge plasma electrolysis is a new generation green technology in this category. It is seen that most of the papers discussing the synthesis of colloidal nanoparticles by glow discharge plasma are published after 2005 and the interest in it is growing due to its applicability in industrial production of nanomaterials at large scale. But, only few results are available now and most of them are on metal nanoparticle preparation, so that more studies are needed to understand the particle growth nature under glow discharge in liquid and its applicability in preparing semi-conductor nanoparticles. Many have tried many methods to synthesize nanoparticles in liquid under glow discharge and a review like this is the need of the time to understand its present status that helps to modify the present situation to a better one. This paper discusses the possibility of glow discharge electrolysis for the preparation of nanoparticles, since the glow discharge electrolysis occupy a special place among the new technologies developed to prepare nanomaterials due to the fact that the discharge allows one to create active species that can react with the selected target molecules very fast and very easily. There are three excellent reviews discussing the discharges and the chemical reactions when the discharge in and in contact with liquid [2–4] and a review on plasma liquid application in nanoscience [5], but those reviews have not given full attention to the colloidal nanomaterial preparations by plasma electrochemical method. Of course, this review will give you an insight into the nanoparticles preparation by glow discharge plasma.
Nanoparticles can be synthesized via solid [6–8], liquid, gas, and plasma phase routes [9–27]. For high purity products, gas-phase synthesis is most effective. Gas-phase synthesis methods are gas-phase condensation [8, 26–28] and flame pyrolysis [29, 30]. In the gas-phase condensation, the material is being vaporized using heated crucibles, electron or laser beam evaporation or sputtering, but when we replace the evaporative source with precursor for decomposition, then the gas-phase condensation is known as chemical vapor condensation or chemical vapor synthesis. In the case of flame pyrolysis, the gaseous or liquid precursors are decomposed by the combustion reaction. Evaporation and condensation are also the basic principles of the thermal plasma-based nanoparticles synthesis. There are several ways to produce the fourth state of matter, plasma such as direct current plasma, microwave plasma, and radio frequency plasma.
A gas is called a plasma when it is partially ionized and having collective behavior [31, 32] by introducing energy that makes electrical breakdown so that the gas become electrically conductive. There are two kinds of plasma, thermal equilibrium plasma and non-thermal equilibrium plasma. In the case of thermal equilibrium plasma, the temperature of all the electron, ion, and neutral species are the same, whereas in the case of non-thermal equilibrium plasma the temperature of all the species are not same, where electron has much higher temperature than other species. The importance of the non-thermal equilibrium plasma is that, non-thermal equilibrium plasma can drive high-temperature chemistry at low ambient temperatures using lower input energy.
Plasma can be produced by applying a potential difference between two electrodes inserted in a gas-filled cell. Electrons are accelerated away from cathode and collide with the gas atoms. The excited species due to the excitation collisions decays to lower levels by glow or emission of light. Ion–electron pair is produced due to ionization collision and the ions release secondary electrons when they are accelerated towards cathode. The release of secondary electrons gives more ionization collision and the plasma sustains. Plasma can be characterized by different quantities such as electron temperature, gas-phase composition, plasma gas enthalpy using characterization techniques like Langmuir probe, enthalpy probe, and spectroscopy.
In the case of DC glow discharge, a continuous potential difference is applied across the cathode and anode and this gives rise to a constant current. Glow discharge plasma [33] is a complicated gas mixture because of the interactions of a number of different plasma species, where the electron possesses energies in the range of 1–10 eV with density of about 109–1011 cm−3 [34].
In 1887, Gubkin [35] demonstrated the possibility of the electrolysis of aqueous solution of metallic salts using glow discharge and a new field of research was started called plasma electrochemistry. Plasma electrochemistry is the study of the interaction of plasma with the electrolyte solution, and it uses plasma to drive the chemical reactions. This paper reviews the nanomaterials synthesis using plasma electrochemical method. Nanomaterials synthesis using the classical electrochemical methods [36] comes under the liquid-phase synthesis and plasma electrochemical method is a fusion of liquid and plasma phases. Electrochemical reactions are taking place in between an electronic conductor (electrodes) and an ionic conductor (electrolyte) with charge transfer across the interface between these two. Electrochemical reactions are carried out using aqueous electrolytes [36], solid electrolytes [37, 38], and molten salts or ionic liquids or room-temperature ionic liquids [39–43]. In the case of plasma electrolysis, plasma can be considered as a conductive fluid because of the presence of ions and electrons.
Plasma electrolysis is a generic term used to describe high-voltage electrochemical processes where the plasma discharge occurs at the electrode–electrolyte interface. Discharge phenomena have been observed in both positive and negative biasing of the electrodes, and depending on the electrode–electrolyte combination. Discharge vary in appearance with the gas used and the electrolyte solution. Many have studied about the discharges in electrode–electrolyte interface [35, 44–62] in the past for the degradation of toxic substances [63–68], but the plasma–liquid interaction is still not well-understood due to complex and dynamical nature of the plasma–liquid interface. Based on the discharge mechanism, electrodes positions with electrolyte, and the nature of electrolytes, the plasma electrolysis can be broadly classified in to three as (1) contact glow discharge electrolysis (CGDE), (2) glow discharge electrolysis (GDE), and (3) ionic liquid glow discharge electrolysis (IL-GDE). Bruggeman and Leys [2] have classified the discharges in liquid and in contact with liquids as (a) direct liquid-phase discharges, (b) discharges in the gas phase with liquid electrodes, and (c) discharges in bubbles in liquids. But the discharges in bubbles in liquids are actually contact glow discharge electrolysis, because both the electrodes are in contact with the electrolyte when we consider the position of electrodes with the electrolyte. Here, we are not going to explain discharge mechanisms in liquid or water electrolytes because it is already well-explained by Bruggeman and Leys [2].
