Abstract
In natural waters, fluoride ions are necessary and beneficial for the human being. At higher level of F− in water, it is toxic and detrimental to human health, leading to serious problems such as dental and skeleton fluorosis. According to the World Health Organization, the acceptable concentrations of fluoride in potable water are in the range of 0.7–1.5 mg L−1. Various treatment technologies for fluoride removal from water have been used such as ion exchange, adsorption and membrane processes. In the present study, removal of fluoride ions from aqueous solutions was investigated using a polyamide thin film composite nanofiltration membrane denoted as HL 2514 T from Osmonics Company. Through this membrane, the mechanism of transport was investigated. The Kedem–Katchelsky model was applied in order to determine phenomenological parameters σ and P s, respectively, the reflection coefficient of the membrane and the solute permeability of ions. The convective and diffusive parts of the mass transfer were quantified. The retention of monovalent and bivalent salts by this membrane shows that it is negatively charged. In the second part, retention of fluoride anions was investigated. Results show that the retention of fluoride by HL membrane exceeds 80%. The influence of the chemical parameters (feed concentration and ionic strength) and the physical parameters (applied pressure and recovery) on the elimination of fluoride was studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoride is one of the most abundant elements of the earth's crust. It is found in the form of fluorine (CaF2), biotite ((Mg,Fe)2Al2(K,H)(SiO4)2), cryolithe (Na3(AlF6)) and fluoro-apatite (Ca10F2(PO4)6). Because these minerals are less soluble in the water, the concentration of the fluoride in the water surface is generally weak. However, the physico-chemical characteristics of certain salts and ground waters (high temperatures for example) in the contact of these rocks make the dissolution of minerals containing fluoride favourable. The presence of fluoride in excess in drinking water is detrimental to human health leading to serious intoxication. Fluoride is necessary and beneficial for the human health in weak concentrations, but toxic with a stronger amount. Indeed, from 0.5 mg/L of fluoride ions, water plays a prophylactic role, but from 0.8 mg/L, the risk of fluorose begins and becomes strong above 1.5 mg/L. The optimum fluoride concentration in drinking water ranges from 0.7 to 1.5 mg/L for temperatures of 12 to 25 °C [1].
Various treatment technologies were studied for removing fluoride from water in which the concentration exceeds the permitted levels [2] such as precipitation [3], adsorption onto activated alumina [4, 5], ions exchange [6, 7] and processes membrane like electrodialysis [8], reverse osmosis [9, 10] and nanofiltration [11, 12]. Among these membrane processes, nanofiltration is the most recent technology; it is a process having many applications and particularly the field water treatment. However, nanofiltration membranes are not expected to reject monovalent ions to a large extend. Nanofiltration membranes have been used when partial rejection may be sufficient to meet the regulations in drinking water.
Nanofiltration, indicated at the beginning like a hybrid filtration [13], uses a gradient of pressure as driving force; it kept this name until the end of the 1980s, where it took its industrial rise with arrived membrane of the composite type [14].
Nanofiltration is a process located between ultrafiltration (using porous membranes) and reverse osmosis (using dense membranes), allowing separation of very small molecules.
This membrane technology uses membranes deriving from that used in reverse osmosis and replaces conventional processes of water softening while working with more low pressure that of reverse osmosis [15, 16].
Nanofiltration was developed thanks to the improvement of membrane materials [17–19]. Indeed the existing membranes of nanofiltration present molecular weight cutoffs for the organic compounds going from 300 to 1,000 Da [20–23], corresponding to a diameter of pore of about a nanometer. These membranes present a specific selectivity of separation for the ionic species, with a strong total retention for salt with weak concentration, with all the same a weaker retention of the monovalent ions than the bivalent ions.
Today, nanofiltration can be found in applications of various industries such as milk industry [24], sugar [25], paper [26] and in the field of desalination [27], separation and purification [28, 29], production of the drinking water [30, 31] and in the treatment of the waste water [32, 33].
