Abstract
In this work, 1 M LiPF6:EC:DEC (v/v = 1/2) was used as a baseline electrolyte where EC is ethylene carbonate and DEC is diethyl carbonate. Ethyl propionate (EP) was used as an additive. The conductivity of the liquid electrolyte was obtained at ambient and elevated temperatures. The highest room temperature conductivity was observed at (8.05 ± 0.16) mS cm−1 for the electrolyte containing 28.6 vol.% EP. Viscosity of the baseline and EP added baseline electrolytes have been measured at room and elevated temperatures. The electrolyte was also characterized by linear sweep voltammetry. The highest conducting electrolyte with 28.6 vol.% EP and the baseline electrolyte were used to fabricate several batteries. The batteries were charged and discharged at room temperature and at −20°C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In general, liquid electrolyte for lithium batteries consists of a lithium salt and solvents. The number of solvents may range from one to four [1–4]. Most of these solvents are carbonate based. Although fluorinated carbonate-based solvents have also been used [5]. Many groups [1–6] have also demonstrated the use of the ester co-solvent as an approach to increase the conductivity of the electrolyte and to improve the performance in Li-ion cells at low temperatures. More specifically, many groups have investigated the use of ethyl propionate in Li-ion battery electrolytes [6, 7]. The solvents are aprotic in nature since the presence of water is detrimental to the performance of a lithium-ion battery.
Liquid electrolytes in general need to meet a minimum number of requirements such as (1) high ionic conductivity to minimize cell resistance, achieve good rate capability, and keep self-discharge to a minimum; (2) high thermal and chemical stability; (3) wide potential window, i.e., defined as the voltage range between the oxidation and reduction decomposition limits of the electrolyte; (4) non-toxic and safe; (5) low melting point to help provide conductivity at low temperatures; (6) high boiling point to prevent pressure build-up in the cell, which may lead to an explosion; and (7) low cost. However, these criteria are not fully achieved. The working temperature range of a lithium-ion battery is limited to −10 to 40 °C [5, 8]. Many works have been done to enable the cell to operate at temperatures less than or equal to −30 °C [9–13]. Sazhin et al. have already studied the effect of adding ethyl propionate to electrolytes such as 1 M LiPF6/EC-DEC and 1 M LiPF6/EC-EMC where EMC is ethyl methyl carbonate. These electrolytes showed attractive performance at −20 °C, good cycle life and rate capability at both room temperature and 50 °C. Ohta and co-workers [7] have reported the performance of 1 M LiPF6/EC-DEC with addition of methyl acetate (MA), ethyl acetate (EA), methyl propionate (MP), and ethyl propionate (EP) Li-ion cells. They reported that cells containing 1 M LiPF6 dissolved in EC-DEC-MP electrolyte exhibits good capacity of 80 mAh at −10 °C as well as good storage characteristics at 60 °C but some gas evolution was observed after a 60 °C storage period.
Lithium hexafluorophosphate (LiPF6) dissolved in non-aqueous solvents based on mixed alkyl carbonate is known to exhibit high conductivity [14–16]. At 40 °C, EC has high dielectric constant (ε = 89.78) and relatively low viscosity (η = 1.90 cP), which are among the criteria for a good solvent. The high dielectric constant enables high salt dissociation and solubility which would lead to high conductivity. EC has a high boiling point of 248 °C, which ensures low vapor pressure and good electrochemical stability. However, the high melting point (36.4 °C) and viscosity requires EC to be blended with other co-solvents in order to produce solutions that are highly conductive over a wide temperature range. DEC has a melting point of −43 °C. Its viscosity is even lower than EC (η = 0.75 cP at 25 °C). The addition of ethyl propionate in this work, though not a very novel approach, with melting point −73 °C and viscosity 0.0532 cP (at 20 °C) should improve the mobility of the lithium ions in the electrolyte particularly in the low temperature range. Conductivity, viscosity, linear sweep voltammetry, and battery characterizations will be carried out in this paper.