Contact glow discharge electrolysis
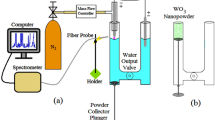
Contact glow discharge electrolysis (CGDE) [44–46, 59, 63, 66, 69, 70] is an electrochemical process, where the plasma is sustained in between an electrode and the surface of surrounding electrolyte. On the other hand, when a high voltage is applied in the conventional electrolysis, it becomes CGDE, where the electrodes are immersed in the electrolyte and it is also called plasma under liquid, that includes discharge produced at the ultrasonic cavity in the liquid also [59, 60, 71]. The experimental setup used for the CGDE and photographs of the discharge are given in Fig. 1. The other approach in the CGDE is keeping either one of the electrodes, cathode, or anode fully immersed in the solution and the other electrode kept as just contacting the surface of the solution [47, 66]. There are two ways to grow particles by this method. One is the dissolution of either one electrode, and the other is from the particle species dissolved in the liquid electrolyte. Growth of the particle is faster when the growth by the particle species of the electrolyte is chosen, since the charged species are available from the beginning of the process, but this process is constrained by the limited reservoir of the species in the liquid electrolyte. When the particle growth by the electrode dissolution is chosen, there is an infinite supply of the growth species, but the growth of the particle is slow. CGDE method is also used for the preparation of oxide or metal films and that process is known as “plasma electrolytic oxidation” or PEO where the film will be deposited in any one of the electrode [69, 71–75].
There is a clear deviation seen from the Faradays’ electrochemical law [48] in the contact glow discharge electrolysis, so that high yield is obtained during the material preparation [49]. It is a well-known fact that novel material productions are not possible by the conventional electrolysis, but even water decomposition [50, 51] is also possible by the CGDE. The reason is nothing but the high energetic charged species and the presence of the two reaction zones such as the liquid near the plasma–electrolyte interface and the plasma around the electrode. No doubt that these two reaction zones are the reason for the high yield [45, 50]. The plasma around the electrode is produced by the dissociation of solvent and solute molecules by appropriate energy transfer and there are a number of radicals, ions, and other active species produced via electron impact dissociation, excitation, and ionization.
The reaction zone is located in a fixed position and constantly renewed during the diffusion of the active species in to the solution. In the reaction zone within the plasma around the electrode, the generation of primary products occurs via ionization in the liquid phase at the expense of the kinetic energy of accelerated particles entering the solution during the discharge.
When the potential between the electrodes is high enough, the solution vaporizes and a gaseous sheath is formed around one electrode [62, 76, 77]. Glow discharge plasma occurs in this gaseous sheath when the gaseous sheath breakdown at high potential. The color of the discharge depends on the constituents of the electrolyte [44, 61] and orange light is seen for the Na2CO3 electrolyte [44]. A typical I–V characteristic of the CGDE is shown in Fig. 2. It is observed from the characteristic curve that the CGDE is undergoing in three processes [3, 4, 65, 66, 69, 70, 78–80]. It is deduced that CGDE obeys ohm’s law until a particular applied voltage only that is the current increased almost proportionally with the rising of the applied voltage. It is known as conventional/classical electrochemical region in the I–V characteristic curve. With a further increase in applied voltage, a thin gas film starts to form [66, 78, 80] and insulate the working electrode from the bulk solution. The equivalent resistance of the gas-solution interface increases sharply due to the lower electrical conductivity of the gas film than the electrolytic solution, which results in a sudden decrease of the electric current. This region in the I–V characteristics is known as unstable region. The voltage at which the current starts to decrease is known as the critical voltage. With a further increase in applied voltage the current starts to increase again and starts to obtain the electrochemical discharges. Colloidal nanoparticles can be synthesized by applying a voltage above the critical voltage. Carbon-encapsulated iron carbide [59, 71], carbon-encapsulated cobalt [60], Ag [77], Ti [77], nickel [77, 78], copper [78, 79, 81], Pt [79], Au [77, 79, 82, 83], TiO2 [76, 80] nanoparticles have been synthesized by this method so far and the details are tabulated in Table 1. TEM image of the nickel particles prepared by this method is shown in Fig. 3 [78].
Nickel nanoparticles prepared by CGDE method ([78], Reproduced with permission from Elsevier)
Lal et al. [79] prepared Cu nanoparticles of sizes 50–400 nm using CuSO4 + H2SO4 electrolyte solution and it is shown in Fig. 4. The same group has prepared Pt particles of 30–200 nm sizes [79] using H2PtCl6 + HClO4 as electrolytic solution. They have also used NaAuCl4 + HClO4 electrolyte for preparing Au nanoparticles [79], and H2PtCl6 + NaAuCl4 + HClO4 electrolyte for Pt + Au nanoparticles. In all the above cases, the discharge lasted only for about 10 μs with 1A current.
Copper nanoparticles prepared by CGDE method ([79], Reproduced with permission of ELSEVIER INC. in the format Journal via Copyright Clearance Center)
Paulmier et al. [80] reports the preparation of TiO2 nanorods with a diameter of about 200 nm using this CGDE method, where the plasma discharge occurs at a voltage of about 500 V at atmospheric pressure. They observed that the density and the orientation of the nanorods perpendicular to the substrate surface are increasing with increase in voltage from 500 to 700 V. They have also reported that amorphous TiO2 was formed at lower voltages and this amorphous is converted to anatase phase as the intensity of plasma is increased. They have used HCl with the precursor solution to control the current. The experiments by increasing the treatment time give longer bended nanofibers with higher disorientation [80].
