Abstract
Composite nanofibrous membranes based on poly (vinyl chloride) (PVC)–poly (methyl methacrylate) (PMMA) were prepared by electrospinning and then they were soaked in liquid electrolyte to form polymer electrolytes (PEs). The introduction of PMMA into the PVC matrix enhanced the compatibility between the polymer matrix and the liquid electrolyte. The composite nanofibrous membranes prepared by electrospinning involved a fully interconnected pore structure facilitating high electrolyte uptake and easy transport of ions. The ion conductivity of the PEs increased with the increase in PMMA content in the blend and the ion conductivity of the polymer electrolyte based on PVC–PMMA (5:5, w/w) blend was 1.36 × 10−3 S cm−1 at 25 °C. The polymer electrolyte based on PVC–PMMA (5:5, w/w) blend presented good electrochemical stability up to 5.0 V (vs. Li/Li+) and good interfacial stability with the lithium electrode. The promising results showed that nanofibrous PEs based on PVC–PMMA were of great potential application in polymer lithium-ion batteries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent developments in PEs have received significant attention for application in lithium-ion batteries because of their advantages in providing lighter and safer batteries with longer shelf-life, leak proof construction, and easy fabrication into a desired shape and size compared with traditional liquid electrolytes [1, 2]. Many investigations have been done in order to achieve high ionic conductivity at ambient temperature. Among these investigations reported, polymer blending is the most feasible technique [3]. This technique not only complements the advantages from other techniques but also improves the electrical and physical properties of PEs [4].
PVC (ε ≈ 3) is a commercially available polymer, which acts as good mechanical stiffener and has easy processibility. In the recent years, PVC-based PEs got their popularity due to their inexpensive price and good compatibility with many polymers [5–7]. One of the problems which restrict the use of PVC-based PEs is that the PEs based on PVC have low ionic conductivity at ambient temperature.
PMMA is a host polymer matrix for the preparation of PEs. The PEs based on PMMA can absorb lots of liquid electrolytes and have good compatibility with the liquid electrolyte. E. Cazzanelli et al. [8] reported that the ionic conductivity of the PEs based on PMMA was in the order of 10−3 S cm−1 at room temperature. Therefore, many researchers [9, 10] blended PMMA with another polymer matrix to improve electrolyte uptake and the ionic conductivity of the PEs.
Electrospinning has received great attention in the past few years due to its simplicity, inexpensiveness, and versatility for fabrication of ultra-fine fibers with diameters in the range of several micrometers to tens of nanometers [11]. Polymer electrolyte membranes prepared by electrospinning have been reported by a great many literatures [12–14]. Such membranes are particularly suitable as host matrix for PEs since the interlaying of fibers generates large porosity with fully interconnected pore structure and large surface area facilitating high electrolyte uptake and easy transport of ions [15]. So, the PEs prepared by electrospinning can achieve high electrolyte uptake and ionic conductivity at room temperature [16].
The PEs based on PVC–PMMA prepared by casting technology have been earlier reported by A. M. Stephan et al. [17] and S. Ramesh et al. [18]. Their results demonstrated that the PEs prepared by casting technology had low ionic conductivity. Thus, it is inferred that the conventional way for the preparation of PEs based on PVC–PMMA could not apply to practice for lithium-ion batteries.
In our study, we have made use of electrospinning to prepare composite PVC–PMMA membranes and explored the beneficial characteristics between PVC and PMMA to make PEs for lithium-ion batteries. The characterization and electrochemical performances of the electrospun PVC–PMMA membranes were investigated. These results showed that PVC–PMMA composite membrane prepared by electrospinning had a good prospect as PEs for lithium-ion batteries.
Experimental
Materials
PVC (WS-1000S) and PMMA (AR, Taiwan) were vacuum-dried for 12 h at 60 °C before use. The solvent N, N-dimethylformamide (DMF, AR) was used as received. The anhydrous tetrahydrofuran (THF, AR) was distilled before use. The salt LiClO4 (AR, Sinopharm chemical Reagent Co., Ltd.) was dried at 80 °C and kept under vacuum for 72 h before use. The plasticizers ethylene carbonate (EC) and propylene carbonate (PC) with high purity (N 99%) were purchased from Shenzhen Capchem Technology Co., Ltd. and used without further purification.
