Abstract
Compared to cardiac parameters and skin conductivities, the photoplethysmogram (PPG) recorded at fingertips and other parts near to peripheral nerve ends have been recently revealed to be yet another sensitive measure for cognitive load assessment. However, there is so far no research on measuring adolescents’ cognitive load using physiological signals. A comprehensive study on the effects of PPG morphometrics over a cohort covering both adolescent and adult students is also absent. In this study, we analyze the morphological features of PPG on cognitive load assessment and compare them between adolescent and adult students. Experiments on two-level arithmetic tasks show that the PPG morphometrics reached the same level of significance on the effect of task difficulty/period as heart rate, and different morphological behaviors were also shown between adolescent and adult students during the cognitive task effects, which may imply their physiological differences across age. Physiological signals recorded by wearable devices are also found to be effective in measuring cognitive load.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cognitive load is considered the load that performing a particular task imposes on the human cognitive system (Meshkati 1988; Paas and Van Merrienboer 1994; Yeh and Wickens 1988). It is a multidimensional construct and reflects the demand for both psychological and physiological resources in complex cognitive tasks. Human learning and performance are largely constrained by limited working memory. The measurement of cognitive load is thus crucial to human–computer interaction (HCI) design (Oviatt 2006; Paas and Van Merrienboer 1994; Sweller et al. 1998), as well as teaching and learning designs, as the control and manipulation of the task according to cognitive load assessment can optimize the learning experience and HCI.
The assessment of cognitive load can be conducted via three dimensions: the task-centered dimension (i.e., stress or task demand (Young et al. 2015)), the human-centered dimension (i.e., mental effort), and the synthesized dimension (i.e., performance (Jahns 1973)). Mental effort, which refers to the cognitive capacity invested in the task (Paas and Van Merrienboer 1994), is essential to measure cognitive load in practice. Mental effort reflects all three causal factors of cognitive load, i.e., task and human characteristics and the interaction between the two (Paas et al. 2003). Mental effort is linked to both cognitive capacity (cognitive resources available) and intentional autonomic arousal. Due to variations in arousal, the same person may perform differently on the same task at different times (Askew 1998). By measuring the cognitive load of teachers and students, teaching or learning effort can be further assessed. Furthermore, combining learners’ mental effort and performance output, teaching effectiveness can be objectively and quantitatively evaluated.
Measures for assessing mental effort include rating scale techniques, behavioral methods, and psychophysiological techniques. The rating scale-based methods are subjective and rely on participants’ memory of their cognitive process, while the other methods are objective and based on the assumption that variations in cognitive functioning can be reflected by behavioral and physiological signals. In addition, physiological signals, such as electroencephalography (EEG), electrocardiography (ECG), blood pressure (BP) and electrodermal activities (EDA, i.e., skin conductivity-based measures), are believed to have promising potential to provide more precise and accurate measurements. However, there are a variety of limitations in sensitivity, reliability, and availability for these measures. Although researchers usually assess mental effort by a combination of multiple measures to improve the assessment, combining multiple measures is not ubiquitously available across applications. There is much inconvenience or inaccuracy in measuring physiological signals such as ECG and blood pressure on a wearable device.
Compared to cardiac parameters and skin conductivities, the photoplethysmogram (PPG) recorded at the fingertips and other parts near peripheral nerve ends has been recently revealed to be yet another sensitive measure for cognitive load assessment (Lyu et al. 2015). PPG is an optically obtained vital sign that is used to show the blood volume changes in the peripheral blood vessels, which reflects both the cardiac activity and the status of vasculature. The low-frequency components of the PPG waveform are attributed to respiration, sympathetic nervous system activity and thermoregulation (Allen 2007). PPG morphometrics have also been correlated with other cardiac parameters (such as ECG and blood pressure) and physiological state (such as age, blood pressure, and heart rate). When cognitive load occurs, the sympathetic nervous system will become excited and elicit a series of reactions, such as increased heart rate and vasoconstriction, and the morphology of PPG will change accordingly. Furthermore, many previous studies (Berntson et al. 1991; Charkoudian and Rabbitts 2009; Hernando et al. 2019; Joyner et al. 2010; Malpas et al. 2001; Pfeifer et al. 1983; Shabanah et al. 1964; Sherwood et al. 2002) also suggested that the PPG acquired at the fingertips or other body parts near peripheral nervous ends can sensitively reflect sympathetic nervous system (SNS) activity.
