Abstract
Proton-conducting polymer complex electrolytes were prepared by incorporation of boric acid, H3BO3 into poly(vinylalcohol), PVA, to form hydrated PVAxH3BO3 where x denotes the number of moles of boric acid per polymer repeat unit. The dried materials were characterized via Fourier transform infrared spectroscopy, thermogravimetry, and X-ray diffraction. The proton conductivity of the hydrated complex electrolytes was measured by AC impedance spectroscopy. PVA2H3BO3 with RH ∼25% was found to be optimum composition that exhibited proton conductivity of 1.3 × 10−3 S/cm at 80 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Proton exchange membranes (PEM) have gained prominence after they become applicable to various technological areas particularly on clean energy systems such as fuel cells. Hydrated perfluorosulfonic acid ionomers, typically Nafion, are the most commonly used membranes that separate into hydrophilic and hydrophobic domains up on swelling [1]. The conductivity occurs via transport of the protons throughout the hydrophilic channels. These membranes have been successfully used in the hydrogen/oxygen fuel cells (PEMFCs) due to their excellent chemical and mechanical stability as well as high proton conductivity [2, 3]. However, there are some limitations such as high cost and the difficulty in recycling or disposal of the perfluorosulfonic acid membranes [4].
Poly(vinylalcohol), PVA, is a water-soluble, polyhydroxy polymer whose physical properties are dependent on the degree of polymerization and degree of hydrolysis. PVA has an excellent chemical resistance; thus, it can be used in many practical applications such as adhesives, textile, and pharmaceutical and biomedical industries due to nontoxic and biodegradable properties [5–7]. PVA can also be modified by chemical crosslinking, which is a highly versatile method to improve the chemical, thermal, and mechanical properties [8]. One of the most common methods is the reaction of hydroxyl groups of PVA with aldehydes to form acetal or hemiacetal [9]. PVA can also physically crosslink with boric acid through hydrogen bonding and ionic interactions in the hydrated state [6, 7].
In the present work, we produced hydrated PVA-Boric acid systems at several stoichiometric compositions and investigated their proton-conducting properties. Moreover, the Fourier transform infrared spectroscopy (FT-IR), thermal, and morphology properties of the dried PVA/H3BO3 samples were studied at several compositions.
Experimental
Poly(vinyl alcohol), PVA (degree of hydrolization ≥98%, M w = 72,000), and boric acid were received from Merck. The complex electrolytes were prepared by mixing of PVA and H3BO3 at several stoichiometric ratios in hot water to get PVAxH3BO3 complex electrolytes, where x is the molar ratio of H3BO3 to polymer repeat unit. Three different polymer electrolytes (PVA1H3BO3, PVA2H3BO3, and PVA3H3BO3) were produced. Water contents of the polymer electrolytes were measured by gravimetric measurements, i.e., comparing the weight changes before and after complexation. The approximate RH values are 30% for PVA1H3BO3, 25% for PVA2H3BO3, and 15% for PVA3H3BO3.
FT-IR Spectra of the dried samples were recorded using Mattson Genesis II spectrophotometer.
Theromogravimetry (TG) measurements were carried out using Perkin Elmer Pyris 1. TG thermogram of PVA was recorded at a temperature-scanning rate of 10 °C/min, under N2 atmosphere.
The thermograms hydrated PVAxH3BO3 electrolytes were recorded by heating the samples from RT to 100 °C at a rate of 5 °C/min and then they hold at this temperature for 30 min under nitrogen atmosphere. Thereafter, the samples were further heated to 650 °C under nitrogen atmosphere at a rate of 10 °C/min.
The structural characterization of the samples were performed using X-ray diffractometer (XRD) model Huber JSO-DEBYEFLEX 1001 diffractometer (Cu Kα radiation) operated at 40 kV and 35 mA. The samples were dried under high vacuum at 80 °C before XRD analysis.
