Abstract
Olivine-structured pure LiNiPO4 and yttrium-doped LiNiPO4 have been synthesized by a Pechini-type polymerizable precursor method. Compound formation temperature is confirmed from thermogravimetry to differential thermal analysis. Powder X-ray diffraction pattern confirmed the formation of phase pure LiNiPO4 compound with an orthorhombic structure with fine crystallite size. Presence of preferred local cation environment is understood from Fourier transform infrared spectroscopy (FTIR) studies. XRD and FTIR studies show that doped yttrium ion entered in the lattice of LiNiPO4. It has been found that the ionic conductivity of LiNiPO4 is enhanced by around two orders of magnitude by doping yttrium. Dielectric spectra show the decrease in dielectric constant with increase in frequency. Dielectric loss spectra reveal that dc conduction contribution predominates in the sample.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pioneering work of Padhi et al. [1], on olivine-structured LiMPO4, has been intensively investigated as a potential candidate cathode material for rechargeable lithium batteries from both economic to environmental points of view [2]. However, one of the main problems of Olivine materials lies in its poor rate capability, which is attributed to its low electronic conductivity and slow kinetics of lithium ion diffusion through the LiMPO4–MPO4 interfaces [3–5]. Two approaches have been attempted to overcome this problem [6]. One is to enhance its electronic conductivity by coating an electron-conducting layer such as conductive carbon, nano-copper, and nano-silver around the particles or by doping with guest cations [7–10]. The other is to minimize the particle size by modifying synthesis conditions [11–13]. Several techniques have been applied to prepare LiMPO4, such as solid-state reaction, co-precipitation, sol–gel preparation, and hydrothermal method. Tian et al. [14] suggested that the yttrium ion doping in LiFePO4 improves its ionic conductivity in comparison with the relative low ionic conductivity observed in the high valence transition metal ions doped sample. Luo et al. [15] added a small amount of La3+ ions in LiFePO4 by solid state synthesis, and found that these ions doping would improve its capacity delivery and cycle performance. Research of Jiezi et al. [16] has shown that substitution of rare Earth ions at Li site of (LiY)FePO4 improves the ionic conductivity. Zhong et al. [17] studied the effect of doping yttrium ion in Li3V2(PO4)3 and observed increase of conductivity due to doping yttrium ion. In this work, an attempt has been made to study the effect of yttrium doping in LiNiPO4 prepared by Pechini-type polymerizable precursor method.
Experimental techniques
Pure LiNiPO4 and doped Li.99Y.01NiPO4 have been prepared by the Pechini-type polymerizable precursor method [18–20]. This method is based on polyesters obtained from metal-chelated citric acid to polyhydroxyl alcohol and was first developed by Pechini. The raw materials LiNO3, Ni(NO3)2.6H2O, Y(NO3)3.6H2O, and NH4H2PO4 are dissolved in double distilled water to prepare doped and undoped LiNiPO4. Citric acid and poly ethylene glycol with a molar ratio of (1:1) has been added as a chelating agent for gelation. Pyrolysis of the mixture obtained is performed at 350 °C for 2 h in a hot plate that yields homogeneous black powder precursors that have been ground into fine powders which has been calcinated at 800 °C for 8 h. This method then offers several advantages compared to the conventional solid-state reaction method to prepare ceramic powders with highly controlled stoichiometry and morphology and also nanosized materials. Thermogravimetric analysis (TGA) and differential thermal analysis (DTA) studies have been performed using Perkin-Elmer Diamond TGA/DTA instrument at a heating rate of 5 °C min−1 in air. XRD patterns have been obtained using a XPERT-PRO X-ray diffractometer PW3050/60 with Cu Ka radiation having a wavelength of 1.5417Å at room temperature. Fourier transform infrared spectroscopy (FTIR) spectroscopy has been performed using FTIR-8400S SHIMADZU spectrophotometer in the wave number range 400–4,000 cm−1. A pressure around 1.2 t is applied to form a pellet with 1 mm thickness and 10 mm diameter. The pellet has been sintered at 600 °C for 3 h. Subsequently, the pellets are polished by fine emery paper to make their faces smooth and parallel. The pellets are finally coated with conductive silver paint. Electrochemical impedance spectroscopy measurements have been carried out using a Solatron 1260 frequency response analyzer combined with a Solatron 1296 electrochemical interface. The impedance spectra are recorded over the frequency range of 1 MHz to 10 Hz at different temperature range.