Despite benefiting from a fast technological development, these mechanisms of transport and separation are not completely cleared up yet [34, 35]. Transfer occurs by convection in ultrafiltration membranes and by diffusion in reverse-osmosis membranes. As nanofiltration process lies between reverse osmosis and ultrafiltration, the transfer can be convective or diffusive.
The first mechanism, of diffusional type similar to that of reverse osmosis, is independent of the flux of solvent and of the pressure; it depends only on the gradient of concentration on both sides of the membrane [36–38]. The second mechanism corresponds to a selective drive of the aqueous solutions by solvent through the membrane: the convection [39–41].
In treating fluoride-rich waters, NF processes can provide a partial defluoridation, and an optimal fluoride content in permeate can possibly be achieved by adjusting the operation conditions. Studies on NF performance on fluoride removal from water are limited [39]. The works of Kettunen et al. [42] and Lhassani et al. [39] were based on pilot studies. No details about the effect of the membrane intrinsic properties on the membrane performance were included. Choi et al. [43] have investigated the influence of co-existing ions on the fluoride rejection by two commercial nanofiltration membranes, considering only the charge effect.
Hu et al. [44] developed a mechanical–mathematical model to interpret the influence of the membrane structure and charge properties on the membrane performance on fluoride removal. The developed model was based on the extended Nernst–Planck equation, the Donnan exclusion model and the Grahame equation. The membrane solution flux and the rejection were investigated by single salt (NaF) experiments for the three commercial membranes (SR, DL and HL). The experiments were carried out at a variety of operation conditions, including pressure and concentration.
Tahaikt et al. [45] investigated the influence of some operational parameters on the defluoridation performance by nanofiltration membrane and compared the performances of two commercial membranes with different pore radii.
The aim of this work is to study the effectiveness of a given nanofiltration membrane for fluoride removal, in view of understanding the underlying mechanisms that may lead to changes in observed retentions. The retention was studied according to operational factors such as the feed concentration, the nature of the associated cation, the ionic strength, the feed pressure and the recovery. The membrane permeability to ultrapure water and the charge of membrane surface were determined. The model of Spiegler–Kedem was applied in order to determine the phenomenological parameters σ and P s in a respective way, the reflection coefficient of the membrane and the solute permeability of the membrane to the aqueous solution. The convective and diffusive values of the parameters of the mass transfer were measured.
Experimental
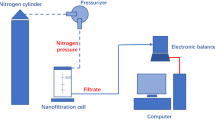
The experiments were performed on a pilot plant, which was carried out in our laboratory and equipped with nanofiltration modules in order to investigate fluoride removal.
The NF tests were carried out with the use of Osmonics spiral module equipped with HL 2514 T membrane. As we are interested by the use of HL membrane, we sought to apply it to fluoride removal and to compare its performances with those obtained with other membranes.
This membrane is of type “thin film composite”, which is a composite membrane since it is manufactured with two layers of different polymers. Its active layer is made out of polyamide and has an asymmetrical structure. The film membrane’s very low thickness is deposited on a macroporous support by means of a polysulfone flexible layer to confer a mechanical resistance. The membrane has a molecular weight cutoff for the organic compounds of molar mass of about 150 to 300 Da. The module HL 2514 TF used for this study is a module of the spiral type. It is approximately 64 mm in diameter and 356 mm in length. The nominal active surface of membrane rolled up in the module is of 0.6 m2. This module is characterised by a flow of production of maximum of 0.83 m3/day to minimise the obstruction of the drainage canals. All experiments were carried out at constant temperature of 25°C. The set-up has been presented in a previous work [46].
Ion analyses were performed by ionic chromatography, coupled to a conductimetric detector.
The amount of fluoride removed (R%) is given as:
Where C 0 and C p are the feed and permeat fluoride concentrations in the solution.
The recovery rate Y is given as:
Where Q 0 and Q p are the initial and permeate flow rates, respectively.