To our knowledge, there are currently no studies measuring adolescent cognitive load using physiological signals. Many studies have shown that adolescents are in the midst of many physiological and psychological changes (Artemenko et al. 2018; Mueller et al. 2017; Romeo 2013). Specifically, adolescence is also a significant period of continued neural maturation in hypothalamic–pituitary–adrenal (HPA) axis reactivity, resulting in heightened stress-induced hormonal responses (Romeo 2013). It is presently unclear whether adolescents have different physiological responses than adults when cognitive load occurs. In this study, we were mainly concentrated on the differences in PPG morphometrics, which are easily accessible and less invasive among measurable physiological signals. Moreover, a comprehensive study on PPG morphometrics across a cohort including both adolescents and adults is still absent. We characterized and defined the morphological features of PPG and verified their psychometric properties in assessing cognitive load for both adolescent and adult students.
In this paper, the sensitivity of PPG morphometrics on cognitive load assessment and the differences between adolescents and adults are analyzed. Experiments on two-level arithmetic tasksFootnote 1 show that PPG morphometrics can reach the same level of significance on the effect of task difficulty/period as heart rate, and different morphological features are also compared between adolescents and adults during the cognitive task. The physiological signals recorded by wearable devices are also found to be promising in measuring cognitive load. We show that the PPG morphometrics are effective in revealing the status of cognitive load and that it is more appropriate to establish separate indices and criteria for adolescents and adults on cognitive load assessment. Our findings may provide some guidance for teaching and HCI design to be more flexible and efficient.
The main contributions of this study are as follows:
-
As an addition to traditional measures, PPG morphometrics were comprehensively studied for assessing cognitive load in adolescent and adult students.
-
Both fingertip PPG and wearable PPG reached significance in differentiating effortful conditions associated with tasks of varying difficulties that were used to impose cognitive loads.
-
The performance and sensitivity of morphometrics on the assessment of cognitive load during cognitive tasks between adolescent and adult students were compared. The results showed that there was a difference in the level of significance between adolescent and adult students across morphometric measures.
The remainder of the paper is organized as follows. First, the background of PPG morphometry and correlated information are systematically presented. Second, the basic methodology and experimental design are introduced. Then, the experimental results and analyses are reported. Finally, the limitations and applicability of this study are presented.
Background
Most of the PPG morphometric measures are visualized in Fig. 1. The upper part of Fig. 1 models a standard PPG waveform by morphometric characterization, and Tables 1 and 2 give the corresponding morphometric measures and meanings for the time domain and frequency domain, respectively, some of which were also similarly formulated in previous research related to PPG feature analysis (Kavsaoglu et al. 2014). The lower part of Fig. 1 shows the corresponding first-order derivative and second-order derivative to the PPG waveform. Tables 3 and 4 give their detailed measures and meanings, respectively.
The original time-domain and frequency-domain measures of a standard PPG waveform have been mentioned in a range of previous studies. To date, there has not been a set of standard symbols and denotations for the subwaves and features found, and we provide some relatively general denotations for them in this study that are slightly different from the previous ones, such as those using x, y, z and IPA for subwaves (Elgendi 2012; Kavsaoglu et al. 2014). For some definitions rarely seen in other publications, we also mark their referred sources in the tables accordingly. In related studies, the first-order derivative indices (Alty et al. 2007; Millasseau et al. 2002) were not as well studied as the second-order derivative indices (Baek et al. 2007; Otsuka et al. 2006; Takazawa et al. 1998), which were first proposed as domains of interest in the 1980s in cardiac medicine professions. In this study, we chose to follow a similar ABCDEF symbol and denotation system with those invented in the cardiac research domain to maintain a similar stylish convention with the PQRST system in ECG studies. The waveforms of the first-order derivative are named a1, b1, c1, and d1. The waveforms of the second-order derivative are named a2, b2, c2, d2, and e2.