Results and discussion
Thermogravimetric and differential thermal analysis
Thermal decomposition of the black powder precursor obtained from the pyrolysis has been examined by means of the thermogravimetric and differential thermal analysis. Figure 1 represents theTGA/DTA curve of yttrium-doped LiNiPO4 precursor. Thermogravimetric analysis of doped and undoped samples exhibits a gradual and an initial weight loss up to about 630 °C due to the initial evaporation of water (below 200 °C) and a subsequent decomposition of precursors (200–630 °C) from the in situ-formed meta stable complex. In other words, the decomposition of citric acid with evolution of CO2, NO2, and NH3 gasses followed by the final decomposition which shows larger exothermic peak observed at 572 °C in the DTA curve, which results in the formation of LiNiPO4 and yttrium-doped LiNiPO4. Herein, the compound formation process starts at a temperature as low as 450 °C and the same gets completed around 630 °C. While further increasing the temperature, the TGA keeps almost a constant value. Meanwhile, the DTA value decreases gradually to 720 °C, and then becomes constant. It may reflect that the ordered olivine structure materials became more complete in this region. However, the samples of the present study has been heat treated up to 800 °C in order to get phase pure and better crystalline product.
XRD analysis
Influence of yttrium doping on crystal structure of cathode materials
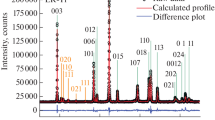
The X-ray diffraction patterns for pure LiNiPO4 and doped Li.99Y.01NiPO4 powders are shown in Fig. 2. The crystal phase of LiNiPO4 and Li.99Y.01NiPO4 are identified to be LiNiPO4 phase with ordered olivine structure indexed by orthorhombic symmetry with Pnma space group. It can be seen from the patterns that all diffraction peaks are very strong, which indicated that the sample has good crystal structure. There has been no other phase except the LiNiPO4 phase, which meant the entire yttrium component entered the lattice of LiNiPO4 [21]. Main peaks for these prepared samples are labeled with (h k l) indexes. It has been observed that the 2θ values obtained are in good agreement with (JCPDS file number 88–1297).
The average crystallite size has been calculated by the Debye–Scherrer method,
where, Δ is the average particle size, λ is the wavelength of Cu Kα, β is the full-width half-maximum of the reflection peak in radians, and θ is the Bragg angle. The crystallite size calculated using the above formula has been found to be 71 and 60 nm for LiNiPO4 and Li.99Y.01NiPO4 prepared by the Pechini-type polymerizable precursor method, respectively.
The lattice parameters of these two samples have been calculated and summarized in Table 1. Doping effect will sometimes increase lattice parameters or decrease depend on doping ion and amount of doping [22–28]. It has been seen that the lattice parameter along b-axis increases in LiNiPO4 as the position of Li+ in the lattice is partially replaced by Y3+, which is favorable to the transmission of Li ions for the Li ion transmitting along b-axis [16, 29]. The reason is that the ion radius of Li+ is 0.076 nm and smaller than 0.09 nm, that of Y3+ and it caused the increase of length of b-axis. The lattice volume of yttrium-doped LiNiPO4 is larger than that of pure LiNiPO4 which may be useful for Li to insert and de-insert process. In the pure LiNiPO4, the cell volume decreases as Ni2+ is oxidized to Ni3+ during de-intercalation of Li+ because the diameter of Ni3+ is smaller than that of Ni2+ as a result, the lattice may collapse and further causes a reduced Li+ diffusion ability. However, for Y-doped sample Li0.99Y0.01NiPO4, Y3+ in the lattice will act as a pillar to prevent the collapse of crystal during charge/discharge cycle.