Experimental methods
Liquid electrolyte preparation
Procured from Techno Semichem Co., Ltd, Korea, 1 M LiPF6:EC:DEC (v/v = 1/2) was used as baseline electrolyte. Ethyl propionate (CH3CH2COOC2H5) was dried using 4 Å molecular sieves (Fisher Scientific) overnight. The molecular sieve was heated at 400 °C for 8 h and cooled to room temperature before use. Various concentrations 0, 9.1, 16.7, 23.1, 28.6, 33.7, and 37.5 vol.% of EP were added into 1 M LiPF6:EC:DEC. Electrolyte formulation was performed under an argon atmosphere in a LABmaster 130 Mbraun glove box.
Characterization
Impedance measurement
Ionic conductivity is a reflection of the total charge species in the electrolyte. High conductivity electrolytes are favorable because they have lower ohmic potential losses. Conductivity, σ can be calculated from the equation,
where t is thickness of sample or liquid, A is the contact area between stainless steel electrode and the electrolyte, and R b is the bulk impedance (or resistance) obtained from the Cole–Cole plot that was derived from impedance measurement. Impedance was measured using the Hioki LCR 5322 bridge that was interfaced to a computer. Impedance measurements were performed in the frequency range between 50 Hz to 100 kHz. The conductivity cell consists of a quartz cuvette containing two stainless steel electrodes. The conductivity cell has a path length of 1.0 cm and the sample was filled to a height of 1.0 cm. Dispensing of the liquid electrolyte in the cuvette was performed under argon atmosphere in a LABmaster 130 Mbraun glove box.
Viscosity measurements
Viscosity measurements were conducted using a rotational viscometer model NDJ-1. The temperature effect of ethyl propionate on the viscosity of the 1 M LiPF6:EC:DEC electrolyte was varied from 30 to 60 °C. All measurements were carried out in the glove box.
Linear sweep voltammetry
The electrochemical stability window of the electrolyte formulation was studied by linear sweep voltammetry (LSV) measurement using the Autolab PGSTAT12 potentiostat–galvanostat. The LSV test was performed using a three-electrode configuration; stainless steel (SS) as the working electrode and a lithium metal as both the reference and counter electrodes. The liquid electrolyte was soaked in a fiber glass mesh. Sample preparation was carried out in the glove box.
Battery fabrication and characterization
The electrode for the lithium-ion batteries were procured from Magna Value Sdn Bhd (Malaysia) and used as received. The cathode (LiCoO2 as the active material) and anode (graphite as the active material) were coated on aluminum and copper foils, respectively. The electrodes were cut using an electrode punching machine in to the size of 5 cm × 5 cm. Poly(ethylene) was used as the separator (thickness = 20 µm). The cells were fabricated by packing the stacked electrodes and separators in a metallized packaging bag. The theoretical capacity of the battery is 250 mAh. The cells were characterized in terms of (1) the room temperature rate capability, (2) number of cycle performance at room temperature, and (3) the charge and discharge characteristics at room temperature and at −20 °C. Charge–discharge measurements and cycling tests were carried out with a Land battery cycler model CT2001A. A freezer was used to maintain the cell temperature at −20 °C.
Results and discussion
Sazhin et al. [6] reported on the addition of EP to 1 M LiPF6 in solutions of EC-DMC, EC-EMC, and EC-DEC. However, the conductivity of the electrolytes were not reported. In this work, we present the conductivity studies on 1 M LiPF6 in EC:DEC:EP electrolytes. Figure 1 shows the room temperature conductivity of the baseline electrolyte added with EP.