Different shapes of gold nanoparticles with size ranges in between 25–50 nm were synthesized from the solution of HAuCl4.4H2O with sodium dodecyl sulfonate as stabilizer by applying voltage from 1,600 to 3,200 V in between two tungsten electrodes in a setup similar to the picture shown in Fig. 1c [82], and Osamu Takai [81] also reported the preparation of 10 nm gold nanoparticles from HAuCl4 with gelatin and KCl in a similar setup by applying a voltage of 2,500 V. It is deduced from their results that the size and shapes of the particles are depended on the time of treatment, applied voltage, and concentration of the dissolved species.
Electrode dissolution method can also be utilized to prepare nanomaterials as described in the previous paragraphs. Nano-balls of Ni, Ti, Ag, and Au [77] as depicted in Fig. 5 have been prepared by the dissolution of the electrodes using the same setup described above, but Ruslan Segiienko [59, 60, 71] and his colleagues have performed the electrode dissolution in a different way, where the discharge was produced with the help of ultrasonic cavitation method. They [59, 60, 71] have used a setup shown in Fig. 1d where the ion electrodes were melted and reacted with the ionized ethanol electrolyte that made carbon-encapsulated iron carbide nanoparticles of sizes ranging in between 5 and 600 nm as given in Fig. 6. Ultrasonic cavitation creates highly localized high temperature and pressure regions that enhance the electrical conductivity, so that plasma discharge can be generated at relatively low electric power, so that they [59, 60, 71] have prepared the metal nanomaterials by electrode dissolution by creating a constant potential difference of 55 V in between the electrodes.
Micrographs of Ni, Ti, Ag, and Au nano balls ([77], Reproduced with permission of AMERICAN INSTITUTE OF PHYSICS in the format Journal via Copyright Clearance Center)
Carbon nanocapsule with an amorphous core ([59], Reproduced with permission of ELSEVIER S.A. in the format Journal via Copyright Clearance Center)
Glow discharge electrolysis
Glow discharge electrolysis (GDE) [52, 53, 65, 84–86] is an electrochemical technique in which the discharge is initiated in the gas in between the metal electrode and the aqueous solution by applying high voltage.
One gets corona discharge when distilled water is used as an electrolyte solution and stainless steel as pin electrode [53, 84] and can observe a liquid elevation before the corona onset due to the Coulomb force. It is found that water surface deformations have a significant influence in the electrical breakdown formation and the current–voltage characteristic is relatively independent of the voltage in a particular current range [53] for filamentary glow when the water acts as cathode, but I–V characteristic exhibits a glow like behavior for higher currents. A cathode spot is seen on the surface of the water and its diameter increases with current. A typical I–V characteristic is shown in Fig. 7.
Dynamics of a glow discharge over a potassium permanganate solution: a smooth surface, b warped surface, c wave perturbation of the surface, d growing perturbation, e turbulent ejection of the plasma–electrolyte mixture into the discharge zone, and f another turbulent ejection of the plasma electrolyte mixture into the discharge zone (with kind permission from Springer Science + Business Media: experimental study of the stability of the interface between a liquid electrolyte and the glow discharge plasma [54] Fig. 1)
Modes of a glow discharge over a copper sulfate solution: a, b smooth surface, c, d solitary wave perturbation, e, f regular ripples, and g, h churning foamed turbulent mixing zone (with kind permission from Springer Science + Business Media: experimental study of the stability of the interface between a liquid electrolyte and the glow discharge plasma [54] Fig. 2)
Vyalykh group [54] conducted a photographic investigation to study the interface of the liquid electrolyte and the plasma of a low-pressure dc glow discharge. The photographs of the plasma–electrolyte interface are shown in Figs. 8 and 9. This group [54] conducted experiments for copper sulfate (CuSO4) water solution and for the water solution of potassium permanganate (KMnO4). A complicated kinetic process is involved at the plasma–electrolyte interface as well as the surface is also affected by intense acoustic oscillations due to the current flow through the electrolyte and the exchange of energy from electronic current to the ionic current. During the discharge over the smooth electrolyte surface, the surface warped and the electrolyte and the plasma underwent a turbulent mixing after intense surface waves. Later, the discharge was extinguished when the mixture was ejected to the upper electrode after the turbulent mixing. This may be due to the coulombic attraction as shown by Bruggeman et al. [53]. But, a slight change is seen in the discharge of the copper sulfate solution that the discharge was quieter and passed through different quasi-steady modes, such as smooth surface, a solitary wave perturbation, regular ripples (Faraday waves) and churning foamed turbulent mixing zone. It is evident from the churning foamed turbulent mixing zone that coulombic attraction also prevails here. But we do not have much theoretical and experimental study available to date to confirm what happens at the plasma–liquid interface. The basic principle in this reaction is that plasma occurs above the surface of the solution and then moves into the solution by transferring energetic plasma particles into aqueous solution and collide with ions in the aqueous solution. That the charged species in the plasma accelerated towards the solution and enters into the solution with high magnitude of kinetic energy so as to make chemical reactions in the solution [65]. An overview of important reactions in the plasma–liquid interactions is well-explained in the review published by Bruggeman and Leys [2]. The most probable reaction may be due to the collision of high energetic electrons with the molecules in the electrolytic solution [65]. The electron density increases with increase in plasma power which increases the plasma volume so that the electrons have greater chance to interact with the molecules in the solution [65] and a similar effect is reported in the CGDE also [79] where the amorphous TiO2 is converted to anatase when the plasma intensity increased to a certain level.