Preparation of nanofibrous membranes
A 20% amount of PVC–PMMA [xPVC–(1 − x) PMMA, where x is 1, 0.7, and 0.5] was dissolved in a mixed solvent of DMF/THF (5:5, w/w) in a closed beaker and then stirred for 6 h at 50 °C until a homogeneous solution was formed. The resulting homogeneous solution was degassed for 15 min to get a bubble-free clear solution. The fibrous membranes were prepared by a typical electrospinning method at room temperature as described in some previous publications [19, 20]. Electrospinning of the homogeneous solution was performed at a flow rate of 3 mL/h with a high voltage of 27 kV at room temperature. A distance of 20 cm was kept between the syringe tip and the collector plate. A fibrous membrane was then deposited on the collector plate. Also, the average thickness of the fibrous membranes was around 0.08 mm, which was measured with a micrometer. Then, the electrospun fibrous membranes were finally dried under vacuum at 60 °C for 12 h. These membranes were designated as PE 10 {PVC–PMMA (10:0, w/w)}, PE 7 {PVC–PMMA (7:3, w/w)}, and PE 5 {PVC–PMMA (5:5, w/w)} to highlight their respective composition.
Characterization of nanofibrous membranes
The surface morphology of the electrospun fibrous membranes was observed with a scanning electron microscope (SEM, Hitachi S-3500, Japan). Thermal analysis was performed by TG (Perkin-Elmer Co., USA) in a nitrogen atmosphere at a heating rate of 20 °C min−1.
The porosity (%) of PVC–PMMA nanofibrous membranes was determined using n-butanol uptake. PVC–PMMA fibrous membranes were immersed in n-butanol for 2 h. The mass of PVC–PMMA fibrous membranes before and after immersion was measured. The porosity (%) of the membranes was calculated using the following equation:
where W i and W a were the weight of the wet and dry membranes, respectively. ρ is the density of n-butanol. V is the apparent volume of the membrane.
The electrochemical measurements of the PEs were obtained by immersing the electrospun nanofibrous membranes in 1 M LiClO4-PC/EC (volume 1:1) solution for 60 min at 25 °C in a dry glove box. The electrolyte uptake was calculated according to the following equation:
where W i and W a were the weight of the wet and dry membranes, respectively.
The ionic conductivities of the PEs were measured through an ionic conductivity cell, which was made by sandwiching a given PE membrane between two stainless steel (SS) blocking electrodes (SS/polymer electrolyte membrane/SS). The ionic conductivity was determined by the impedance measurements in the temperature between 25 and 75 °C, using a Zahner Zennium electrochemical analyzer (Germany). The frequency of testing ranged from 100 mHz to 100 kHz at an AC amplitude of 5 mV. The ionic conductivity could be calculated from the following equation:
where R b, L, and S are bulk resistance (Ω), thickness (cm), and effective area (cm2) of the polymer electrolyte membranes, respectively. R b was obtained from the high frequency intercept on the real axis. The effective area was determined by the circular stainless steel blocking electrodes and the thickness was around 0.08 mm.
Electrochemical stability was determined by linear sweep voltammetry at the scanning rate of 5 mV s−1, using a stainless steel electrode as working electrode and a lithium metal as reference and counter-electrode. The measurement was operated by a CHI 660a electrochemical analyzer (CH Instrument Inc., USA) over the potential range of 2.5–6.0 V versus Li+/Li.
The interfacial resistance (R f) between the PEs and lithium metal electrode was measured by the impedance response of Li/polymer electrolyte membrane/Li cells over the frequency range 100 mHz to 100 kHz at an AC amplitude of 5 mV.
