Abstract
Ion-conducting solid polymer blend electrolytes based on polyvinyl chloride (PVC)/poly methyl methacrylate (PMMA) complexed with sodium perchlorate (NaClO4) were prepared in various concentrations by solution cast technique. The features of complexation of the electrolytes were studied by X-ray diffraction (XRD) and Fourier transform infrared (FTIR) spectroscopy. DC conductivity of the films was measured in the temperature range 303–398 K. Transference number measurements were carried out to investigate the nature of charge transport in the polymer blend electrolyte system. The electrical conductivity increased with increasing dopant concentration, which is attributed to the formation of charge transfer complexes. The polymer complexes exhibited Arrhenius type dependence of conductivity with temperature. In the temperature range studied, two regions with different activation energies were observed. Transference number data showed that the charge transport in this system is predominantly due to ions. Optical properties like absorption edge, direct band gap, and indirect band gap were estimated for pure and doped films from their optical absorption spectra in the wavelength region 200–600 nm. It was found that the energy gap and band edge values shifted to lower energies on doping with NaClO4 salt.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, studies on the electrical and optical properties of polymers have attracted much attention in view of their applications in electronic and optical devices [1, 2]. The electrical properties are aimed to understand the nature of the charge transport prevalent in these materials while the optical properties are aimed at achieving better reflection, antireflection, and polarization properties. Electrical and optical properties of the polymers can be suitably modified by the addition of dopant depending on their reactivity with the host matrix. Among the first and most studied host matrices for solid polymer electrolytes is polyethylene oxide (PEO), which dissolves high concentrations of a wide variety of salts to form polymeric electrolytes [3]. Investigations on polymer electrolytes have focused primarily on the enhancement of ionic conductivities at room temperature. Several methods, such as copolymerization, plasticization, blending, and addition of ceramic fillers/additives have been used to modulate the conductivity of the polymer electrolytes. Among the polymer electrolytes, PMMA-based electrolyte has a special significance in view of its well-known chemistry and cheaper method of processing as laminates. To get PMMA amenable, choice of blending with PVC has been suggested [4]. Although there are many polymer blends, those containing PVC are among the most important from both scientific and commercial point of views [5, 6]. A relatively small number of studies have been reported on the development of PVC/PMMA-based polymer electrolyte systems [7–9].
The present work aims to investigate the PVC/PMMA matrix to improve its electrical and optical performance. Because Na+ is a fast conducting ion in a number of crystalline and amorphous materials, its incorporation in a polymeric system may be expected to enhance its electrical and optical performance. This paper presents the results of investigations on the structure, optical, and electrical properties of NaClO4 complexed PVC/PMMA solid polymer blend electrolyte films.
Experimental
Films (thickness, approximately 150 μm) of (PMMA+PVC) blend and NaClO4 salt complexed (PMMA+PVC) blend were prepared in weight ratios (66.5:28.5:5), (63:27:10), and (59.5:25.5:15) by solution cast technique using tetrahydrofuran (THF) as the solvent. Appropriate amounts of PMMA, PVC, and NaClO4 were dissolved in THF and the solutions were magnetically stirred for 10–12 h at room temperature to obtain a homogeneous solution. The stirred solutions were cast onto polypropylene dishes and allowed to evaporate slowly at room temperature followed by vacuum drying. The final product was vacuum dried at 45 °C thoroughly under a vacuum of 10−3 mbar to remove residual solvent, if any. All films were stored in an evacuated dry box.
The X-ray diffraction (XRD) studies were performed by means of the SEIFERT X-ray diffractometer. IR spectra profiles were obtained using a Perkin-Elmer FTIR spectrophotometer in the range 500–4,000 cm−1 in the transmittance mode. The DC conductivity was studied as a function of temperature and composition in the range 303–398 K using a conductivity cell designed in our laboratory. The total ionic transference numbers (t ion, t ele) were evaluated by means of Wagner’s polarization technique [10]. In this technique, freshly prepared polymer blend electrolyte films were polarized in the configuration Na/polymer blend electrolyte/C under a DC bias of 1.5 V. The resulting current was monitored as a function of time. The optical absorption profiles of these samples were recorded at room temperature in the wavelength range 200–600 nm using the UV–VIS–NIR (Model UV-3100) spectrophotometer. From this data, the optical constants such as band edge, optical band gap (both direct and indirect) were determined.