The conductivity of 1 M LiPF6:EC:DEC (v/v = 1/2) as baseline electrolyte is (5.24 ± 0.16) mS cm−1. The conductivity of the baseline electrolyte was double-checked using the conductivity meter model Con 150, Eutech Instrument. The result obtained was (6.31 ± 0.16) mS cm−1. Prior to this measurement the conductivity meter was calibrated using a KCl solution with a known conductivity of 1.41 mS cm−1. This measurement was performed at 25 °C. The conductivity of 1 M LiPF6:EC:DEC (v/v = 1) is ∼8.0 mS cm−1 as estimated from the work of Xiang et al. [18] at 25 °C and from the work of Morita and co-workers [19] the conductivity of 1 M LiPF6:EC:DEC (v/v = 2/1) is 8.5 mS cm−1 at 20 °C. The conductivity of the EP added system increases gradually at low concentrations, passes through a maximum and decreases at EP concentrations greater than 28.6 vol.%. The maximum conductivity observed is (8.05 ± 0.16) mS cm−1. The standard error ranges from 2% to 8%. Therefore, the baseline electrolyte with 28.6 vol.% EP was considered as the highest conducting electrolyte. At this EP concentration, the volume percentage of EC has dropped to ∼24% and the volume percentage of DEC has dropped to ∼47%. Although these solvents contribute to the low viscosity of the electrolyte solution the increase in EP content resulted in further decrease in viscosity of the electrolyte. The decrease in viscosity results in the increase in fluidity of the solution [17], which in turn increases the mobility of the ions. Hence, conductivity increases with increase in EP concentration up to 28.6 vol.%. The decrease in conductivity is attributed to the dilution of the electrolyte with EP that leads to a lower density in the number of Li+-transporting ions [17].
The conductivity of the electrolyte solutions was also measured as a function of temperature in the range between −30 and 50 °C as shown in Fig. 2.
Overall, the conductivity was found to increase with increasing temperatures in the range studied. The conductivity of the EP added electrolyte is higher than the baseline electrolyte at the same temperature. According to Xiang et al. [18], they observed a solid precipitation in 1 M LiPF6/EC-DEC(1:1) electrolyte at low temperatures (<−25 °C) which may be due to the freezing of EC. The lower conductivity of the baseline electrolyte at −30 °C may therefore be attributed to the solidification of EC which could have trapped the surrounding lithium ions and hence reduced the number of free lithium ions that is transported in the liquid phase. In the electrolyte added with EP in this work, the amount of EC has been lowered to ∼24 vol.% from 33 vol.% in the baseline electrolyte. The presence of EP in the baseline has prevented such precipitation. Therefore, there is no reduction in concentration of lithium ions and the conductivity is higher compared to the baseline electrolyte at −30 °C. It is to be noted that the volume content of EC is 50% in the baseline electrolyte of Xiang and co-workers [18], which is higher than the volume content of EC in the baseline electrolyte of the present work. This could also be the reason why such precipitation was not observed. From the figure, it can be observed that the conductivity of EP added baseline electrolyte at room temperature 25 °C is ∼8.0 mS cm−1 while at 50 and −30 °C the conductivity is 12.6 and 1.1 mS cm−1, respectively. The obtained values are found to be close to those reported by Xiang et al. [18] in the system 1 M LiPF6:EC:DEC (v/v = 1/1) with additive dimethyl methylphosphonate. Xiang and co-workers reported the conductivity values at 25, 50, and −30 °C as 8, 10, and 2 mS cm−1, respectively. Morita et al. [19] have developed a polymeric gel electrolyte comprising poly(vinylidene-co-hexafluoropropylene) and LiPF6 in EC/DEC (v/v = 2/1) and trimethyl phosphate for rechargeable lithium battery systems. The ionic conductivity of 7.1 mS cm−1 was obtained at 20 °C which is comparable with the conductivity of 7.9 mS cm−1 at the same temperature in our work. Ohta and co-workers reported the conductivity of baseline electrolyte 1 M LiPF6:EC:DEC (v/v = 1/2) added with ester-based solvent such as MA, EA, MP, and EP. The ionic conductivity of 7.6, 4.4, 5.2, and 4.1 mS cm−1, respectively were obtained at −10 °C. In our work for the same temperature the conductivity of baseline electrolyte and baseline electrolyte added with 28.6 vol.% of EP are 2.0 and 3.70 mS cm−1, respectively.