The blood volume changes in peripheral blood vessels that PPG records reflect both the heart activities and the blood vessel status; therefore, the PPG signals are actually tightly correlated to ECG and blood pressure, which are widely used for studying the heart and circulatory systems. A range of studies have revealed the potential of PPG in indicating heart diseases as well as hypertension. In addition, PPG has also been widely used to indicate blood vessel health status, such as those observed with aging (Baek et al. 2007; Bortolotto et al. 2000; Millasseau et al. 2002), stiffness (Alty et al. 2007; Millasseau et al. 2002; Shimazu et al. 1986), and blockages (Awad et al. 2007; Bortolotto et al. 2000; Otsuka et al. 2006; Takazawa et al. 1998). Moreover, aging has been associated with increased vascular stiffness and peripheral resistance and reduced vasoconstriction in response to sympathetic stimulation (Hogikyan and Supiano 1994), which could be a possible reason that PPG morphometrics behave differently when cognitive load occurs.
The time relationship between the PPG and ECG is illustrated in Fig. 2, where the delay between the ECG R wave and the peak of the PPG pulse is defined as T. It has also been defined as the pulse arrival time (PAT) and can be used to estimate blood pressure (BP) combined with heart rate (Cattivelli and Garudadri 2009). Blood pressure can also be inferred from PPG morphometric measures. Therefore, many algorithms (Imholz et al. 1988; Kachuee et al. 2017; Peter et al. 2014) for noninvasive blood pressure continuous measurement using PPG have been proposed. These algorithms use many features mentioned in the previous section and include the augmentation index and inflection point area ratio (Millasseau et al. 2002; Padilla et al. 2008; Wang et al. 2009). The calculation methods of these features may be slightly different across papers but involve consistent basic concepts. Some researchers have modeled blood flow changes from the heart to peripheral blood vessels (Goldwyn and Watt 1967; van de Vosse and Stergiopulos 2011). These models explained the information contained in PPG changes in some way.
PPG morphology is also correlated with the sympathetic nervous system (SNS) activity, which controls the contraction and relaxation of peripheral blood vessels. The general response to both physical and psychological stress is the activation of the sympathetic nervous system with inhibition of the parasympathetic nervous system (Wolf 1997). When stressed, there will be increases in heart rate, left ventricular ejection fraction, cardiac output and vasodilation. Some of these physiological manifestations are highly related to the measures of tpi, AUC, IPA, sVRI, etc. It has been proven that PPG can also efficiently reflect the SNS because peripheral vasoconstriction is controlled only by the SNS (Lyu et al. 2015; Zhang et al. 2018). There are also many physiological signals that can reveal stress levels, including heart activity, breathing, and blinking, but they are all controlled by the parasympathetic nervous system (Berntson et al. 1991; Farmer et al. 2014) and are not as sensitive as the signals controlled only by the sympathetic nervous system. Therefore, it may be inappropriate to consider measures such as tpi as sensitive measures. Some morphometrics such as IPA and sVRI that reflect pure SNS activities may be sensitive enough. Consequently, PPG could be as direct and sensitive to SNS activities as electrodermal activities (EDA) (Boucsein 2012), but this is still to be further researched.
Methodology
The study presents a classic arithmetic calculation task, which requires the subject to have basic mathematical operation ability. Participants were required to perform continuous subtraction within a specified period, where the subtractions remain the same, such as consecutive subtractions of 7, 13, 77, etc. Adults and minors have slightly different topics. The experimental data of the subjects, including task scores, experimental performance and physiological signals, i.e., PPG signals, were collected in real time during the experiment through arithmetic task performance, experimental recordings and portable wearable physiological signal detection equipment. After the experiment, the participants completed the self-report sheet. Finally, the above data were comprehensively analyzed, and the experimental results were obtained.