The team of R. Mohan Sankaran [26, 87–89] has designed a microplasma method to prepare nanomaterials by electrode dissolution and nucleation from the electrolyte solution, this method comes under the GDE based on the classification used here, since one of the electrodes is in contact with the electrolyte and other is plasma. They have prepared silver nanoparticles by the electrode dissolution (Ag anode) [26] with a stabilizer in an H-shaped setup as shown in Fig. 10a, further Au and Ag nanoparticles were also prepared by the growth of the particle species dissolved in the electrolyte HAuCl4 and AgNO3 using the setup shown in Fig. 10b.
Ionic liquid glow discharge electrolysis
Ionic liquid glow discharge electrolysis (IL-GDE) is developed by the group of Frank Endres [43, 53, 56], who developed the electrodeposition of nanoscale materials in ionic liquid. Here, the discharge is initiated in the gas in between the metal electrode and the ionic liquid solution. This group [43, 53, 56] named it as plasma electrochemical deposition (PECD) at the interface of plasma/ionic liquid by considering plasma as an electrode. Ionic liquids are defined as molten salts with melting point below 100 °C with large number of cations and anions. Frank Endres et al. defined the ionic liquid as “an ionic material that is liquid below 100 °C” [56]. Ionic Liquids (IL) have high ionic conductivity, low viscosity, high thermal stability, wide electrochemical window up to 7 V, and have negligible vapor pressure as well as they are environmentally friendly [56]. The IL-GDE concept is based on the reduction of precursor material dissolved in IL with free electrons from the plasma. Here, the plasma considered as an electrode because of the deposition of the materials at the interface of ionic liquid and plasma like electroplating at one electrode. The negligible vapor pressure of the IL helps to make an extended and stable plasma [42, 90] cloud rather than a localized corona discharge and the larger electrochemical window helps to electro-deposit materials that cannot be deposited from aqueous solutions. A photograph of ionic liquid glow discharge and the schematic representation of ionic liquid glow discharge electrolysis are shown in Fig. 11.
a Photograph of ionic liquid glow discharge plasma, b schematic drawing of ionic liquid glow discharge plasma electrochemical setup. (Reproduced by permission of The Royal Society of Chemistry, DOI: 10.1039/b614520e)
Understanding the stability of ionic liquids when they bring in contact with plasma is very important, so that Yong-Bing Xie et al. [91] have studied the stability of [BMIM]Cl, [BMIM][PF6] and [BMIM][BF4] ionic liquids under glow discharge plasma exposure for 10 min when neither of the electrodes are in contact with the ionic liquid. They [91] have produced argon plasma by applying a DC voltage of 1.0 kV at a pressure of 100 Pa in between two stainless steel electrodes of diameter 40 mm kept 100 mm away while maintaining the flow rate as 10 ml/min in a quartz tube of 40 mm diameter. They [91] have used Fourier transform infrared and NMR spectroscopy to determine the structural changes in the ionic liquid after the application of the glow discharge. After comparing chemical shift values of the samples before plasma treatment and after treatment, Yong-Bing Xie et al. [91] and the team of Frank Endres [92] have concluded that the structure of imidazolium ionic liquids is stable under the influence of these plasmas due to the low temperature of the glow discharges.
But, de-composition of ionic liquid is [55, 58] reported when any one of the metal electrode is in contact with the ionic liquid.
Sebastian A. Meiss and M. Poelleth’s [55, 57] group synthesized silver nanoparticles using IL-GDE for about 10 min. From their observations, the reactions started at the IL–argon plasma interface and it spread all over the solution, which was observable from the dark color of the solution. During the reaction time, a change in the pink/blue emission of argon plasma is observed and then the reaction started at the IL–plasma interface.
The physicochemical properties of ionic liquids also influences the particles growth and size [58] and it was confirmed by preparing copper nanoparticles in different air and water stable IL 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl) amide ([EMIm]Tf2N) and 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl) amide ([Py1,4] Tf2N). Where, the decomposition of the IL takes place at the anode and the materials starts to deposit at the IL–plasma interface, at the gaseous electrode [55, 58].
Metal nanoparticles of platinum, palladium [58], silver [39, 40, 58] can also be synthesized using the same technique by dissolving metal salts in different ionic liquids; the dissolved metal salts can be reduced with the help of free electrons from plasma [56]. Germanium particles of sizes about 50 nm were prepared using the electrolyte GeCl2C4H8O2 + [EMIm]TFSA as anode with an applied voltage in between 450 V and 500 V for about 30 min [90] where the plasma is ignited at 1,000 V with a current of 10 mA at 100 Pa. So that the electrons are accelerated towards the surface of ionic liquid electrolyte. The resulting Ge particles are shown in Fig. 12.