Results and discussion
Membrane characterisation
Membrane permeability
The membranes of nanofiltration used in liquid separation can be usually considered as an ideal porous environment. These result in a proportionality between the solvent flux obtained J W and the transmembrane pressure applied ∆P, which is written according to the Darcy law:
with
- R m :
-
the intrinsic resistance of the membrane
- μ :
-
the dynamic viscosity of water
- L p :
-
pure water permeability
- ΔP :
-
transmembrane pressure
The membrane permeability of water determined using Eq. 2 is equal to 7.3 L h−1 m−2 bar−1. This value is retained as reference for the continuation of our work. For the same HL membrane, a value of 9.014 L h−1 m−2 bar−1 was obtained by Ku et al. [44].
Salt retention measurements
A porous membrane of nanofiltration is never neutral, but carrying a charge. Interactions of electrostatic type also take part in the transfer [47]. On the other hand, in reverse osmosis, the membranes are supposed to be nonporous, and consequently, the flux of transfer of aqueous solution is essentially diffusive [48].
The study of transfer of aqueous solution through the porous membranes of nanofiltration requires the knowledge of the nature and the density of membrane material load, which are important parameters. The methods of measurements of the loads must be adapted to the specificity of these membranes. Peeters et al. [49] indicated that the measurements of the retention of salts proved that the behaviour of the majority of the membranes of nanofiltration could be classified in two principal categories:
-
The membranes for which the model of exclusion of Donnan seems to play an important role. If the membrane is negatively charged, the sequence of retention is the following \( {R_{{\text{N}}{{\text{a}}_2}{\text{S}}{{\text{O}}_4}}}>{R_{\text{NaCl}}}>{R_{{\text{CaC}}{{\text{l}}_2}}} \); if it is positively charged, the sequence of retention is \( {R_{{\text{CaC}}{{\text{l}}_2}}}>{R_{\text{NaCl}}}>{R_{{\text{N}}{{\text{a}}_2}{\text{S}}{{\text{O}}_4}}} \).
-
The membranes for which the retention was determined by the difference of the coefficients of diffusion between various salts.
The retention of the three salts NaCl, Na2SO4 and CaCl2 by the nanofiltration membrane in function of the concentration is provided in Fig. 1.
Figure 1 makes it possible to classify the rates of retention of these three salts according to the following order:
In case of the retention sequence, we can conclude that \( {R_{{\text{N}}{{\text{a}}^{+}}}}>{R_{{\text{C}}{{\text{a}}^{\text{2 + }}}}} \) and \( {R_{{\text{SO}}_4^{\text{2 - }}}}>{R_{{\text{C}}{{\text{l}}^{-}}}} \), i.e. the retention for the bivalent anion is the highest, whereas that of the bivalent cation is the lowest. This is a typical behaviour of a negatively charged membrane [49].
The high retention for the sodium sulphate and the lower retention for calcium chloride are in accordance to the Donnan exclusion model. This model indicates that the co-ions (ions with the same charge as the membrane) are repelled by the membrane, whereas counter ions (ions with the opposite charge as the membrane) are attracted.
In addition, it is observed that the retention of salts does not vary in a simple manner with energy of hydration of the ions. This indicates a preponderance of the exclusion of Donnan effect on the retention of salts by the membrane compared to the steric effect.
It should be noted that the sequence of retention obtained indicated that the size of the pores of the membrane is relatively high and is the same as a nanometer. In the case of a membrane charged negatively and with size of the pores, the low (about 0.5 nm) size of the hydration radii of the ions intervenes, and the order of retention between monovalent and bivalent cations can be reversed. A study was carried out by Verliefde et al. [50] on a HL membrane where it confirmed that the latter is negatively charged by the determination of the zeta potential.
Transport equations
The solute flux through the NF membranes was defined by the model of Kedem–Katchalsky, where it described the transport of ion through these membranes by irreversible thermodynamics in order to take into consideration the coupling of both flux.