Local cation environment by FTIR
Generally, vibrational modes are attributed to the motion of cations with respect to their oxygen neighbors are sensitive to the point group symmetry of the cations in the oxygen host matrix [30], and hence, the local environment of the cations in a lattice of close-packed oxygens can be studied by FTIR spectroscopy [31]. Figure 3 shows the FTIR spectra of Li.99Y.01NiPO4 with wave number ranging from 400 to 4,000 cm−1.The bands observed in the region 1,400–400 cm−1 are characteristic of phosphate ion [PO4]−3 which are in free state existing in tetrahedral (Td) symmetry.
In an ideal case, the phosphate ion exhibits four fundamental modes of vibrations at 1,148(ν3), 1,053 (ν1), 648 (ν4), and 474 (ν2) cm−1 [32]. Of these, ν1 is non-degenerate; ν2 is doubly degenerate, whereas ν3 and ν4 are triply degenerate. Among these four vibrations, only ν3 and ν4 vibrations are infrared active.
In general, in most of the cases, phosphate ion is distorted from the ideal Td symmetry. This removes the degeneracy of the infrared-active vibrations (ν3 and ν4) and also allows the non-active vibrations ν1 and ν2 to absorb energy in the infrared region. The frequencies of these types of vibrations in different compounds are always within the same frequency range, so that an unambiguous assignment of the high energy modes of the sample is possible. Usually, the asymmetrical ones are at higher wave numbers than the symmetrical ones. The vibrational band at around 548 cm−1 may be attributed to Li-O symmetric stretching [33]. No obvious difference is observed in the FTIR patterns of the yttrium-doped LiNiPO4 with undoped LiNiPO4. There are no other vibrations except LiNiPO4, which meant the entire yttrium component has entered the lattice of LiNiPO4 as confirmed by XRD results also.
Impedance analysis
Nyquist plots consist of a depressed semicircle in high frequency region and a straight line in low frequency region at various temperatures for LiNiPO4 and Li.99Y.01NiPO4 as shown in Fig. 4. These high frequency region semicircles are due to parallel combination of bulk resistance (R b) and bulk capacitance (C b) of the materials. The straight line in the low frequency is attributed to the diffusion of the lithium ions into the bulk of the electrode material or so-called Warburg diffusion. The depression of the semicircle below the real axis shows the non-Debye nature of the material, which is quite a common property of fast ionic conductors. The bulk resistance of the sample has been calculated from the low frequency interception of real axis using the program EQ [34]. The numerical value of the diameter of the semicircle on the Z real axis is approximately equal to the charge transfer resistance (R ct), therefore, it can be seen that there is a marked decrease in R ct after doping yttrium ion.
The ionic conductivity of the samples has been calculated by the following equation.
- σ:
-
ionic conductivity
- d :
-
thickness of the sample
- S :
-
area of the sample
- R b :
-
bulk resistance
The bulk conductivity has been found to be 9.34 × 10−9 Scm−1 for LiNiPO4 and 9.772 × 10−7 Scm−1 for Li.99Y.01NiPO4 for room temperature. At 300 °C, the bulk conductivity is 2.34 × 10−7 Scm−1 for LiNiPO4 and 2.068 × 10−5 Scm−1 for Li.99Y.01NiPO4. The associated capacitance has been calculated from the relation ωRC = 1 at the arc maximum (Fig. 4) and it corresponds to a value of pF, that is attributed to a conduction process through the bulk of the material [35].
Conductivity analysis
The ionic conduction in the material requires the ions to move by hopping mechanisms between allowed sites. The AC conductivity σ (ω) usually reaches to a frequency-independent plateau at low frequencies and dispersive phenomena at high frequencies. The conductivity study of doped and undoped LiNiPO4 cathode materials at various temperatures is shown in Fig. 5 and it is found to obey Jonscher’s power law [36]. The Jonscher’s power law equation may be written as
where n is the frequency exponent in the range 0 < n <1:
The frequency-independent conductivity is observed in the low-frequency region, which indicates the DC conductivity of the material. In the high-frequency region, the power-law feature σ(ω) ∞ Aωn is observed. DC conductivity value has been calculated by extrapolating the plateau region to Y-axis.