The color change during the preparation of Ge nanoparticles and the prepared Ge nanoparticles ([90], Reproduced with permission from Elsevier)
Copper nanoparticles of 11 nm [93] were prepared by dissolving copper in 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide ([EMIm]Tf2N and copper nanoparticles of 26 nm [93] by dissolving copper in 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide([Py1,4]Tf2N) by igniting plasma at 1,000 V at 100 Pa with a current of 10 mA for 30 min. After the ignition, the voltage dropped from 1,000 to 450–500 V and the resulting particles are shown in Fig. 13. This work [93] indicates that the nature of ionic liquid also affects the particles size when they are prepared by the plasma electrolytic method.
a Copper nanoparticles prepared in [EMIm]Tf2N and b copper nanoparticles prepared in [Py1,4]Tf2 ([93], Reproduced by permission of the PCCP Owner Societies, DOI: 10.1039/b906567a)
Theoretical aspects of plasma–liquid interface
A priori, electrolytes are purely ionic conductor and metallic electrodes are electron conductor, but plasma is a fluid of ionic and electronic conductors. It is assumed that the glow discharge plasma as an electronic conductor since the electrons have high kinetic energy while neglecting the inhomogeneity of the glow discharge plasma, so that the glow discharge plasma can be considered as an electrode. But, when the plasma sheath becomes poor in electrons under a negative polarization and thus ionic conduction predominates and thus the plasma sheath act as an electrolyte.
Charged species from plasma diffuses to the plasma boundaries and leads to a negative charging of the plasma walls and leaves a positive space charge in front of the wall, called sheath. This sheath can be treated as the diffusion potential in conventional electrolytes.
When we are considering plasma as an electrolyte, this means that there are pairs of electrolytes that are in mutual contact, that forms liquid electrolyte–plasma electrolyte interfaces and this interface is not stable because these are immiscible and this interface is in between two immiscible electrolytes. But from the reports of Vyaluk et al. [54], it is seen that the ions in both phases diffuse across the plasma electrolyte–liquid electrolyte interface and disappearing the interface (evident from the movement of liquid electrolyte to the upper electrode after a turbulent mixing), which means it brings a potential equilibrium between these two phases. Even though, from the IL-GDE [53, 56], these inhomogeneous immiscible electrolyte systems preserve its interface. Because of the difference in charge driving forces in these dissimilar media, there are some nontransferring components in each of the phases and there is a Galvani potential (in the case of Galvanic cell) at the interfaces between these two electrolytes and that cannot be measured [94]. More detailed studies are needed to unravel the secret of the plasma-liquid interface and their interactions to form nanomaterials.
In conclusion, it is observed that only few studies are available about the nanoparticle preparation in liquid under plasma and this is not enough to tell the nucleation and the growth process of nanoparticles in liquid under plasma.
References
Feynman RP (1959) There’s plenty of room at the bottom—an invitation to enter a new field of physics, Presentation at the California Institute of Technology
Bruggeman P, Leys C (2009) Non-thermal plasmas in and in contact with liquids. J Phys D: Appl Phys 42:053001. doi:10.1088/0022-3727/42/5/053001
Brisset J-L, Moussa D, Doubla A, Hnatiuc E, Hnatiuc B, Kamgang Youbi G, Herry J-M, Naїtali M, Bellon-Fontaine M-N (2008) Chemical reactivity of discharges and temporal post-discharges in plasma treatment of aqueous media: examples of gliding discharge treated solutions. Ind Eng Chem Res 47:5761–5781
Yerokhin AL, Nie X, Leyland A, Matthews A, Dowey SJ (1999) Plasma electrolysis for surface engineering. Surf CoatTechnol 122:73–93
Graham WG, Stalder KR (2011) Plasmas in liquids and some of their applications in nanoscience. J Phys D: Appl Phys 44(174037):8. doi:10.1088/0022-3727/44/17/174037
Andrievski RA (1994) The synthesis and properties of nanocrystalline refractory compounds. Russ Chem Rev 63:431–448
Swihart MT (2003) Vapor-phase synthesis of nanoparticles. Curr Opin Colloid Interface Sci 8:127–133
Hahn H (1997) Gas phase synthesis of nanocrystalline materials. Nanostructured Mater 9:3–12
Rao N, Girshick S, Heberlein J (1995) Nanoparticle formation using a plasma expansion process. Plasma Chem Plasma Process 15:581–606
Ananthapadmanabhan PV, Sreekumar KP, Venkatramani N, Sinha PK, Taylor PR (1996) Characterization of plasma-synthesized alumina. J Alloys Compd 244:70–74. doi:10.1016/S0925-8388(96)02440-1
Sakka Y, Okuyama H, Uchikoshi T, Ohno S (2002) Synthesis and characterization of Fe and composite Fe-TiN nanoparticles by dc arc-plasma. J Alloys Compd 346:285–291. doi:10.1016/S0925-8388(02)00514-5