The flux of solvent and the flux of solute are given by Eqs. 3 and 4.
with
- J s :
-
flux of solute
- Δπ :
-
difference of the osmotic pressure
- P s :
-
solute permeability
- σ :
-
reflexion coefficient
with C 0, C P and C m being, respectively, the feed concentration, the permeate concentration and the concentration in the membrane.
The solute flux is the sum of a diffusive flux J diff, which depends only on the concentration on both sides of the membrane and of a convective flux J conv, which is a purely physical flux. The latter depends only on the solvent flux and the pressure applied:
with
Therefore,
However, according to the principle of mass conservation,
From Eqs. 6 and 7, we can deduce the expression from the concentration of permeate according to flux:
The graphic representation of C p versus the reverse of the permeate flux will make it possible to quantify separately both parts of the solutes mass transfer occurring in NF: convection and diffusion.
According to Kedem–Katchalsky, the retention is given by the following equation:
with
The values of the parameter σ are calculated starting from the relation of Pusch [51]:
where L D represents the osmotic permeability coefficient.
Plotting the experimental values of (1/R) versus (1/J v) permits to calculate σ.
In Fig. 2, we represented the permeate concentration variation according to the reverse of flux. The curves obtained are of linear lines and they check the theory well.
For each salt, one deduces the value from diffusive flux J diff starting from the slope of the curve \( {C_{\text{p}}} - f\left( {1/{J_{\text{v}}}} \right) \) and the value of the concentration in the permeate due to the C conv convection starting from the original ordinate.
Figure 3 represents the variation of the reverse of retention according to the reverse of flux for various salts. The curves obtained are also of linear lines and are checking well the relation 8. The value of σ corresponds to the inverse of original ordinate of the curve \( 1/R = f\left( {1/{J_{\text{v}}}} \right) \).
The values of the parameters of J diff and C conv like those of σ and P s are grouped in Table 1.
Table 1 shows that the values of P s depend on the nature of the anion and the cation. The reflection coefficient σ is a characteristic of the convective transport of the solute. The value of σ is about equal to 1 for different salts. High values of σ were obtained by Murthy et al. [52] using a thin film composite polyamide membrane for NaCl and Na2SO4 salts.
This result indicates that the convection solute transport is totally hindered or that no transport by convection takes place at all. This is the case for ideal RO membranes where the membranes have a dense structure and no pores are available for convective transport. The retention may however be lower than 100% because solute transport may take place by solution diffusion. As it has been shown that NF membranes have pores, a reflection coefficient below 100% will be found if the solutes are small enough to enter the membrane pores. Of course, the actual solute retention will be smaller than the reflection coefficient because of the contribution of diffusion [53]. The anion sulphate and the cation calcium, which are more solvate, give the weakest P s values compared to the less solvate monovalent ions. It also appears that the diffusive component of the flux of permeate decreases with the increase in the energy of hydration. Pontalier et al. [54] report that the hydration can be regarded as forces necessary to extract the aqueous solution from solvent in order to push it inside the pores of the membrane. In this way, it would require more energy to extract sulphates and to push them inside the pores in comparison with the other anions.
Fluoride retention
Effect of physicals parameters
Effect of feed pressure
The figure illustrates the evolution of the retention of the fluoride according to the pressure applied for various fluoride concentrations.
As was known from the equations above, the retention of fluoride increases with pressure; this can be seen in Fig. 4. For all the concentrations in fluoride ion studied, we note that the retention increases while increasing the feed pressure until a pressure applied of 10 bar. Beyond an applied pressure of 10 bar, stability on the level of the retentions for different concentrations was observed.
Thus for pressures lower than 10 bar, the mass transfer is the convection type in accordance with Eq. 3. On the other hand, for pressures higher than 10 bar, the transfer becomes diffusive, and the retention is not affected any more by the pressure.
In light of these results, the value of 10 bar was retained as an optimal pressure, and beyond this pressure, the diffusion prevails with regard to the convection.
Effect of recovery
Figure 5 illustrates the evolution of the retention of the fluoride ions according to the recovery rate for two different concentrations of fluoride.
According to Fig. 5, when the recovery increased, the retention decreases for the studied feed concentrations.