The viscosity, η of baseline electrolyte and baseline electrolyte with addition of 28.6 vol.% EP was measured in the temperature range from 30 to 60 °C. The graph of ln η versus 1,000/T in Fig. 3 shows that viscosity of the electrolyte decreases with temperature.
It can also be observed that the baseline electrolyte is more viscous than that of EP added electrolyte. Hence, the addition of EP has lowered the viscosity of the baseline electrolyte. The overall increase in conductivity with temperature is therefore attributed to the decrease in viscosity according to the equation
Here Z is the ion valency, n is number density of mobile ions, e is electron charge, and r is the radius of the lithium ion (0.76 Å). By substituting the relationship σ = neμ in the above equation, the ionic mobility, μ can be calculated. The ionic mobility dependence on temperature is depicted in Fig. 4.
Since the graph is a straight line, the ln μ–T relationship is Arrhenian and can be written as
Here μ o is the pre-exponential factor, E m is the activation energy of migration in eV, k is Boltzmann constant, and T is absolute temperature.
The number density of mobile ions, n also can be calculated using equation \( \sigma = \frac{{Zn{e^2}}}{{6\pi \eta {\kern 1pt} \;r}} \). The number density of mobile ions dependence on temperature is depicted in Fig. 5.
From the graph, it can be observed that the number density of mobile ions at 30 °C for baseline electrolyte and EP added electrolyte is 1.13 × 1020 cm−3 and 1.59 × 1020 cm−3, respectively. This result is in reasonably good agreement with the study by Arai et al. [20], whereby the number density of mobile ions at room temperature in the system 1 M LiPF6 dissolved in PC/EC/DEC (5/30/65) is 1.61 × 1020 cm−3. Knowing μ, the diffusion coefficient, D can be calculated and is depicted in Fig. 6.
The graph is linear with R 2 = 0.98 and hence, D and T are related by the equation:
Here D o is pre-exponential factor and E D is the activation energy of diffusion. The value of μ o, E m , η o, E η , D o, and E D for the two systems are tabulated in Table 1.
From the table, it can be observed that EP added electrolyte has activation energy of 1.6 × 10−20 J for ion migration. This is in reasonably good agreement with the activation energy of the lithium ion (2.9 × 10−20 J) in the system of 1 M LiPF6:γ-butyrolactone [21].
The LSV results for the 1 M LiPF6:EC:DEC baseline electrolyte, is as shown in Fig. 7.
It can be seen that the current does not register any significant increase when the potential is increased to 4.5 V. A small background current was recorded in the LSV trace which is quite constant in the voltage range from 3.0 to 4.5 V. Such observation has been reported by Zhou et al. [22] from the LSV studies on P(AN-MMA) electrolyte. This background current according to Georen and Lindbergh [23] is caused by non-Faradaic processes such as double-layer charging and increases with sweep rate. According to Zhou and co-workers [22], the small change in current (such as between 3 to 4.5 V, in the present work) is due to changes of the surface of the stainless steel electrode used in the LSV experiment and the significant change in current (above 4.5 V) is due to the decomposition of the electrolyte. In the attempt to find a suitable material for probing electrolyte stability limits, Georen and Lindbergh [23] tested platinum, stainless steel, glassy carbon, nickel, and graphite electrodes. In order to determine the voltage at which electrolyte decomposition started to occur, they have defined a stability limit in terms of a current density. The stability limit was defined to be the potential where a current density of 50 μA cm−2 was reached. The limit only serves as a tool to determine the breakdown voltage and is not considered a strict reaction rate limit. The results in Fig. 7 indicate that the electrolyte decomposition occurs when the potential exceeds ∼4.5 V, but according to Georen and Lindbergh [23] the breakdown reactions of the electrolyte start to occur at 6.2 V. Figure 8 depicts LSV trace for the EP added baseline electrolyte.