Karthikeyan J, Berndt CC, Tikkanen J, Reddy S, Herman H (1997) Plasma spray synthesis of nanomaterial powders and deposits. Mater Sci Eng A 238:275–286
Vollath D, Sickafus KE (1993) Synthesis of nanosized ceramic nitride powders by microwave supported plasma reactions. Nanostruct Mater 2:451–456
Vollath D, Vinga Szabó D, Hauβelt J (1997) Synthesis and properties of ceramic nanoparticles and nanocomposites. J Eur Ceram Soc 17:1317–1324. doi:10.1016/S0955-2219(96)00224-5
Troitskiy VN, Domashnev IA, Kurkin EN, Grebtsova OM, Berestenko VI, Balikhin IL, Gurov SV (2003) Synthesis and characteristics of ultra-fine superconducting powders in the Nb-N, Nb-N-C, Nb-Ti-N-C systems. J Nanopart Res 5:521–528. doi:10.1023/B:NANO.0000006072.30306.1f
Gitzhofer F (1996) Induction plasma synthesis of ultrafine SiC. Pure Appl Chem 68:1113–1120
Dundas PH, Thorpe ML (1970) Titanium dioxide production by plasma processing. Chem Eng Prog 66:66–71
Sugasawa M, Kikukawa N, Ishikawa N, Kayano N, Kimura T (1998) Synthesis of Y-Fe-O ultrafine particles using inductively coupled plasma. J Aerosol Sci 29:675–686. doi:10.1016/S0021-8502(97)00463-1
Girshicka SL, Chiua CP, Munoa R, Wua CY, Yanga L, Singh SK, McMurry PH (1993) Thermal plasma synthesis of ultrafine iron particles. J Aerosol Sci 24:367–382. doi:10.1016/0021-8502(93)90009-X
Guo JY, Gitzhofer F, Boulos MI (1995) Induction plasma synthesis of ultrafine SiC powders from silicon and CH4. J Mater Sci 30:5589–5599. doi:10.1007/BF00356691
Mizoguchi Y, Kagawa M, Suzuki M, Syono Y, Hirai T (1994) Synthesis of ultrafine particles and thin films of BaFe12O19 by the spray-ICP technique. Nanostruct Mater 4:591–596. doi:10.1016/0965-9773(94)90068-X
Nutsch G, Boer J, Herrmann A, Weiss K-H (1997) Nanoparticle formation using an inductively coupled radio frequency plasma, in ISPC 13, edited by C. K. Wu, Peking University Press, Bejing, China (1997), 1642–1647; http://134.147.148.178/ispcdocs/ispc13/content/13/13-1642.pdf
Bouyer E, Müller M, Henne RH, Schiller G (2001) Thermal plasma processing of nanostructured Si-based ceramic materials. J Nanopart Res 3(5-6):373–376. doi:10.1023/A:1012546917064
Kumar R, Cheang P, Khor KA (2001) RF plasma processing of ultra-fine hydroxyaptite powders. J Mater Process Technol 113:456–462. doi:10.1016/S0924-0136(01)00611-2
Akashi K (1985) Progress in thermal plasma deposition of alloys and ceramic fine particles. Pure Appl Chem 57:1197–1206
Chiang W-H, Richmonds C, Mohan Sankaran R (2010) Continuous-flow, atmospheric-pressure microplasmas: a versatile source for metal nanoparticle synthesis in the gas or liquid phase. Plasma Sourc Sci Tech 19(034011):8. doi:10.1088/0963-0252/19/3/034011
Chiang W-H, Mohan Sankaran R (2007) Microplasma synthesis of metal nanoparticles for gas-phase studies of catalyzed carbon nanotube growth. Appl Phys Lett 91:121503
Granqvist CG, Buhrman RA (1976) Ultrafine metal particles. J Appl Phys 47:2200–2219
Pratsinis SE (1998) Flame aerosol synthesis of ceramic powders. Progr Energ Combust Sci 24:197–219
Wooldridge MS (1998) Gas-phase combustion synthesis of particles. Prog Energ Combust Sci 24:63–87
Boulos MI, Fauchais P, Pfender E (1994) Thermal plasmas—fundamentals and applications. Plenum Press, New York. ISBN 0-306-44607-3
Chen FF (1974) Introduction to plasma physics. Plenum Press, New York
Bogaerts A, Neyts E, Gijbels R, van der Mullen J (2002) Gas discharge plasmas and their applications. Spectrochimica Acta B 57:609–658
Ogumi Z, Uchimoto Y, Takehara Z-I (1995) Electrochemistry using plasma. Adv Mater 7(3):323–325. doi:10.1002/adma.19950070318
Gubkin A (1887) Reduced matrix interferences compared to flames. J Ann Phys Chem 32:114–115
Wang X-F, Jing-Juan Xu, Chen HY (2008) Dendritic CdO nanomaterials prepared by electrochemical deposition and their electrogenerated chemiluminescence behaviors in aqueous systems. J Phys Chem C 112(18):7151–7157. doi:10.1021/jp711093z
Vennekamp M, Janek J (2005) Control of the surface morphology of solid electrolyte films during field driven growth in a reactive plasma. Phys Chem Chem Phys 7:666–677
Vennekamp M, Janek J (2001) Plasma electrochemical growth of ion-conducting AgBr and AgCl Solid State Ionics. 141–142, 71–80
He P, Liu H, Li Z, Liu Y, Xiudong Xu, Li J (2004) Electrochemical deposition of silver in room temperature ionic liquids and its surface enhanced Raman scattering effect. Langmuir 20(23):10260–10267. doi:10.1021/la0484801
Zein El Abedin S, Endres F (2009) Electrodeposition of nanocrystaline silver films and nanowires from the ionic liquid 1 ethyl 3 methylimidazolium trifluromethylsulfonate. Electrochim Acta 54:5673–5677
Zein El Abedin S, Saad AY, Farag HK, Borisenko N, Liu QX, Endres F (2007) Electrodepostion of selenium, indium and copper in air and water stable ionic liquid at variable temperatures. Electrochim Acta 52:2746–2754