The retention of fluoride ions was considerably affected by the recovery rate and varies from 98.3% for a recovery rate of 10% to 94.3% for a recovery rate of 70%, showing the effect of the polarisation concentration. It seems that the transfer is primarily convective and that the contribution of diffusion to the ion transport is negligible. This reduction in the rate of retention is the consequence of the reduction of the speed of tangential circulation and of the appearance of a layer of polarisation at the strong recovery rate.
Effect of chemical parameters
Effect of ionic strength
The effect of ionic strength on the retention of fluoride ions was studied by the addition of various concentrations of NaCl between 0.01 and 0.2 M.
Figure 6 shows that the increase in the NaCl concentration is accompanied by a reduction in the retention of fluoride ions. Indeed, when the NaCl concentration increases, there are more sodium ions, which neutralise partially the negative charges of the membrane which facilitates the passage of the fluoride ions.
The decrease in the charge of the membrane involves a decrease in the retention of the ions of negative charge since the electrostatic effect membrane-aqueous solution became weak, and consequently, the effect of exclusion of Donnan is reduced.
Effect of feed concentration
The effect of the feed concentration of fluoride on the retention was investigated as shown in Fig. 4. This figure shows that the variation of the concentration has a great effect on the rate of retention for various pressures applied. Indeed, the retention of the fluoride ions decreases with the increase in the concentration varying from 99% for a concentration of 3 × 10−3 mol/L to 90% for a concentration of 10−1 mol L−1 for a 10-bar transmembrane pressure applied. This reduction is attributed to the shielding phenomenon. Indeed, the fixed charges of the active layer of the membrane are neutralised partially by the counter ions of the electrolyte. This phenomenon is all the more marked that its counter ions are numerous. Thus, an increase in the salt concentration would decrease the electrostatic interaction between the ions and the membrane [55].
Another study [56] accounted for the decrease in the retention by the increase in the osmotic pressure of the solution, and consequently the effective pressure decrease on the membrane, and in the decline in the flux of solvent, the solute is divided in a volume of less important solvent, and the permeate will be concentrated more, i.e. the retention would be less important.
Comparison between fluoride anions and different anion retentions
Figure 7 shows the retention of NaCl, NaF, NaNO3 and Na2SO4 with different feed pressure, the same feed concentration C 0 = 2 × 10−2 mol L−1 in Na+ ions and the recovery rate Y = 15%.
The order of retention of these salts by the nanofiltration membrane follows the following sequence:
Membrane HL being negatively charged, bivalent ions is retained better than the monovalent ions. The membranes of negative charges tend to push back in a more important way the multivalent anions than the monovalent anions.
One can call upon the effects of size of the solvated ions to explain the variations of retention between the anions associated with the same cation, i.e. an anion is all the more retained as its radii of hydration is high.
Although the measured radii of the nitrate ions is practically equal to those of the chloride ions, the nitrate ions are slightly retained compared to the chloride ions. The anomalously low rejection of the nitrate ions by NF membrane is consistent with literature reports for other NF/RO membrane [57].
In recent studies, Tansel et al. [58] reported that the small monovalent ions (F−) flow through the gel matrix with water molecules attached, whereas the large monovalent ions (Cl−) adsorb to the nonpolar surface of the gel, a process requiring partial dehydration of the ion and implying that these ions bind the immediately adjacent water molecules weakly [59]. Water cluster binding studies show that the ions possessing high charge density bind larger water clusters more strongly [60]. The transition from strong to weak hydration occurs at a radius of about 1.78 Å for the monovalent anions.
Tansel et al. [58] concluded that during membrane filtration, the high shear strain on the ions could force them to align with the flow and also forcing them to acquire smaller effective cross sections. When the filtration is performed in dead end mode, the ions with strong hydration bonds may not able to detach from the hydration layers and may be too big to pass through the membrane pores. However, the ions with weak hydration bonds can lose some or all the water of hydration and can fit through the membrane pores.