Hypotheses
According to the relationships between PPG morphometrics and cognitive load described in “Background” section, we suppose that some measures can be used to assess cognitive load and stress. We designed an experiment with two task difficulties and two testing periods to evaluate the following specific hypotheses:
-
Hypothesis A—Period effect: Some measures will show significant differences between different periods (i.e., pre-task and in-task).
-
Hypothesis B—Difficulty effect: Some measures will show significant differences based on difficulty (i.e., easy and hard).
-
Hypothesis C—SNS activities: Some measures related to SNS activities will show significant differences between periods or difficulties.
Participants
A total of 36 participants were recruited in this experiment, including 17 adults (9 men and 8 women; average age: 22.4 years) and 19 adolescents (10 boys and 9 girls; average age: 16.8 years). All of them were university students or high school students.
All subjects were physically and mentally healthy, and their hearing and vision (including corrected vision) were normal. They were all right-handed and had never participated in related cognitive experiments. Before the experiment, they had carefully read and signed the informed consent form approved by the ethics committee of the Department of Psychology of Tsinghua University. Additionally, both minors and their guardians had carefully read and signed the informed consent form and the form for underage (8–17 years old) participants. Each adult participant was paid 50 yuan with an additional bonus of 13 to 30 yuan added according to the experimental performance. Each adolescent participant was awarded a set of hand-painted postcards and a notebook for participation in the experiment.
Apparatus and settings
The experiment used a standard clinical finger-clip PPG sensor (sampling frequency 200 Hz) and a wearable wristwatch (sampling frequency 20 Hz) to collect the PPG and HR signal of the subject. The sensor was uniformly placed on the left index finger of the subject, and the wristwatch was uniformly worn under the left wrist joint of the subject. The two experiments were carried out in a laboratory environment with normal light levels and constant temperature and humidity. The task was presented on a laptop screen. The participants watched relaxing videos and operated a standard keyboard to input their answers to the arithmetic problems. The sound was played through SONY headphones, and the volume was set in advance to a moderate level.
Stimuli and protocol
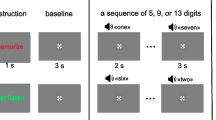
The experiment consists of two parts: the explanation phase and the formal experiment. Each participant was tested separately. After the formal test began, the physiological signals of the subjects were recorded. During the explanation phase, the participants were required to understand the purpose and steps of the experiment while relaxing. The formal experiment consists of five parts, as shown in Fig. 3.
First, the baseline test was conducted, in which the subjects were asked to sit for 1 min and 30 s with closed eyes and 1 min and 30 s with open eyes for baseline blink data collection as indicated by the experimental test program. The participants then watched a 5-min video. After the video, the subjects were asked to perform the first calculation task (difficult mode). The initial subtraction was 73 from 5000 for both adults and minors. The subject needed to calculate the solution within 10 s of the specified time. If the calculation was correct, then 4927 became the minuend for the next question, from which 73 was subtracted, and so on. The calculation task duration was 5 min, during which each correct answer resulted in 5 points and errors removed 3 points, and the system recorded the final score of the test.
After the first mental arithmetic task, the participants relaxed and rested again while watching a video. The video content was immediately followed by the previous video for 10 min. After this second rest period, the subject performed the second calculation task. This task was the simple mode; the initial number was 4000, and the subtracted number was 13 for adults and 7 for minors. The subject needed to perform continuous subtraction for the total time of 5 min. The limit time for each question was 10 s, and the scoring method was the same as in the first task.
At the end of the experiment, the participants completed the questionnaire for the self-report and scored the difficulty and cognitive load of the two mental tasks on a scale of 1 to 4. Regarding difficulty, a score of 1 indicated that the task was very simple, and a score of 4 indicated that the task was very difficult. Regarding cognitive load, a score of 1 indicated that the subject felt no pressure during the task, and a score of 4 indicated that the subject was full of stress during the task.
Analysis and results
In this study, the data were divided into different segments according to the experimental process. The study calculated the average values of the PPG and HR data under the different segments as dependent variables for subsequent analysis. Repeated measures ANOVA was performed, in which the period was divided into two stages: pre-task and in-task. The difficulty of the task was divided into two levels: easy and hard. The period and difficulty were the main factors in the analysis. The main effect of period or difficulty on the cognitive load was assessed according to whether there was a significant difference in the mean value of the different variables across periods or difficulties.