It has been observed that conductivity increases due to doping yttrium ion. The improvement of the ionic conductivity might be explained as follows. Yttrium doping can reduce the grain size, decrease the Li+ diffusion resistance, and improve the conductivity. Also, the impregnation of yttrium leads to creation of vacancies of lithium ions which is beneficial to the diffusion of lithium ions and yttrium can reduce the interaction force between Li+ and Li+, which makes the Li+ intercalation/de-intercalation easier. Similar effect has been reported by Tian et al. in yttrium-doped LiFePO4 [14] and also by Luo et al. [15] in lanthanum doped LiFePO4 cathode material. Nazar et al. [37] stated that preparation of LixNiPO4 by heating carbon-containing precursors at temperatures between 600 and 900 °C in air has lead to the formation of Li4P2O7 and Ni2P2O7, minor contaminants and heat treatment under argon at 600 °C resulted in deep black materials consisting of LiNiPO4, and minor fractions of Ni3P (along with Li4P2O7 and Li2Ni3P4O14). Their studies shows the formation of secondary phase which leads to the enhancement of conductivity. But our study shows, no formation of secondary phase in our samples.
Dielectric analysis
The frequency dependence of the dielectric function ε(ω) of a solid reflects the dynamic response of the constituents of the solid. The complex permittivity is given by
where ε′ is the real part of complex permittivity or dielectric constant and ε″ is the dielectric loss. Figure 6a shows that the real part of the dielectric permittivity decreases with increase in frequency. At low frequencies due to space charge accumulation a gradual increase in the dielectric constant has been observed. At higher frequencies, the change in the direction of the electric field lines is too fast to be followed by the charged ions and hence the dielectric constant decreases. The plot of log ε″ vs. logω is shown in Fig. 6b. The slope of the straight line has been found to be in the range of −0.8 to −0.95. Hence, log ε″ vs. logω plot with a slope value near to −1 indicates that the dc conduction contribution is predominant in the present sample. The absence of peak in the dielectric loss spectra also indicates the long-range ionic conductivity dominance in dielectric response at low frequencies [38]. The deviation from the unity may be attributed to the space charge effects at low frequencies.
Dissipation (loss) factor tanδ is given by
Figure 6c shows the frequency dependence of tanδ at various temperatures. The tanδ is found to increase with frequency at different temperatures, pass through a maximum value (tanδmax), and thereafter decreases. Tanδmax gets shifted to higher frequency as the temperature is increased. This behavior suggests that the system can be represented by a parallel RC element [39].
From Fig. 6a–c, it is understood that ε′, ε″ and tanδ increases with increase in temperature and doping yttrium ion. The increase in the dielectric constant may be attributed to the migration and polarization of Li+ ions [40]. Thus, the dielectric dispersion observed with temperature and frequency variation confirms the electrical charge hopping mechanism which governs both the charge transport and relaxation phenomenon in the present sample.
Conclusion
Olivine-structured pure LiNiPO4 and yttrium doped have been successfully synthesized by a Pechini-type polymerizable precursor method and yttrium has entered into the lattice of LiNiPO4 completely. The yttrium doping favors the expansion of the lattice volume and provides more space for lithium ion intercalation/de-intercalation. The impregnation of yttrium can reduce the grain size and resulted in the formation of vacancies, which were favorable to the enhancement of the ionic conductivity of the LiNiPO4 system. The ionic conductivity of LiNiPO4 is enhanced by around two orders of magnitude by doping yttrium. Dielectric loss spectra indicate that the DC conduction contribution is predominant in the present samples.