The addition of EP has further increased the background current. Following the criterion suggested by Georen and Lindbergh [23], the electrolyte breakdown voltage should occur at ∼4.0 V. This shows that the current density of 50 μA cm−2 is too low for demarcation of breakdown voltage. A hump is also observed in the voltammogram shown in Fig. 8. Such peaks are attributed to phase transitions occurring in the electrode material. Solvent oxidation therefore occurs at above 4.6 V. Geoffroy et al. [24] carried out LSV measurements of EPC (ethyl-propyl carbonate)-EC-LiPF6 (1 M) at a LiCoO2 composite electrode and obtained two peaks between 4.2 and 4.8 V. The oxidation voltage occurs at 5.1 V. The results in Fig. 7 show that the EP added electrolyte has an electrochemical window within the working operation voltage of a lithium-ion cell, i.e. from 2.5 to 4.3 V [25].
Figure 9 (a and b) show the charge and discharge characteristics for cells containing baseline electrolyte and EP added electrolyte at room temperature.
The capacity drops with higher current drain. The difference in capacity when the baseline cell was discharged at 0.14 and 1.4 C is ∼20% (with respect to capacity at 0.4 C) and for the cell containing EP added electrolyte the capacity of the cell dropped by ∼4%. From this result, the cell with EP added electrolyte have excellent capacity even at high rate current.
For discharge studies at different temperatures, the cells were was left for 4 h before being charged and discharged to ensure the surrounding temperature is in equilibrium with that of the cell. In this work, the temperatures chosen were 25 and −30 °C at 35 mAh. The discharge characteristics of LiCoO2/baseline/graphite and LiCoO2/baseline electrolyte + 28.6 vol.% EP/graphite are shown in Fig. 10 (a and b).
The discharge from 4.2 to 3.0 V at 25 °C (Fig. 10a and b), shows that the capacity of the cell containing baseline electrolyte is 178 mAh which is ∼30% lower than the theoretical capacity of the cell and cell with EP added electrolyte the capacity is 246 mAh which is ∼2% lower than the theoretical capacity of the cell. These results showed that EP is able to improve performance of the cell using 1 M LiPF6 in EC:DEC (v/v=1/2) at room temperature. At −20 °C, the capacity of the cell containing baseline electrolyte and EP added electrolyte are 158 (∼63%) and 186 mAh (∼74%), respectively.
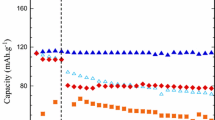
The cycling performance of LiCoO2/baseline electrolyte/graphite and LiCoO2/EP added baseline electrolyte/graphite cells, Fig. 11a shows no significant difference in the discharge capacity over number of cycles at 25 °C.
However, there is a drop in capacity for the cell with baseline electrolyte by ∼12% at −20 °C (Fig. 11b). Thus, it can be inferred that EP added baseline electrolyte should be stable on the LiCoO2 positive electrode and graphite negative electrode [18]. It maybe inferred that EP helps to stabilize the SEI layer and prevents exfoliation from the surface of the carbonaceous anode [18, 26]. At −20 °C, the discharge capacity of cells containing EP added baseline electrolyte and baseline electrolyte dropped by 2% and 12%, respectively, with respect to the initial capacity. From these results, it can be said that with EP incorporated into the baseline electrolyte, the performance of lithium-ion cell is improved especially at −20 °C. In Fig. 11a the capacity of the cell can be seen to increase slightly during the first few cycles which could be attributed to the improved electrode–electrolyte contact by the EP additive [27]. Similar behavior can be observed in the cell containing baseline electrolyte but the effect is less pronounced.
Conclusions
The conductivity of baseline electrolyte increased with addition of 28.6 vol.% of EP. The value is (8.05 ± 0.16) mS cm−1. The number density of mobile ions at 30 °C for baseline electrolyte and EP added electrolyte is 1.13 × 1020 and 1.59 × 1020 cm−3, respectively. With the incorporation of EP into baseline electrolyte, the viscosity decreased. Viscosity of electrolyte also decreased as temperature increased. The battery performance increased when 28.6 vol.% of EP was added into the baseline electrolyte. The improved performance of cell containing EP added in baseline electrolyte may be due to the low melting point and viscosity of EP.