Results and discussion
XRD analysis
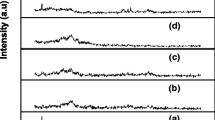
To investigate the complexation of sodium perchlorate salt with the polymer blend, XRD studies were performed. Figure 1a–f shows the XRD patterns of pure PVC, PMMA, NaClO4 salt, and PVC/PMMA-based composite polymer electrolytes, respectively. Comparison of the XRD patterns of complexed polymer blend films with those of pure PVC, PMMA, and NaClO4 reveals the following differences. The amorphous nature of PMMA and the crystalline nature of NaClO4 salt are observed. Peaks observed for 2θ values around 26° are less intense and slightly displaced in complexed polymer blend films compared to those in pure PVC film. This indicates that the addition of NaClO4 salt causes a decrease in the degree of crystallinity and a simultaneous increase in the amorphicity of the complexed films. The crystalline peaks for 2θ values at 15°, 32°, and 62° corresponding to NaClO4 (Fig. 1f) are absent in polymer complexes. This indicates the absence of any excess (uncomplexed) salt in the complexed polymer blend electrolyte films. Absence of sharp peaks at higher concentrations of NaClO4 salt indicates the dominant presence of amorphous phase. This amorphous nature is responsible for greater ionic diffusivity resulting in high ionic conductivity. This observation confirms that complexation has taken place in the amorphous phase.
FTIR studies
Infrared spectral analysis monitors the vibrational energy levels in the region of different molecules. FTIR spectroscopic studies on (PVC+PMMA) complexes are expected to throw light on mainly two kinds of information: (1) the conformation of the polymer chain and (2) the position of the ion in the polymer chains, which are essentially the fingerprints of different molecules and structures [11]. The infrared spectra would be sensitive in both situations whether the complexation has occurred in crystalline or amorphous phase.
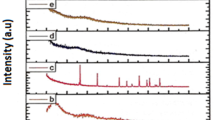
The FTIR spectra of pure PMMA, PVC, NaClO4, and polymer complexes are shown in Fig. 2a–f. In PMMA (Fig. 2a), CH3 asymmetric stretching vibration appears at 2,942 cm−1 and is shifted to 2,950, 2,956, and 2,954 cm−1 in the complexes containing 5%, 10%, and 15% NaClO4, respectively. The peak at 3,010 cm−1 assigned to O–CH3 asymmetric stretching of PMMA is shifted to 2,995, 2,993, and 2,990 cm−1 in the complexes. In PMMA, which is a saturated polymeric ester, C=O symmetrical stretching frequency gives rise to an intense peak at 1,742 cm−1 [12], which is shifted to 1,746 cm−1 in the complexes. The absorption peaks at 1,488, 1,172, and 753 cm−1 are assigned to CH2 scissoring, twisting, and rocking modes of PMMA and are shifted to 1,484, 1,162, and 752 cm−1, respectively, in the complexes. The vibrational frequency at 1,205 cm−1 is assigned to C–O stretching of PMMA and is shifted to 1,068 cm−1 in the complexes. The band at 940 cm−1 is attributed to the symmetrical stretching of the C–O bond in the C–O–C linkage of PMMA [13]. The absorption peak at 630 cm−1 attributed to amorphous PMMA is shifted to 628 cm−1 in the complexes. Characteristic peaks of PMMA at 842, 668, and 482 cm−1 are shifted to 843, 679, and 648 cm−1, respectively, in the complexes.
In PVC (Fig. 2b), the bands of the spectrum in the region of 600–700 cm−1 are characteristic of C–Cl stretching vibrations. CH2 deformation vibration band of PVC is observed at 1,429 cm−1 while the band at 961 cm−1 is assigned to the weak trans CH wagging. The bands at 696 and 636 cm−1 are attributed to the cis CH wag vibration. The band at 1,074 cm−1 is assigned to the CH rocking vibration in pure PVC. The band at 1,654 cm−1 is assigned to the C=C stretching vibration in pure PVC, which got shifted to 1,702 cm−1 in the polymer complexes. The band of pure PVC at 2,872 and 1,718 cm−1 got shifted to 2,865 and 1,702 cm−1, respectively, in the polymer complex.
The characteristic frequencies of NaClO4 (Fig. 2f) at 1,618, 939, and 629 cm−1 are shifted to 1,637, 990, and 628 cm−1 in the polymer complexes, respectively, while the peak at 480 cm−1 disappeared in the polymer complex. From the above analysis, it may be inferred that complexation has taken place.