Before the analysis, it was necessary to perform a spherical test on the correlation between the repeated measurement data. If the test result was p > 0.05, there was no correlation between the repeated measurement data. The measurement data conformed to the Huynh–Feldt condition, which can be processed by one-way ANOVA. If the test result was p < 0.05, it indicated that there was a correlation between the measured data, so the data cannot be processed by one-way ANOVA. All statistical analyses in this study were performed in SPSS 25.0 with a significance of 0.05 and two-tailed testing.
Performance data
For the scores of performance and self-assessment cognitive load, we used the paired t test to examine significant level of difference under different difficulty. Figure 4 shows the performance and self-report cognitive load in two mental arithmetic tasks, where the significant level is also marked with stars. The scores in the hard mode (\(134 \pm 55\)) were significantly lower (\(p < 0.001, t\left( {16} \right) = - 8.91\)) than the scores in the easy mode (\(253 \pm 61\)), and the results of the self-reports showed that the performance scores were negatively correlated with cognitive load across difficulties. The self-assessment scores of cognitive load in the hard task (\(2.65 \pm 0.79\)) were higher than those in the easy task (\(1.65 \pm 0.49\)), with a significance level of \(p < 0.001,t\left( {16} \right) = 4.12\).
Figure 5 provides the performance results of the adolescent participants. The scores in the hard mode (\(52 \pm 62\)) were significantly lower (\(p < 0.001, t\left( {18} \right) = - 18.15\)) than those in the easy task (\(298 \pm 67\)), and the performance scores showed a negative correlation with the cognitive load according to the self-report, where scores in the hard task (\(3.00 \pm 0.82\)) were higher than those in the easy task (\(1.58 \pm 0.77\)), with a significant difference (\(p < 0.001,t\left( {18} \right) = 5.30\)). Both sets of results showed that the task difficulty set in this experiment was reasonable. In summary, the two figures prove the rationality and effectiveness of the experimental design; that is, the cognitive load caused by the simple task was low, and the cognitive load caused by the difficult task was higher.
Physiological data
By repeated measures ANOVA, we found some measures showing significant differences across periods and difficulties. Table 5 reports the average and corresponding standard deviations of the different physiological measures for the adult subjects during the task. The subscript ‘(F/W)’ indicates the source of data: ‘(F)’ means the finger-clip PPG sensor and ‘(W)’ means the wearable wristwatch. These measures included sVRI, AUC, tpi, t1/tpi, ta1/tpi, tb1/tpi, ta2/tpi, and tb2/tpi (note that \(t_{pi}\) is equivalent to heart rate). Repeated-measures ANOVA of the 2 periods (pre-task and in-task) × 2 difficulties (easy and hard) was performed for each of those measures and used to first examine the physiological responses during different testing periods. The analysis results are presented in Tables 7 and 8 and include p and F values for the main effects of period and difficulty and \(\eta^{2}\) for the effect sizes, which were the partial eta squared values in SPSS.
Table 8 reports the within-subject effects of different measures from the adults across the different periods and difficulties. Combined with the results in Tables 1, 2, 3, and 4, we obtained some measures that showed significant period and difficulty effects on cognitive load: tpi(F), tpi(W) and ta2/tpi significantly decreased (p < 0.05), and t1/tpi(F), ta1/tpi, tb1/tpi and tb2/tpi significantly increased (p < 0.05), while the subjects performed the tasks.
Meanwhile, we note that sVRI and AUC, which are related to SNS activities, were significantly different across periods or difficulties in the adults. However, the AUC is also dually controlled by PNS. It is directly correlated with heart rate. Indicators such as IPA were not significant for any effect. According to the heart rate measure tpi(W) (F (1, 16) = 23.25) collected by the wearable device, it had significant period effects on cognitive load. In particular, tpi(W) significantly decreased (p < 0.01), which was consistent with the results of physiological measures collected from the clinical finger clip.