References
Smart MC, Ratnakumar BV, Surampudi S (1999) 14th battery conference on applications and advances. Long Beach, CA, pp 55–60
Ratnakumar BV, Smart MC, Surampudi S (2001) J Power Sources 97–98:137–139
Smart MC, Ratnakumar BV, Whitcanack L, Chin K, Rodriguez M, Surampudi S (2002) Aerosp Electron Syst Mag, IEEE 17:16–20
Smart MC, Ratnakumar BV, Whitcanack L, Chin KB, Surampudi S, Croft H, Tice D, Staniewicz R (2003) J Power Sources 119–121:349–358
Smart MC, Ratnakumar BV, Ryan-Mowrey VS, Surampudi S, Prakash GKS, Hu J, Cheung I (2003) J Power Sources 119–121:359–367
Sazhin SV, Khimchenko MY, Tritenichenko YN, Lim HS (1999) J Power Sources 87:112–117
Ohta A, Koshina H, Okuno H, Murai H (1995) J Power Sources 54:6–10
Smart MC, Whitacre JF, Ratnakumar BV, Amine K (2007) J Power Sources 168:501–508
Smart MC, Ratnakumar BV, Huang C-K, Surampudi S (1998) SAE Aerospace Power Systems Conference Proceedings P-322 p 7–14
Plicta EJ, Behl WK (1998) 38th Power Sources Conference, Cherry Hill, NJ, June 8–11, 444
Bruce G, Schunacher A, Marcoux L (1998) 38th Power Sources Conference, Cherry Hill, NJ, June 8–11, 500
Herreyre S, Huchet O, Barusseau S, Perton F, Bodet JM, Biensan Ph (2000) J Power Sources 97–98:576
Shiao H-C, Chua D, Lin H-P, Slane S, Salomon M (2000) J Power Sources 87:167–173
Takami N, Ohsaki T, Inada K (1992) J Electrochem Soc 139:1849
Nanjundiah C, Goldman JL, Dominey LA, Koch VR (1988) J Electrochem Soc 135:2914
Webber A (1991) J Electrochem Soc 138:2586
Zhu A, Wang J, Han L, Fan M (2009) Chem Eng J 147:27–35
Xiang HF, Jin QY, Chen CH, Ge XW, Guo S, Sun JH (2007) J Power Sources 174:335–341
Morita M, Niida Y, Yoshimoto N, Adachi K (2005) J Power Sources 63:238–242
Arai J, Matsuo fujsaki AT, Ozawa K (2009), doi.10.1016/j.jpowsour.2009.04.001
Chagnes A, Carre B, Willmann P, Lemordant D (2002) J Power Sources 109:203–213
Zhou DY, Wang GZ, Li WS, Li GL, Tan CL, Rao MM, Liao YH (2008) J Power Sources 184:477–480
Georén P, Lindbergh G (2003) J Power Sources 124:213–220
Geoffroy I, Chagnes A, Carre B, Lemordant D, Biensan P, Herreyre SJ (2002) Power Sources 112:191–198
Shim, E-G, Nam T-H J-G, Kim H-S, Moon S-I (2008) J Power Sources 175:533–539
Xu HY, Xie S, Wang QY, Yao XL, Wang QS, Chen CH (2006) Electrochim Acta 52:636–642
Chew SY, Sun J, Wang J, Liu H, Forsyth M, MacFarlane DR (2008) Electrochim Acta 53:6460–6463
Acknowledgement
We would like to thank the University of Malaya for financial support and also Ministry of Higher Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kufian, M.Z., Majid, S.R. Performance of lithium-ion cells using 1 M LiPF6 in EC/DEC (v/v = 1/2) electrolyte with ethyl propionate additive. Ionics 16, 409–416 (2010). https://doi.org/10.1007/s11581-009-0413-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-009-0413-6