Electrical conductivity
The temperature dependence of DC electrical conductivity (log σ vs 1,000/T) for different compositions of (PVC+PMMA+NaClO4) polymer electrolyte system along with pure (PVC+PMMA) in the temperature range 303–398 K is shown in Fig. 3. It was observed that the room temperature conductivity of the polymer blend increased by almost two orders of magnitude for a 10% concentration of NaClO4 salt. The increase in conductivity with increasing dopant concentration may be explained on the basis of charge transfer complexes (CTCs) in the host lattice. These charge transfer complexes increase the electrical conductivity by providing additional charges in the lattice. At low salt concentrations, the increase in conductivity is large but at higher concentrations, the increase in conductivity is modest. This is generally due to the formation of ion aggregates, which do not take part in the conduction process [14].
The conductivity increased with temperature and followed an Arrhenius behavior throughout with two regions (region-I and region-II) having different activation energies. This may be due to a transition from a semicrystalline phase to an amorphous phase [15]. According to Druger et al. [16, 17], in polymer electrolytes, change of conductivity with temperature is due to the segmental motion, which results in an increase in the free volume of the system. This increase in free volume would facilitate the motion of ionic charge [18]. The existence of two regions in the conductivity vs temperature plots has been observed in a number of polymer electrolytes [19, 20]. The DC conductivity (σ) as per Arrhenius relation can be expressed as
where σ 0 is a proportionality constant, E a is the activation energy, and K is the Boltzmann constant.
The activation energies, evaluated from the slopes of these plots, show a decrease in their value with increasing dopant concentration (Table 1). The decrease in activation energies may be explained on the basis of fact that the incorporation of small amounts of the dopant forms charge transfer complexes in the host lattice [21]. These charge transfer complexes increase the electrical conductivity by providing additional charges in the lattice. This results in a decrease of activation energy.
Transference number
The conductivity measurements will be inadequate if performed without the information on possible electronic and ionic transport numbers. In Wagner’s polarization technique, the DC current is monitored as a function of time on application of a fixed DC voltage of 1.5 V across the cell in the configuration Na/(polymer blend electrolyte)/C. After polarization, the current vs time plots were obtained (Fig. 4) and the transference numbers were calculated using the equations
where I T is the total current (ionic+electronic) at the start (t = 0) and I S is the current on saturation (electronic current only). The calculated transference numbers, t ion and t ele, are given in Table 1. For all the compositions of the (PVC+PMMA+NaClO4) electrolyte system, the values of ionic transference numbers (t ion) are in the range 0.94–0.97. This suggests that the charge transport in these polymer blend electrolyte films is predominantly due to ions; only a negligible contribution comes from the electrons.
Optical absorption
The study of optical absorption gives information about the band structure of solids. Insulators/semiconductors are generally classified into two types: (1) direct band gap and (2) indirect band gap. In direct band gap semiconductors, the top of the valence band and the bottom of the conduction band both lie at same zero crystal momentum (wave vector). If the bottom of the conduction band does not correspond to zero crystal momentum, then it is called an indirect band gap semiconductor. In indirect band gap materials, transition from valence to conduction band should always be associated with a phonon of the right magnitude of crystal momentum.
Optical absorption studies on pure and doped films were carried out to determine the optical constants such as optical band gap (E g) and the position of the fundamental band edge. The absorption coefficient was determined from the spectra using the formula:
where A is the absorbance and d is the thickness of the film. When a direct band gap exists, the absorption coefficient has the following dependence on the energy of the incident photon [22, 23].
where E g is the band gap, C is a constant dependent on specimen structure, υ is the frequency of incident light, and h is the Planck’s constant.
Thus, a plot of (αhν)2 vs hν (photon energy), as shown in Fig. 5, should be linear. The intercept on the energy axis on extrapolating the linear portion of the curves to zero absorption value may be interpreted as the value of the band gap. For pure (PVC+PMMA) polymer electrolyte, the direct band gap lies at 4.24 eV while for doped films, the values are found to decrease from 4.24 to 4.05 eV (Table 1).
For indirect transitions, which require phonon assistance, the absorption coefficient has the following dependence on the photon energy [22, 23].
where E p is the energy of the phonon associated with the transition, and A, B are constants depending on the band structure.
The indirect band gaps were obtained from the plots of (αhν)1/2 vs hν (photon energy) as shown in Fig. 6. For pure (PVC+PMMA) polymer electrolyte, the indirect band gap lies at 4.14 eV, while for doped films, the values are found to decrease from 4.14 eV to 3.88 eV.