Results and discussion
Morphology
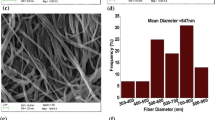
Figure 1 shows the SEM images of electrospun membranes. Much important information can be obtained in the morphology of electrospun membranes. Firstly, the fibers in electrospun membranes are well-interconnected with a large number of voids and cavities. The well-interconnected structures are able to uptake large amounts of liquid electrolyte. Secondly, the average fiber diameter (AFD) decreases with increasing PMMA content in the composite. The diameter values of PE 10 fibers are 1.6–2.4 μm and that of AFD is 1.8 μm (Fig. 1a). The diameter values of PE 7 fibers are in the range of 1.4–2.0 μm and the AFD is 1.6 μm (Fig. 1b). The diameter values of PE 5 fibers are in the range of 1.2–1.6 μm and the AFD is 1.4 μm (Fig. 1c). PE 5 has a narrower diameter range as compared to the other membranes. So, PE 5 has a more uniform fiber diameter. The lower diameter of the fiber in membranes with PMMA can be attributed to the substantial decrease in the viscosity that results from the blending of polymer solution with the addition of PMMA. The high viscosity of the electrospinning polymer solution results in the ejection of a larger fluid jet from the needle and a consequent deposition of fibers with a larger diameter [15]. Thirdly, there are no bead-fibers in electrospun membranes. It can be attributed to several combinational factors: the high polarity of DMF (dipole moment of 3.80D and dielectric constant of 36.7), low volatility of THF (boiling point of 64 °C), polar nature of PVC and PMMA [21]. These results lead to an enhancement of electrolyte uptake.
Thermal analysis
Figure 2 shows the thermal stability of the electrospun fibrous membranes. The occurrence of stable weight for temperatures of up to 100 °C for all the weights of thin membranes indicates that the samples have almost dried up. All the thin membranes have shown two obvious decomposition stages; however, PVC has a weight loss of 1.8% at 112 °C. This is mainly due to the evaporation of residual solvent. All the samples have a basically unanimous decomposition at 265 °C, but the weight losses for PE 10, PE 7, and PE 5 are different; they are 64%, 55%, 40%, respectively. These weight losses are mainly attributed to the dehydrochlorination of PVC. It is observed that the first weight loss decreases with the addition of PMMA. The second decomposition of PVC occurs at 410 °C and has a weight loss of 20%. The unzipping process of PVC leads to the weight loss. We find that the second decomposition of PE 5 shifts to an earlier temperature (370 °C) than that of PVC (410 °C). This is mainly due to the degradation of unsaturated group in PMMA. These results imply that electrospun fibrous membranes have a good thermal stability.
Porosity and electrolyte uptake
Electrospun membranes show an interconnected porous network structure with varying porosity. This is inferred from porosity measurements as shown in Table 1. The porosity (%) of electrospun membranes increases with increasing PMMA (wt.%) content. The increase of porosity (%) seems to be originated from the decrease of the average diameters of fibers [22]. Among the electrospun membranes, PE 5 shows high porosity (~86.0%).
Figure 3 shows the uptake of the liquid electrolyte (1 M LiClO4 in EC/PC (1:1, v:v)) as a function of PMMA content in the blend. The electrolyte uptake value of each membrane is also presented in Table 1. The fully interconnected pore structure of these membranes induces fast liquid penetration into the membrane, and hence the uptake process gets stabilized within a span of only 20 min. Electrolyte uptake is found to increase with the increase in PMMA content in the blend. This is mainly due to the good compatibility between PMMA and the liquid electrolyte [9]; besides, the increase in porosity can also be used to illustrate the increase of electrolyte uptake. The higher electrolyte uptake can make an improvement in ionic conductivity.
Ionic conductivity
The AC impedance plots of the PEs at 25 °C are shown in Fig. 4. The straight lines inclined towards the real axis represent the electrode/electrolyte double-layer capacitance behavior. This response is typical of the electrolyte with a major contribution towards total resistance from bulk resistance (R b) and only a minor contribution from the grain boundary resistance [23]. R b of the electrolyte was obtained from the high frequency intercept on the real axis. The ionic conductivity of the PEs which can be calculated according to Eq. 3 is listed in Table 2.