Conclusion
The retention of various salts by a membrane of nanofiltration HL showed that the membrane is negatively charged. The model of Spiegler–Kedem showed a good correlation between the experimental values of various salts and the theoretical model. It allowed the calculation of the transport parameters as well as the contribution of the diffusion and the convection for the transfer of solute.
Nanofiltration membrane HL allowed fluoride retention exceeding 80%. This retention depends on the physical (pressure applied and recovery rate) and chemical parameters (feed concentration and ionic strength).
At higher pressure, fluoride retention is practically unaffected by pressure because the passage of this ions is mainly due to diffusion. Convection has virtually no effect. By contrast at lower pressure, the fluoride retention was much more influenced by pressure; this implies that the transfer is primarily convective.
The selectivity of the membrane for different ions is of chemical origin. The results indicate that the smaller the ion, the better it is retained. This is derived from the salvation energy of the ions by water. Since fluoride ions are more solvated, they are better retained than chloride and nitrate.
References
Pontié M, Rumeau M, Ndiaye M, Diop CM (1996) Sur le problème de la fluorose au Sénégal: bilan des connaissances et présentation d’une nouvelle méthode de défluoruration des eaux de boisson. Cahiers Santé 6:27
Mazounie P, Mouchet P (1984) Available processes for fluoride removal from drinking waters. Revue française des Sciences de l’eau 3(1):29–51
Singh G, Kumar B, Sen PK, Majumdar J (1999) Removal of fluoride from spent pot liner leachate using ion exchange. Water Environ Res 71(1):36–42
Fink GJ, Lindsay FK (1936) Activated alumina for removing fluoride from drinking water. Ind Eng Chem 28(8):947
Bulusu KR, Nawlakhe WG (1990) Defluoridation of water with activated alumina continuous contacting system. Ind J Environ Health 32(3):197–218
Castel C, Schweizer M, Simonnot MO, Sardin M (2000) Selective removal of fluoride ions by a two-way ion-exchange cyclic process. Chem Eng Sci 55:3341–3352
Reardon EJ, Wong Y (2000) A limestone reactor for fluoride removal from wastewaters. Environ Environ Sci Technol 34(15):3247–3253
Amor Z, Bariou B, Mameri N, Toky M, Nicolas S, Elmidaoui A (2001) Fluoride removal from brackish water by electrodialysis. Desalination 133:215–223
Bouguecha S, Dhahbi M (2002) The role of membrane technologies in supplying drinking and industrial water in Tunisia: conventional process and new trends. Desalination 151:75–86
Ndiaye PI, Moulln P, Dominguez L, Millet JC, Charbit F (2005) Removal of fluoride from electronic industrial effluent by RO membrane separation. Desalination 173:25–32
Simons R (1993) Trace element removal from ash dam waters by nanofiltration and diffusion dialysis. Desalination 89:325–341
Hu K, Dickson JM (2006) Nanofiltration membrane performance on fluoride removal from water. J Membr Sci 279:528–529
Eriksson P (1988) Nanofiltration extend the range of membrane filtration, Environ. Progress 7(1):58–62
Conlon WJ (1985) Pilot field test data for prototype ultra low reverse osmosis elements. Desalination 56:203–226
Conlon WJ, Mc Clellan SA (1989) Membrane softening: a treatment process comes of age. Journal of AWWA 11:47–51
Taylor S, Mulford LA, Duranceau SJ, Barrett WM (1989) Cost and performance of a membrane pilot plant. Journal of AWWA 81(11):52–60
Van der Bruggen V, Schaep J, Wilms D, Vandecasteele C (1999) J Membr Sci 156:29–41
Jian X, Daia Y, He G, Chen G (1999) Preparation of UF and NF poly (phthalazine ether sulfone ketone)membranes for high temperature application. J Membr Sci 161:185–191