The position of the absorption edge was estimated by extrapolating the linear portions of the α vs hν plots (Fig. 7) to zero absorption value. For pure films, the absorption edge lies at 4.17 eV and for doped films, the values are found to decrease from 4.17 to 4.06 eV.
It is clear from Table 1 that the direct and indirect band gaps showed a decrease on doping with NaClO4. Similar behavior was also seen in the activation energies obtained from the DC conductivity data as shown in Table 1. The magnitudes of the activation energies obtained from the DC conductivity data are small in comparison with optical band gap energies. This is due to the fact that their nature is different. While the activation energy corresponds to the energy required for conduction from one site to another, the optical band gap corresponds to the interband transition.
The decrease in optical band gap and activation energy on doping may be explained on the basis of the fact that the incorporation of small amounts of dopant forms CTCs in the host lattice. A correlation can be seen in Table 1 between the values of the band gaps and the activation energies as a function of NaClO4 concentration. The variation of band gap values for the polymer electrolyte films with different salt ratios is in agreement with the results reported on other polymeric materials [24, 25]. In general, it can be concluded that the value of the optical band gap decreases with increasing dopant concentration. The presence of NaClO4 helps in increasing the ionic contribution for charge transport.
Conclusions
The XRD study reveals the amorphous nature of the polymer electrolytes. The complex formation in polymer electrolyte system has been confirmed from FTIR studies. The electrical study indicates that the polymer blend can be effectively doped with sodium perchlorate to enhance its conductivity. The increase in conductivity with increasing concentration of dopant is attributed to the formation of charge transfer complexes. Transference number data shows that the conductivity is mainly due to ions rather than electrons. The UV–vis studies confirm charge transfer complex formation between the salt and polymer blend. Optical absorption edge and optical energy gap (both direct and indirect) showed a shift toward lower energies with increased dopant concentration.
References
Acosta JL, Morales E (1996) Solid State Ion 85:85
Kim JY, Kim SH (1999) Solid State Ion 124(1–2):91
Acosta JL, Morales E (1996) J Appl Polym Sci 60:1185
Rhoo HJ, Kim HT, Park JK, Hwang TS (1992) Electrochim Acta 42:1571
Pielichowski K, Hametron I (2000) Eur Polym J 36:171
Neiro SMS, Dragunski DC, Rubira AF, Muniz EC (2000) Eur Polym J 36:583
Ramesh S, Yahaya AH, Arof AK (2002) Solid State Ion 152:291
Stephan AM, Saito Y, Muniyandi N, Renganathan NG, Kalyanasundaram S, Elizabeth RN (2002) Solid State Ion 148:467
Uma T, Mahalingam T, Stimmeng U (2004) J Mater Sci 39:2897
Wagner JB, Wagner C (1957) J Chem Phys 26:1597
Nakamoto K (1986) Infrared and Raman spectra of inorganic and coordination compounds. Wiley, New York
Deepa M, Sharma N, Vasheney P, Agnibotry SA, Chandra R (2000) Ionics 6:408
Deepa M, Sharma N, Vasheney P, Agnibotry SA, Chandra R (1963) Idem, chemical applications of infrared spectroscopy. Academic, New York, p 189
Bruce PG (1991) Synth Met 45:267
Miyamoto T, Shibayama K (1973) J Appl Phys 44:5372
Druger SD, Nitzam A, Ratner MA (1983) J Chem Phys 79:3133
Druger SD, Ratner MA, Nitzam A (1985) Phys Rev B 31:3939
Park CH, Kim DW, Prakash J (2003) Solid State Ion 159:111
Sreekanth T, Jaipal Reddy M, Subba Rao UV (2001) J Power Sources 93:268
Staryga E, Swiatek J (1979) Thin Solid Films 56:311
Ramu CH, Naidu YRV, Sharma AK (1994) Ferroelectrics 159:275
Davis DS, Shalliday JS (1960) Phys Rev 118:1020
Thutupalli GM, Tomlin SG (1976) J Phys D Appl Phys 9:1639
Yoshino K, Manada Y, Sawada K, Morita S, Taka Hashi H, Sugi Moto Y, Onoda M (1989) J Phys Soc Jpn 58:1320
Al-Ramadin Y (2000) Opt Mater 14:287
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Achari, V.B., Reddy, T.J.R., Sharma, A.K. et al. Electrical, optical, and structural characterization of polymer blend (PVC/PMMA) electrolyte films. Ionics 13, 349–354 (2007). https://doi.org/10.1007/s11581-007-0124-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-007-0124-9