Proton conductivity measurements were carried out in the Max-Planck Institute for Polymer Research, Mainz/Germany using a SI 1260-Schlumberger impedance analyzer. The hydrated samples were sandwiched between gold blocking electrodes and the conductivity was measured from 0 to 100 °C within the frequency range from 10 Hz to 1 MHz. The experiments were carried out under nitrogen atmosphere. Frequency-dependent AC conductivities (σ ac (ω)) were measured using Eq. (1).
where ω (=2πf) is the angular frequency, ɛ o is the vacuum permittivity \( {\left( {\varepsilon _{{\text{o}}} = {{\text{8}}{\text{.852}} \times {\text{10}}^{{{\text{ - 14}}}} {\text{ F}}} \mathord{\left/ {\vphantom {{{\text{8}}{\text{.852}} \times {\text{10}}^{{{\text{ - 14}}}} {\text{ F}}} {{\text{cm}}}}} \right. \kern-\nulldelimiterspace} {{\text{cm}}}} \right)} \), and ɛ″ is the imaginary part of dielectric permittivity.
Results and discussion
Polymer electrolytes were prepared by incorporation of H3BO3 into PVA at several stoichiometric ratios in water. Boric acid and borates form very stable complexes with diols of PVA, forming hydronium ions (Fig. 1) [6, 7, 16]. In the meanwhile, the solvent water gets immobilized into matrix and hydrated PVA/H3BO3 systems were produced.
In acid-doped polymer electrolytes, there should be excess acid to attain high conductivity. However, the excess acid leaches out by the liquid water, which is inevitably change the conductivity of the membrane and decreases the efficiency of the fuel cell during operation [10–12]. In that respect, polymer-boric acid complexation or crosslinking seems to be alternative pathway which may inhibit proton solvent exclusions.
FT-IR spectra for the PVA and dried PVA1H3BO3 are compared in Fig. 2. Characteristic main chain absorptions of the PVA are located at 3,350, 2,934, and 1,282 cm−1, corresponding to ν O–H, ν C–H, and ν–C–C–O–, respectively (Fig. 2a). The peak at 1,145 cm−1 (C–O, ν = 1,090–1,150 cm−1) can be attributed to C–O stretching [13]. In Fig. 2b, the intense peak at 665 cm−1 was attributed to vibration of B–O bond. In addition, the absorption band near 1,340 cm−1 was assigned to the antisymmetric stretching vibration of the B–O bond, ν as (B–O) [14]. The band at 1,020 cm−1 was due to the stretching of BO–C [14, 15], which confirms the condensation reaction of PVA with boric acid. The existence of B–O–B linkage was proved by the peak located at 768 cm−1, indicating the condensation boric acid with itself.
Figure 3 shows TG thermograms of the pristine PVA and PVA2H3BO3 which are recorded from room temperature to 650 °C, under nitrogen atmosphere. As seen, the onset of the degradation temperature of PVA is approximately 225 °C. Hydrated sample, PVA2H3BO3, was first heated from ambient temperature to 100 °C at a rate of 5 °C/min and kept at this temperature for half an hour to see the exponential weight loss because of the evaporation of humidity as well as condensation reactions polymer electrolytes. Further increase of the temperature at a heating rate of 10 °C/min leads to a progressive degradation above 360 °C. Clearly, the addition of H3BO3 results in the formation crosslinked polymers above 100 °C which shifted the degradation temperature of PVA about 135 °C to higher temperatures. The total weight change because of loss of free water and condensation (when T > 100 °C) were found to be 45% for PVA1H3BO3, 42% for PVA2H3BO3, and 38% for PVA3H3BO3. By subtracting the percent weight changes from the free water contents (experimental part), one can conclude the respective weight losses because of condensation reactions at higher temperatures, i.e., 15% for PVA1H3BO3, 17% for PVA2H3BO3 and 23% for PVA3H3BO3. Clearly, high temperature condensation increased in parallel with the boric acid composition.
TG profile for pure PVA (broken line) at a temperature scanning rate of 10 °C/min. The thermogram of PVA2H3BO3 (solid line) includes three steps: (1) heat from RT to 100 °C at 5 °C/min, (2) hold for 30.0 min at 100 °C, and (3) heat from 100 °C to 650 °C at 10 °C/min. All the experiments were carried out under nitrogen atmosphere
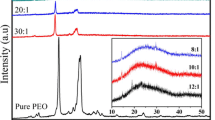
The structures of the PVA and the dried crosslinked systems PVA1H3BO3 and PVA2H3BO3 were investigated using XRD analysis (Fig. 4). PVA shows a broad band centered at around 20° (2θ) indicated the semicrystalline structure of the homopolymer. The XRD patterns of the dried PVA1H3BO3 and PVA2H3BO3 samples shows that the intensity of the broad peak of PVA decreased with increasing H3BO3 concentration. This behavior can be attributed to the chemical crosslinking that increased the amorphous character of the final product.