References
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) J Electrochem Soc 144:1188
Wang XM, Sone Y, Kuwajima S (2005) J Power Sources 14:313
Yamada A, Hosoya M, Chung SC, Kudo Y, Hinokuma K, Liu KY, Nishi Y (2003) J Power Sources 119–121:232
Manickam M, Singh P, Thurgate S, Prince K (2006) J Power Sources 158:646
Shi SQ, Quyang CY, Xiong ZH, Liu LJ, Wang ZX, Li H, Wang DS, Chen LQ, Huang XJ (2005) Phys Rev B Solid State 71:144404
Koltypin M, Aurbach D, Nazar L, Ellis B (2007) J Power Sources 174:1241
Park KS, Son JT, Chung HT, Kim SJ, Lee CH, Kang KT, Kim HG (2004) Solid State Commun 129:311
Jayaprakash N, Kalaiselvi N (2007) Electrochem Commun 9:620
Zhu BQ, Li XH, Wang ZX, Guo HJ (2006) Mater Chem Phys 98:373
Ni JF, Zhou HH, Chen JT, Zhang XX (2005) Mater Lett 59:2361
Salah AA, Mauger A, Julien CM, Gendron F (2006) Mater Sci Eng 129:232
Kim DH, Kim JK (2007) J Phys Chem Solids 68:734
Croce F, Epifanio AD, Hassoun J, Deptula A, Olczac T (2002) Electrochem Solid-State Lett 5:A47
Tian Y, Kang X, Liu L, Xu C, Qu T (2008) J Rare Earths 26:279
Luo S, Tian Y, Li H, Shi K, Tang Z, Zhang Z (2010) J Rare Earths 28:439
Jiezi H, Jian X, Xinbing Z, Hongming Y, Xin Z, Gaoshao C, Jiangping T (2009) J Mater Sci Technol 25:3
Zhong S, Liu L, Jiang J, Li YA, Wang J, Liu J, Li Y (2009) J Rare Earths 27:134
Savitha T, Selvasekarapandian S, Ramya CS (2008) J Solid State Electrochem 12:857–860
Han YS, Kim HG (2000) J Power Sources 88:161–168
Guo ZP, Zhong S, Wang GX, Walter G, Liu HK, Dou SX (2002) J Electrochem Soc 149:A792–A795
Xiangwei Wu, Wen Z, Xiaogang Xu, Han J (2008) Solid State Ionics 179:1779–1782
Shenouda AY, Liu HK (2009) J Alloy Comp 477:498–503
Lin Z-P, Zhao Y-J, Zhao Y-M (2009) Chin Phys Lett 26:038202
Liu H, Cao Q, Fu LJ, Li C, Wu YP, Wu HQ (2006) Electrochem Commun 8:1553–1557
Sun CS, Zhou Z, Xu ZG, Wang DG, Wei JP, Bian XK, Yan J (2009) J Power Sources 193:841–845
Yang R, Songa X, Zhaoa M, Wang F (2009) J Alloy Comp 468:365–369
Wang G, Cheng Y, Yan M, Jiang Z (2007) J Solid State Electrochem 11:457–462
Wang Y, Yang Y, Xin Hu, Yang Y, Shao H (2009) J Alloy Comp 481:590–594
Jiang J, Ouyang CY, Li H, Wang ZX, Huang XJ, Chen LQ (2007) Solid State Commun 143:144
Rougier CJ, Nazri GA, Julian C (1997) Mater Res Soc Symp Proc 453:647
Rouier A, Nazri GA, Julian C (1997) Ionics 3:170
Ravikumar RVSSN, Chandrasekhar AV, Reddy BJ, Reddy YP, Ikeda K (2002) Cryst Res Technol 37(10):1127
Weiwei H, Roger F (1996) Solid State Ionics 86–88:395
Boukamp BA (1986) Solid State Ionics 18–19:136
Irvine JTC, Sinclair DC, West AR (1990) Adv Mater 2:138
Jonscher AK (1977) Nature 267:673
Subramanya Herle P, Ellis B, Coombs N, Nazar LF (2004) Nat Mater 3:147
Bohnke O, Emery J, Fourquet JL (2003) Solid State Ionics 158:119
Simmons JG, Nadkarni GS, Lancaster MC (1970) J Appl Phys 41:538
Orliukas A, Dindune A, Kanepe Z (2003) Solid State Ionics 157:177
Acknowledgment
One of the authors (M. Prabu) is thankful to authorities of the Department of Science and Technology/Japan Science and Technology for offering a JRF position in the DST/JST project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prabu, M., Selvasekarapandian, S., Kulkarni, A.R. et al. Structural, dielectric, and conductivity studies of yttrium-doped LiNiPO4 cathode materials. Ionics 17, 201–207 (2011). https://doi.org/10.1007/s11581-011-0535-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-011-0535-5