It can be found that the ionic conductivity of the PEs in our study is higher than that of the PEs based on PVC–PMMA reported in other references [17, 18]. This can be explained because the fibrous membranes prepared by electrospinning can supply large surface area fibers and fully interconnected porous structures, so they can uptake large amounts of liquid electrolyte to enhance ionic conductivity. This conclusion was the same with the point reported by J.H. Cao et al. [24] that higher electrolyte uptake resulted in higher ionic conductivity. We can also find that the ionic conductivity of the PEs increases with the increase in PMMA content in the blend. The increasing amount of electrolyte uptake obtained from the former electrolyte uptake study may improve the ionic conductivity.
To further understand the electrochemical properties of the PEs, the temperature dependence of the ionic conductivity of the PEs is shown in Fig. 5. A typical behavior of PEs was observed in that the ionic conductivity increased when the temperature went up. This is attributed to the expansion of the polymer matrix. The expansion of the polymer matrix resulting from the higher temperature produced the local empty space and expanded the free volume, which promoted the polymer segments and carrier ions to transfer [25]. Also, the relationship between log (σ) and 1/T was approximately linear; the conductive behavior was basically according to the Arrhenius equation \( \sigma = A{ \exp }\left( { - {E_{\text{a}}}{/}RT} \right) \), where R, σ, A, and T are the gas constant, the conductivity of polymer electrolyte, the pre-exponential index, and the testing temperature, respectively. Therefore, the activation energy (E a) can be calculated from the slope of the imitated straight line according to the Arrhenius equation. The E a order for the PEs is as follows: 17.0 kJ mol−1 (PE 10) <19.6 kJ mol−1 (PE 7) <20.3 kJ mol−1 (PE 10). It suggests that the E a has a little increase with the increase in PMMA content in the blend.
Electrochemical stability
The electrochemical stability window of the PEs based on the electrospun PVC–PMMA membranes is shown in Fig. 6. The polymer electrolyte based on PVC without PMMA exhibits an anodic stability of up to 4.5 V. With the incorporation of PMMA in the PVC, the electrochemical stability is found to be enhanced with the increase in PMMA content in the blend. The stability order for the PEs is as follows: 4.5 V (PE 10) <4.8 V (PE 7) <5.0 V (PE 5). The partial swelling of the fibrous matrices with a large surface area significantly contributed to the increase in the stability of the electrolyte solution under electrochemical environments [26]. The high anodic stability of the PEs should make them potentially compatible with cathode materials such as LiCoO2, LiMn2O4, and LiFePO4, which were commonly used in lithium-ion batteries.
Interfacial characteristics
Compatibility with the lithium metal anode is an important issue to guarantee an acceptable performance in electrochemical devices. Figure 7 shows the initial impedance behaviors of PEs based on electrospun PVC–PMMA membranes. The impedance spectra is in the form of a semicircle with the real axis intercept at the high frequency end denoting bulk electrolyte resistance (R b) and that at the low frequency end denoting the electrode/electrolyte interfacial resistance (R f) [15]. It can be found that the R f of the PEs decreases with the increase in PMMA content in the blend. The R f order for the PEs is as follows: 317 Ω (PE 10) <191 Ω (PE 7) <99 Ω (PE 5). It is attributed to a good stabilization between the lithium metal and PMMA. Therefore, PE 5 has a good interfacial stabilization with lithium metal anode.
The variation of interfacial resistance of PE 5 with storage time is shown in Fig. 8. It is seen that the total interfacial resistance (R f) of PE 5 increases with the increase of the storage time until a stable state is obtained, which is attributed to the growth of a passivating film on the Li electrode surface due to the reaction of Li metal with PE 5. The result illustrates that PE 5 has favorable interfacial properties with lithium metal anode after 14 days of storage. This would make PE 5 a very promising candidate for safe, reliable, and long-lasting lithium-ion batteries.
Conclusions
Composite fibrous membranes based on PVC–PMMA were prepared by electrospinning. The SEM micrograph showed that there were no bead-fibers in fibrous membranes. The addition of PMMA promoted electrolyte uptake and ionic conductivity of the polymer electrolyte. The polymer electrolyte based on PVC–PMMA (5:5, w:w) blend was 1.36 × 10−3 S cm−1 at 25 °C. The temperature dependence of ionic conductivity can be explained on the basis of the Arrhenius model at 25–75 °C. The electrochemical stability window of PE 5 was stable up to 5.0 V (vs. Li+/Li). The PVC–PMMA composite fibrous PEs showed good interfacial stability with the lithium electrode. Thus, PVC–PMMA composite fibrous membranes prepared via electrospinning were a good candidate to be used as the polymer matrix of PEs for polymer lithium-ion batteries.