Whu JA, Baltzik BC, Sirkar KK (1999) Modeling of nanofiltration-assisted organic synthesis. J Membr Sci 163:319–331
Cadotte J, Forester R, KIM M, Paterson R (1988) Nanofiltration membranes broaden the use of membrane separation technology. Desalination 70:77–83
Boye A, Guizard C, Larbot A, Cot L, Arangeon A (1991) A olyphosphazene membrane active in nanofiltration, inorganique membranes ICIM2–91. Trans Tech Publications, Montpellier II
Chern SH, Chang DJ, Liou RM, Hsu CS, Lin SS (2002) Preparation and separation properties of polyamide nanofiltration membrane. J Appl Polym Sci 83:1112
Mehiguence K, Garba G, Taha S, Gondrexon N, Dorange G (1999) Influence of operating conditions on the retention of copper and cadmium in aqueous solutions by nanofiltration: experimental results and modelling. Sep Purif Technol 15:181–187
Timmer JMK, Kromkamp J, Robbertsen T (1994) Lactic acid separation from fermentation broths by reverse osmosis and nanofiltration. J Membr Sci 92:185–197
Wadley S, Brouckaert CJ, Baddock LAD, Buckley CA (1995) Modelling of nanofiltration applied to the recovery of salt from waste brine at sugar decolourisation plant. J Membr Sci 102:163–175
Bertran S, Gaid A, Paillard H (1997) Application de la nanofiltration dans le traitement d’une eau résiduaire de papeterie. Procédés à membranes: maitrise du cycle de l’eau dans l’industrie. Pollutec, Paris
Wang XL, Zhang CH, Ouyang PK (2002) The possibility of separating saccharides from a NaCl solution by using nanofiltration in diafiltration mode. J Membr Sci 204:271–281
Wang XL, Ying AL, Wang WN (2002) Nanofiltration of L-phenylalanine and L-aspartic acid aqueous solutions. J Membr Sci 196:59–67
Li SL, Li C, Liu YS, Wang XL, Cao ZA (2003) Separation of L-glutamine from fermentation broth by nanofiltration. J Membr Sci 222:191–201
Wang DX, Wang XL, Tomi Y, Ando M, Shintani T (2006) Modeling the separation performance of nanofiltration membranes for the mixed salts solution. J Membr Sci 280:734–743
Wang DX, Wu L, Liao ZD, Wang XL, Ando M, Shintani T (2006) Modeling the separation performance of nanofiltration membranes for the mixed salts solution with Mg2+ and Ca2+. J Membr Sci 284:384–392
Jiraratananon R, Sungpet A, Luangsowan P (2000) Performance evaluation of nanofiltration membranes of nanofiltration membranes for treatment of effluents containing reactive dye and salt. Desalination 130:177–183
Ducom G, Cabassud C (1999) Interests and limitation of nanofiltration for the removal of voltaic organic compounds in drinking water production. Desalination 124:115–123
Tsuru T, Izumi S, Yoshioka T, Masashi M (1999) Nanofiltration performance of neutral solutes by inorganic porous membranes. International Congress on Membranes and Membranes processes (ICOM), Toronto, pp 12–18
Yaroshchuck AE (1999) Rejection mechanisms of NF membranes: in what way can they be identified through advanced characterization of membrane transport properties. International Congress on Membranes and Membranes processes (ICOM), Toronto
Rumeau M (1995) La nanofiltration: principes, performances et applications. Inf Chim 373(11):93–98
Lhassani A, Rumeau M (1999) Solute transfer through membranes and selectivity in nanofiltration. Tribune de l’Eau 52:13–17
Pontie M, Sarro O, Rumeau M (1998) Eau Ind Nuis 210:57
Lhassani A, Rumeau M, Benjelloun D, Pontie M (2001) Selective demineralization of water by NF, application to the defluorination of brackish water. Water Res 35:3260–3264
Lhassani A, Benjelloun D, Rumeau M (2000) Interpretation attempt of transfer mechanism of salts in nanofiltration. Tribune de l’Eau 53:100–107