Proton conductivities of the hydrated polymer electrolytes, PVAxH3BO3, were measured using impedance spectroscopy. Frequency dependence of AC conductivity of the PVA2H3BO3 at different temperatures is shown in Fig. 5. It can be observed that the conductivity is almost constant when F > 104 Hz. However, deviations occur at lower frequencies because of electrode polarization. DC conductivity is derived from the plateaus by linear fitting and extrapolating to zero frequency.
Figure 6 shows the temperature dependence of proton conductivity of the gel electrolytes at different compositions as well as different water contents. It is obvious that the proton conductivities of the samples change with the temperature and composition.
The conductivity of hydrated PVA2H3BO3 is higher than PVA1H3BO3 where water content is higher for the latter. However, the proton conductivity of PVA3H3BO3 is slightly lower than that of PVA2H3BO3 at lower temperatures and the deviation is more pronounced when T > 60 °C. This behavior can be attributed to low water content of PVA3H3BO3 electrolyte as mentioned above. From these results, it can be concluded that PVA2H3BO3 is the optimum chosen composition, which holds approximately 25% humidity and exhibits better conductivity. In these complex systems, proton transport is expected to occur throughout the water phase. The complex electrolyte PVA2H3BO3 showed a remarkable conductivity of 1.3 × 10−3 S/cm at 80 °C. Clearly, conductivity decreases because of water loss when T > 80 °C and continuous humidification is required to keep stable conductivity.
Conclusions
The hydrogels of PVA-H3BO3 were produced and the proton conducting properties were investigated. The physical properties were also changed with the boric acid composition as well as water content. We found that hydrated polymer electrolyte PVA2H3BO3 revealed a conductivity of 4 × 10−4 S/cm at RT and 1.3 × 10−3 S/cm at 80 °C. Clearly, PVAxH3BO3 is a low temperature proton conductor and similar to perfluorosulfonic acid membranes, it becomes an insulator in the dry state. However, the dried and crosslinked PVA-H3BO3 systems can be used as novel host material for anhydrous proton conductors where the protogenic solvents can be substituted into the matrix during synthesis.
References
Kreuer KD, Dippel Th, Meyer WH, Maier J (1993) Mater Res Soc Symp Proc 293:273
Rikukawa M, Sanui K (2000) Prog Polym Sci 25:1463
Motupally S, Becker AJ, Weidner JW (2000) J Electrochem Soc 147(9):3171
Schuster MFH, Meyer WH (2003) Annu Rev Mater Res 33:233
Reis EF, Campos FS, Lage AP, Leite RC et al (2006) Mater Res 9:185
Peppas NA, Mongia NK (1997) Eur J Pharm Biopharm 43:52
Hashimoto S, Furukawa K (1987) Biotechnol Bioeng 30:52
Kim DS, Yun TI, Seo MY, Cho HI et al (2006) Desalination 200:634
Martinelli A, Matic A, Jacobsson P, Börjesson L, Navarra MA, Fernicola A, Panero S, Sacrosati B (2006) Solid State Ionics 117:2431
Xing BZ, Savadogo O (1999) J New Mater Electrochem Syst 2:95
Alberti G, Casciola M (2001) Solid State Ionics 145:3
Kufacı M, Bozkurt A, Tülü M (2006) Solid State Ionics 117:1003
Cotaes J (2000) Interpretation of infrared spectra. A practical approach. In: Meyers RA (ed) Encyclopedia of analytical chemistry. Wiley, New York
Pennarun PY, Jannasch P, Papaefthimiou S, Skarpentzos N, Yianoulis P (2006) Thin Solid Films 514:258
Mondal S, Banthia AK (2005) J Eur Ceram Soc 25:287
Cotton FA, Wilkinson G, Gaus PL (1995) Basic inorganic chemistry, 3rd edn. Wiley, New York
Acknowledgements
The project is supported by TÜBİTAK (contract grant number: 105M345). Thanks to C. Sieber from MPI-Polymer Research, Mainz for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Şenel, M., Bozkurt, A. & Baykal, A. An investigation of the proton conductivities of hydrated poly(vinyl alcohol)/boric acid complex electrolytes. Ionics 13, 263–266 (2007). https://doi.org/10.1007/s11581-007-0100-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-007-0100-4