Zein El Abedin S, Polleth M, Meiss SA, Janek J, Endres F (2007) Ionic liquid as green electrolytes for the electrodeposition of nanomaterials. Green Chem 9:549–553
Endres F (2002) Ionic liquids: solvents for the electrodeposition of metals and semiconductors. Chem Phys Chem 3:144–154
Yan ZC, Li C, Lin WH (2006) Experimental study of plasma under-liquid electrolysis in hydrogen generation. Chin J Process Eng 6(3):396–401
Bruggeman P, Schram D, González MÁ, Rego R, Kong MG, Leys C (2009) Characterization of a direct dc-excited discharge in water by optical emission spectroscopy. Plasma Sourc Sci Tech 18(025017):13
Gao J, Wang A, Fu Y, Wu J, Ma D, Guo X, Li Y, Yang W (2008) Analysis of energetic species caused by contact glow discharge electrolysis in aqueous solution. Plasma Sci Tech 10:1. doi:10.1088/1009-0630/10/1/07
Gao J (2006) A novel technique for waste water treatment by contact glow discharge electrolysis. Pak J Biol Sci 9(2):323–329
Hicking A, Ingram MD (1964) Contact glow-discharge electrolysis. Trans Faraday Soc 60:783–793
Susanta KSG, Rajeshwar S, Ashok KSA (1998) Study on the origin of non-faradaic behavior of anodic contact glow discharge electrolysis. J Electrochem Soc 145(7):2209–2213
Polyakov OV, Badalyan AM, Bakhturova LF (2003) The yields of radical products in water decomposition under discharges with electrolytic electrodes. High Energy Chem 37(5):322–327
Polyakov OV, Badalyan AM, Bakhturova LF (2002) The water degradation yield and spatial distribution of primary radicals in the near-discharge volume of an electrolytic cathode. High Energy Chem 36(5):280–284
Development of electrolyte cathode glow discharge atomic emission spectroscopy for the analysis of elements at trace and ultra trace levels (2009) CCM, Hyderabad, BARC news letter, 14, 301
Bruggeman P, Ribezl E, Degroote J, Vierendeels J, Leys C (2008) Plasma characteristics and electrical break down between metal and water electrodes. J Optoelectron Adv Mater 10(8):1964–1967
Vyalykh DV, Dubinov AE, Mikheev KE, Lashmanov YuN, L’vov IL, Sadovo SA, Selemir VD (2005) Experimental study of the stability of the interface between a liquid electrolyte and the glow discharge plasma. Tech Phys 50(10):1374–1375
Meiss SA, Rohnke M, Kienle L, Abedin Sherif Zein El, Endres F, Janek J (2007) Employing plasmas as gaseous electrodes at the free surface of ionic liquids: deposition of nanocrystalline silver particles. Chemphyschem 8:50–53. doi:10.1002/cphc.200600582
Endres F, MacFarlane D, Abbott A (2008) Electrodeposition from ionic liquids. Wiley-VCH Verlag Gmbh&Co.KgaA
Poelleth M, Meiss A, Rohnke M, Kienle L, Zein El Abedin S, Endres F, Janek J (2007) Deposition of metal nanoparticles at ionic-liquid/plasma interfaces, 28th ICPIG, July 15–20, Topic number: 13
Brettholle M, Höfft O, Klarhöfer L, Mathes S, Maus-Friedrichs W, Zein El Abedin S, Krischok S, Janek J, Endres F (2010) Plasma electrochemistry in ionic liquids: deposition of copper nanoparticles. Phys Chem Chem Phys. doi:10.1039/b906567a
Sergiienko R, Shibata E, Akase Z, Suwa H, Nakamura T, Shindo D (2006) Carbon encapsulated iron carbide nanoparticles synthesized in ethanol by an electric plasma discharge in an ultrasonic cavitation field. Mater Chem Phys 98:34–38
Sergiienko R, Shibata E, Zentaro A, Shindo D, Nakamura T, Qin G (2007) Formation and characterization of graphite-encapsulated cobalt nanoparticles synthesized by electric discharge in an ultrasonic cavitation field of liquid ethanol. Acta Mater 55:3671–3680
Burakov V, Tarasenko N, Nevar AA, Nedelko VI (2009) Spectroscopic characterization of electrical discharge plasma in liquids used for nanoparticles fabrication, X17–16.29th ICPIG, July 12–17
Schaper L, Graham WG, Stalder KR (2011) Vapour layer formation by electrical discharges through electrically conducting liquids—modelling and experiment. Plasma Sourc Sci Tech 20:034003. doi:10.1088/0963-0252/20/3/034003
Gai K (2006) Aqueous benzoquinone degradation induced by plasma with glow discharge electrolysis. Can J Anal Sci Spectrosc Volume 51, No. 4
Gai K (2006) Aqueous diphenyl degradation induced by plasma with glow discharge electrolysis. J Chin Chem Soc 53:627–632
Harada K, Terasawa J, Suzuki S (1978) Syntheses of uracil and thymine by contact glow-discharge electrolysis. Naturwissenschaften 65(9):259
Harada K, Suzuki S, Ishida H (1977) Syntheses of amino acids from unsaturated aliphatic carboxylic acid by contact glow discharge electrolysis, 300–31 (Japan), 18 Specialia Experientia 34/1
Gai K, Dong Y-J (2005) Plasma induced degradation of azobenzene in water. J Chin Chem Soc 52(273–276):273
Yan ZC, Li C, Lin WH (2009) Hydrogen generation by glow discharge plasma electrolysis of methanol solutions. Int J Hydrog Energy 3(4):48–55
Paulmier T, Bell JM, Fredericks PM (2007) Deposition of nano-crystalline graphite films by cathodic plasma electrolysis. Thin Solid Films 515(5):2926–2934