Garba Y, Taha S, Gondrexon N, Cabon J, Dorange G (2000) Mechanisms involved in cadmium salts transport through a nanofiltration membrane: characterization and distribution. J Membr Sci 168:135–141
Kettunen R, Keskitalo P (2000) Combination of membrane technology and limestone filtration to control drinking water quality. Desalination 131:271–283
Choi S, Yun A, Hong S, Ahn K (2001) The effect of co-existing ions and surface characteristics of nanomembranes on the removal of nitrate and fluoride. Desalination 133:53–64
Hu K, Dickson JM (2006) Nanofiltration membrane performance on fluoride removal from water. J Membr Sci 279:529–538
Tahaikt M, El Habbani R, Ait Haddou A, Achary I, Amor Z, Taky M, Alami A, Boughriba A, Hafsi M, Elmidaoui A (2007) Fluoride removal from groundwater by nanofiltration. Desalination 212:46–53
Mnif A, Bouguecha S, Hamrouni B, Dhahbi M (2007) Coupling of membrane processes for brackish water. Desalination 203:331–336
Causserand C (2006) Filtration membranaire (OI, NF, UF). Caractérisation des membranes. Technique de l’ingénieur J2792:10
Aimar P (2006) Filtration membranaire (OI, NF, UF). Mise en œuvre et performance. Technique de l’ingénieur J2793:9
Peeters JMM, Boom JP, Mulder MHV, Strathmann H (1998) Retention measurements of nanofiltration membranes with electrolyte solutions. J Membr Sci 145:199–209
Verliefde ARD, Heijman SGJ, Cornelissen ER, Amy G, Van der Bruggend B, van Dijk JC (2007) Influence of electrostatic interactions on the rejection with NF and assessment of the removal efficiency during NF/GAC treatment of pharmaceutically active compounds in surface water. Water Res 41:3227–3240
Pusch W (1977) Determination of transport parameters of synthetic membranes by hyperfiltration experiments, part I: derivation of transport relationship from linear relations of thermodynamics of irreversible process. Ber Bunsenges Phys Chem 81:269–276
Murthy ZVP, Shard KG (1999) Sodium cyanide separation and parameter estimation for reverse osmosis thin film composite polyamide membrane. J Membr Sci 154:89–103
Schaep J, Van der Bruggend B, Vandecasteele C, Wilms D (1998) Influence of ion size and charge in nanofiltration. Sep Purif Technol 14:155–162
Pontalier PY, Ismail A, Ghoul M (1997) Mechanism for the selective rejection of solutes in nanofiltration membranes. Sep Purif Technol 12:175–181
Afonso MD, de Pinho MN (2000) Transport of MgSO4, MgCl2, and Na2SO4 across an amphoteric nanofiltration membrane. J Membr Sci 179:137–154
Bannoud AH, Darwich Y (2007) Elimination des ions fluorure et manganèse contenus dans les eaux par nanofiltration. Desalination 206:449–456
Paugam L, Diawara CK, Schlumpf JP, Jaouen P, Quéméneur F (2004) Transfer of monovalent anions and nitrates especially through nanofiltration membranes in brackish water conditions. Sep Purif Technol 40:237–242
Tansel B, Sager J, Rector B, Garland J, Strayer RF, Levine L, Roberts M, Hummerick M, Bauer J (2006) Significance of hydrated radius and hydration shells on ionic permeability during nanofiltration in dead end and cross flow modes. Sep Purif Technol 51:40–47
Washabaugh MW, Collins KD (1986) The systematic characterization by aqueous column chromatography of solutes which affect protein stability. J Biol Chem 261:12477–12485
Havel J, Högfeldt E (1995) Evaluation of water sorption equilibrium data on Dowex ion exchanger using WSLET-MINUIT program. Scripta Fac Sci Nat Univ Masaryk Brun Chem 25:73–84
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mnif, A., Ben Sik Ali, M. & Hamrouni, B. Effect of some physical and chemical parameters on fluoride removal by nanofiltration. Ionics 16, 245–253 (2010). https://doi.org/10.1007/s11581-009-0368-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-009-0368-7