Table 6 shows the results of the within-subject effects across periods and difficulties for the adolescents. We found that all measures tpi, t2/tpi, t3/tpi, and tb1/tpi showed significant effects of period and difficulty. Specifically, tpi (F (1, 18) = 43.796, p < 0.01) showed a significant negative period effect (p < 0.05) on cognitive load, while the remaining measures presented positive period effects (p < 0.05) on cognitive load. In contrast with the easy task, tpi significantly decreased (F (1, 18) = 19.120, p < 0.01) in the hard task, which meant that the participants had a higher heart rate. The remaining measures t2/tpi, t3/tpi, and tb1/tpi showed lower mean values in the easy task. These data showed significant positive effects of difficulty. In addition, the results also proved that the task difficulty of this experiment was reasonable and distinguishable; that is, the cognitive load was higher in the hard task, and the cognitive load was lower in the easy task.
According to Table 7, the measures related to pure SNS activities, such as sVRI and AUC, were only significant across periods. Combined with the results in Table 8, we found that only the AUC measure was significant across periods, which meant that the cardiac output was significantly increased during the task.
Discussion
Discussion of the results
This study tested hypotheses of period effects, difficulty effects, and SNS activities. First, the significant differences in self-assessment and performance scores showed that the difficulty setting of the cognitive task was reasonable, and the participants had a relatively higher cognitive load in the hard task than in the easy task. After the separate hypotheses test for adults and adolescents, we found that the measures tpi, t2/tpi, t3/tpi, and tb1/tpi for the adolescents and AUC, tpi, t1/tpi, ta1/tpi, tb1/tpi, ta2/tpi, and tb2/tpi for the adults showed significant effects across both periods and difficulties. However, the measures sVRI, AUC, t1/tpi, ta1/tpi and tb2/tpi for the adolescents and sVRI for the adults showed only a significant effect across periods.
The common measures tpi, tb1/tpi were significant across both periods and difficulties for the adults and adolescents, which implied that adults and adolescents may have responded to stress in a similar way, i.e., increasing heart rate and b1 time ratio. The measure sVRI was significant only across periods for both the adults and adolescents, which indicated that this index may only be applicable as a measure of whether the sympathetic nervous system was excited. Due to the limited external stimuli in this study, the activation of the SNS can be taken as the evidence of cognitive load on the subjects.
The uncommon measures here may reveal the differences between adults and adolescents under similar stress conditions. The measures t2/tpi, t3/tpi for the adolescents and AUC, ta1/tpi, ta2/tpi, and tb2/tpi for the adults both showed significance across periods and difficulties, and the adolescent measures AUC, t1/tpi, ta2/tpi, and tb2/tpi showed significance only across periods. Some possible explanations for these differences are as follows:
-
(1)
The adolescents put as much mental effort as possible into the experiment even if the task demand was low, which led to significance only across periods and roughly the same distribution of AUC, t1/tpi, ta2/tpi, and tb2/tpi across different difficulties.
-
(2)
The cardiovascular system and autonomic nervous system in the adults were more mature and had higher vascular stiffness than the adolescents. This difference might have caused the t3/tpi, which is related to the time ratio of the retrograde flow, to be not significant across periods or difficulties for the adults.
It should be noted that some measures showed different significance levels in the adults and adolescents. We may design different indices for them to perform cognitive load assessments in the context of teaching methods and material design. Based on the existing research, HRV, respiration interval, pupil size and skin conductance show significant differences across difficulties and periods (Brouwer et al. 2014). With the exception of skin conductance, these measures are not sensitive enough. Our exploration of PPG morphometrics might help to add more sensitive indicators to monitor cognitive load. The cognitive load effects on PPG morphometrics were analyzed and compared between adolescent and adult students. This study might help us understand more about the physiological responses to cognitive load in adolescents and can be used as a supplement to related research fields at the theoretical level. Additionally, we successfully migrated the laboratory measurements to a real-world situation through the verification experiment of portable wearable devices, providing an experimental basis for the subsequent measurement of physiological signals in real-world contexts to measure cognitive load.