Paulmier T, Bell JM, Fredericks PM (2006) Development of a novel cathodic plasma/electrolytic deposition technique, Part 2: physicochemical analysis of the plasma discharge. Surf CoatTechnol. doi:10.1016/j.surfcoat.2006.07.066
Sergiienko R, Shibata E, Akase Z, Suwa H, Shindo D, Nakamura T (2006) Synthesis of Fe-filled carbon nanocapsules by an electric plasma discharge in an ultrasonic cavitation field of liquid ethanol. J Mater Res 21(10):2524–2535
Niea X, Meletis EI, Jiang JC, Leyland A, Yerokhin AL, Matthews A (2002) Abrasive wearycorrosion properties and TEM analysis of Al2O3 coatings fabricated using plasma electrolysis. Surf CoatTechnol 149:245–251
Khan RHU, Yerokhin A, Li X, Dong H, Matthews A (2010) Surface characterisation of DC plasma electrolytic oxidation treated 6082 aluminium alloy: effect of current density and electrolyte concentration. Surf CoatTechnol. doi:10.1016/j.surfcoat.2010.04.052
Yerokhin AL, Nie X, Leyland A, Matthews A (2000) Characterisation of oxide films produced by plasma electrolytic oxidation of a Ti6Al4V alloy. Surf CoatTechnol 130:195–206
Chu P-J, Shu-Yuan W, Chen K-C, He J-L, Yerokhin A, Matthews A (2010) Nano-structured TiO2 films by plasma electrolytic oxidation combined with chemical and thermal post-treatments of titanium, for dye-sensitised solar cell applications. Thin Solid Films. doi:10.1016/j.tsf.2010.06.046
Schaper L, Stalder KR, Graham WG (2011) Plasma production in electrically conducting liquids. Plasma Sources Sci Technol 20(034004):6. doi:10.1088/0963-0252/20/3/034004
Toriyabe Yu, Watanabe S, Yatsu S, Shibayama T, Mizuno T (2007) Controlled formation of metallic nanoballs during plasma electrolysis. Appl Phys Lett 91:041501
Wüthrich R, Allagui A (2010) Building micro and nanosystems with electrochemical discharges. Electrochim Acta 55:8189–8196
Lal A, Bleuler H, Wüthrich R (2008) Fabrication of metallic nanoparticles by electrochemical discharges. Electrochem Commun 10:488–491
Paulmier T, Bell JM, Fredericks PM (2008) Plasma electrolytic deposition of titanium dioxide nanorods and nano-particles. J Mater Proc Tech 208:117–123
Takai O (2008) Solution plasma processing (SPP). Pure Appl Chem 80(9):2003–2011. doi:10.1351/pac200880092003
Hieda J, Saitoa N, Takai O (2008) Exotic shapes of gold nanoparticles synthesized using plasma in aqueous solution. J Vac Sci Technol, A 26(4):854–857
Jwo C-S, Tien D-C, Teng T-P, Chang H, Tsung T-T, Liao C-Y, Lin C-H (2005) Preparation and UV characterization of TiO2 nanoparticles synthesized by SANSS. Rev Adv Mater Sci 10:283–288
Bruggeman P, Liu JJ, Degroote J, Kong MG, Vierendeels J, Leys C (2008) DC excited glow discharges in atmospheric pressure air in pin-to-water electrode systems. J Phys D: Appl Phys 41:215201
Yan J-H, Du C-M, Li X-D, Sun X-D, Ni M-J, Cen K-F, Cheron B (2005) Plasma chemical degradation of phenol in solution by gas–liquid gliding arc discharge. Plasma Sourc Sci Tech 14:637–644. doi:10.1088/0963-0252/14/4/001
Petr Lukes, Water treatment by pulsed streamer corona discharge, Ph.D. Thesis, Prague 2001, Institute of Chemical Technology, Prague
Chang F-C, Richmonds C, Mohan Sankaran R (2010) Microplasma-assisted growth of colloidal Ag nanoparticles for point-of use surface-enhanced Raman scattering applications. J Vac Sci Technol, A 28(4):L5–L8
Mariotti D, Mohan Sankaran R (2010) Microplasmas for nanomaterials synthesis: a review on microplasmas. J Phys D: Appl Phys 43:323001. doi:10.1088/0022-3727/43/32/323001
Richmonds C, Mohan Sankaran R (2008) Plasma-liquid electrochemistry: rapid synthesis of colloidal metal nanoparticles by microplasma reduction of aqueous cations. Appl Phys Lett 93:131501
Sayed A, Aala A, Al-Salmana R, Al-Zoubia M, Borissenkoa N, Endresa F, Höffta O, Prowalda A, Zein El Abedin S (2011) Interfacial electrochemistry and electro deposition from some ionic liquids: in situ scanning tunneling microscopy, plasma electrochemistry, selenium and macroporous materials. Electrochim Acta. doi:10.1016/j.electacta.2011.02.063
Xie Y-B, Liu C-J (2008) Stability of ionic liquids under the influence of glow discharge plasmas. Plasma Process Polym 5:239–245
Hofft O, Endres F (2011) Plasma electrochemistry in ionic liquids: an alternative route to generate nanoparticles. Phys Chem Chem Phys 13:13472–13478. doi:10.1039/c1cp20501c
Brettholle M, Hofft O, Klarhofer L, Mathes S, Maus-Friedrichs W, Zein El Abedin S, Krischok S, Janekd J, Endres F (2010) Plasma electrochemistry in ionic liquids: deposition of copper nanoparticles. Phys Chem Chem Phys 12:1750–1755
Bagotsky VS (2005) Fundamentals of electrochemistry, 2nd edition, John Wiley & Sons, Inc., pp 22–28
Acknowledgments
The author is highly indebted to Dr. Asar Ahmad, IIT, Kanpur for his timely help in collecting the literatures for this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kareem, T.A., Kaliani, A.A. Glow discharge plasma electrolysis for nanoparticles synthesis. Ionics 18, 315–327 (2012). https://doi.org/10.1007/s11581-011-0639-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-011-0639-y