References
Tarascon JM, Armand M (2001) Nature 414:359–367
Stephan AM, Nahm KS (2006) Polymer 47:5952–5964
Rajendran S, Mahendran O, Kannan R (2002) Fuel 81:1077–1081
Ahmad Z, Al-Awadi NA, Al-Sagheer F (2007) Polym Degrad Stabil 92:1025–1033
Ramesh S, Winie T, Arof AK (2007) Eur Polym J 43:1963–1968
Rajendran S, Babu RS, Sivakumar P (2008) J Membr Sci 315:67–73
Vickraman P, Aravindan V, Selvambikai M, Shankarasubramanian N (2009) Ionics 15:433–437
Cazzanelli E, Mariotto G, Appetecchi GB, Croce F, Scrosati B (1995) Electrochim Acta 140:2379–2382
Nicotera I, Coppola L, Oliviero C, Castriota M, Cazzanelli E (2006) Solid State Ion 177:581–588
Ding YH, Zhang P, Long ZL, Jiang Y, Xu F, Di W (2009) J Membr Sci 329:56–59
Yee WA, Nguyen AC, Lee PS, Kotaki M, Liu Y, Tan BT, Mhaisalkar S, Lu XH (2008) Polymer 49:4196–4203
Li X, Cheruvally G, Kim JK, Choi JW, Ahn JH, Kim KW, Ahn HJ (2007) J Power Sources 167:491–498
Bansal D, Meyer B, Salomon M (2008) J Power Sources 178:848–851
Kim JK, Manuel J, Chauhan GS, Ahn JH, Ryu HS (2010) Electrochim Acta 55:1366–1372
Raghavan P, Zhao XH, Kim JK, Manuel J, Chauhan GS, Ahn JH, Nah C (2008) Electrochim Acta 54:228–234
Xiao QZ, Li ZH, Gao DS, Zhang HL (2009) J Membr Sci 326:260–264
Thirunakaran R, Renganathan NG, Sundaram V, Pitchumani S, Muniyandi N, Gangadharan R, Ramamoorthy P (1999) J Power Sources 81:752–758
Ramesh S, Liew CW, Morris E, Durairaj R (2010) Thermochimi Acta 511:140–146
Frenot A, Chronakis IS (2003) Curr Opin Colloid Interface Sci 8:64–75
Manesh KM, Santhosh P, Gopalan A, Lee KP (2007) Anal Biochem 360:189–195
Dong H, Nyame V, MacDiarmid AG, Jones WE (2004) J Polym Sci B: Polym Phys 42:3934–3942
Kim JR, Choi SW, Jo SM, Lee WS, Kim BC (2004) Electrochim Acta 50:69–75
Raghavan P, Zhao XH, Manuel J, Chauhan GS, Ahn JH, Ryub HS, Ahn HJ, Kim KW, Nah C (2010) Electrochim Acta 55:1347–1354
Cao JH, Zhu BK, Xu YY (2006) J Membr Sci 266:446–453
Cui ZY, Xu YY, Zhu LP, Wang JY, Xi ZY, Zhu BK (2008) J Membr Sci 325:957–963
Gopalan AI, Santhosh P, Manesh KM, Nho JH, Kim SH, Hwang CG, Lee KP (2008) J Membr Sci 325:683–690
Acknowledgement
This work was financially supported by the Key Laboratory of Environmentally Friendly Chemistry and Applications of Ministry of Education item (Grant No. 09B101).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhong, Z., Cao, Q., Wang, X. et al. PVC–PMMA composite electrospun membranes as polymer electrolytes for polymer lithium-ion batteries. Ionics 18, 47–53 (2012). https://doi.org/10.1007/s11581-011-0615-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-011-0615-6