The portable wearable device can greatly reduce the cost and difficulty of collecting physiological signals. The portable device collects physiological signals in real time in a real context. Although the accuracy of the data collected by portable wearable devices is lower than that by laboratory instruments, wearable devices are more convenient and can greatly expand the sample size and data volume, which is not possible using traditional laboratory equipment. The development of wearable physiological signal measurement technology can greatly improve the ubiquity of relevant studies or applications.
Limitations
Although the experimental results showed the possibility of cognitive load measurement using PPG in adolescents and adults, this study still had the following limitations:
-
(1)
The dataset used in this study involved only adolescents with an average age of 16.8 and adults with an average age of 22.4. We may further expand the age range and the quantity of the subjects to observe alterations in the stress responses with age.
-
(2)
We note that tpi(W) was not as accurate as tpi(F). The measures from wearable wristwatches might not be usable. Further evaluation of wearable devices with different precisions is needed for ubiquitous and daily applications. The experimental conditions can still be improved to be more adaptive for circumstances in daily life.
-
(3)
Regarding the SNS activity measures, sVRI was only significant across periods, and IPA showed no significance, which implied that sVRI was a more effective measure than IPA, which may be used as an indicator of exercise (Wang et al. 2009). The level of SNS activation might have no indicators among PPG morphometrics. In other words, the PPG morphometric measures may not reflect the level of pure SNS activities unlike EDA measures. Nevertheless, there are some sensitive measures, such as t2/tpi, tb1/tpi, that can show cognitive load or stress levels. However, we still need to validate their correlation with SNS activities in future work.
-
(4)
In addition, the performance and sensitivity of PPG morphometric measures during cognitive task performance between adolescents and adults were compared, and cardiovascular and cognitive neuroscience theories are still needed to further explore and explain the mechanism.
Conclusions
In this paper, we comprehensively analyzed the PPG morphometrics in the context of cognitive load assessment and compared them between adolescent and adult students. The experiment, based on two levels of task difficulty and two stages of testing periods, showed that there were differences in the levels of significance between adolescents and adults. For example, the measures t2/tpi and t3/tpi did not show significance on any effect in the adults, although they showed significance in the adolescents. Our findings also suggested that measures such as sVRI, AUC, tpi, and t1/tpi might be sensitive, reliable, and usable indices for cognitive load assessment. However, sVRI, AUC, and t1/tpi do not currently distinguish cognitive load from stress despite their differences based on experimental results in both adults and adolescents. These measures may reflect but cannot distinguish the level of pure SNS activities. In future work, we will focus on the current limitations of this method and study its extension for ubiquitous applications.
Notes
The arithmetic calculation is used in a large literature in physiology and biophysiology as a task to study stress via mental effort (Ritter et al. 2007).
References
Allen J (2007) Photoplethysmography and its application in clinical physiological measurement. Physiol Meas 28:R1–R39. https://doi.org/10.1088/0967-3334/28/3/r01
Alty SR, Angarita-Jaimes N, Millasseau SC, Chowienczyk PJ (2007) Predicting arterial stiffness from the digital volume pulse waveform. IEEE Trans Biomed Eng 54:2268–2275. https://doi.org/10.1109/tbme.2007.897805
Artemenko C, Soltanlou M, Ehlis A-C, Nuerk H-C, Dresler T (2018) The neural correlates of mental arithmetic in adolescents: a longitudinal fNIRS study. Behav Brain Funct. https://doi.org/10.1186/s12993-018-0137-8
Asada HH, Shaltis P, Reisner A, Rhee S, Hutchinson RC (2003) Mobile monitoring with wearable photoplethysmographic biosensors. IEEE Eng Med Biol Mag 22:28–40. https://doi.org/10.1109/memb.2003.1213624
Askew S (1998) Transforming learning. Continuum, London
Awad AA, Haddadin AS, Tantawy H, Badr TM, Stout RG, Silverman DG, Shelley KH (2007) The relationship between the photoplethysmographic waveform and systemic vascular resistance. J Clin Monit Comput 21:365–372. https://doi.org/10.1007/s10877-007-9097-5
Baek HJ, Kim JS, Kim YS, Lee HB, Park KS (2007) Second derivative of photoplethysmography for estimating vascular aging. Paper presented at the 6th international special topic conference on information technology applications in biomedicine
Berntson GG, Cacioppo JT, Quigley KS (1991) Autonomic determinism—the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychol Rev 98:459–487. https://doi.org/10.1037/0033-295x.98.4.459
Bortolotto LA, Blacher J, Kondo T, Takazawa K, Safar ME (2000) Assessment of vascular aging and atherosclerosis in hypertensive subjects: second derivative of photoplethysmogram versus pulse wave velocity. Am J Hypertens 13:165–171. https://doi.org/10.1016/s0895-7061(99)00192-2
Boucsein W (2012) Electrodermal activity. Springer, Berlin
Brouwer A-M, Hogervorst MA, Holewijn M, van Erp JBF (2014) Evidence for effects of task difficulty but not learning on neurophysiological variables associated with effort. Int J Psychophysiol 93:242–252. https://doi.org/10.1016/j.ijpsycho.2014.05.004
Brumfield AM, Andrew ME (2005) Digital pulse contour analysis: investigating age-dependent indices of arterial compliance. Physiol Meas 26:599–608. https://doi.org/10.1088/0967-3334/26/5/003
Cattivelli FS, Garudadri H (2009) Noninvasive cuffless estimation of blood pressure from pulse arrival time and heart rate with adaptive calibration. In: Sixth international workshop on wearable and implantable body sensor networks, Proceedings. https://doi.org/10.1109/p3644.34
Charkoudian N, Rabbitts JA (2009) Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clin Proc 84:822–830. https://doi.org/10.4065/84.9.822
Chua CP, Heneghan C (2006) Continuous blood pressure monitoring using ECG and finger photoplethysmogram. In: Conference proceedings: annual international conference of the IEEE engineering in medicine and biology society annual conference, vol 1, pp 5117–5120
Dawber TR, Thomas HE, McNamara PM (1973) Characteristics of dicrotic notch of arterial pulse-wave in coronary heart-disease. Angiology 24:244–255. https://doi.org/10.1177/000331977302400407
Elgendi M (2012) On the analysis of fingertip photoplethysmogram signals. Curr Cardiol Rev 8:14–25
Farmer AD, Al Omran Y, Aziz Q, Andrews PL (2014) The role of the parasympathetic nervous system in visually induced motion sickness: systematic review and meta-analysis. Exp Brain Res 232:2665–2673. https://doi.org/10.1007/s00221-014-3964-3
Goldwyn RM, Watt TB (1967) Arterial pressure pulse contour analysis via a mathematical model for clinical quantification of human vascular properties. IEEE Trans Biomed Eng BM14:11. https://doi.org/10.1109/tbme.1967.4502455
Hernando A et al (2019) Autonomic nervous system measurement in hyperbaric environments using ECG and PPG signals. IEEE J Biomed Health Inform 23:132–142. https://doi.org/10.1109/jbhi.2018.2797982
Hogikyan RV, Supiano MA (1994) Arterial alpha-adrenergic responsiveness is decreased and SNS activity is increased in older humans. Am J Physiol 266:E717–E724
Imanaga I, Hara H, Koyanagi S, Tanaka K (1998) Correlation between wave components of the second derivative of plethysmogram and arterial distensibility. Jpn Heart J 39:775–784
Imholz BPM, Vanmontfrans GA, Settels JJ, Vanderhoeven GMA, Karemaker JM, Wieling W (1988) Continuous non-invasive blood-pressure monitoring—reliability of Finapres device during the Valsalva maneuver. Cardiovasc Res 22:390–397. https://doi.org/10.1093/cvr/22.6.390
Jahns D (1973) A concept of operator workload in manual vehicle operations. Forschungsinstitut Anthropotechnik, Meckenheim West Germany (Technical report No 14)
Joyner MJ, Charkoudian N, Wallin BG (2010) Sympathetic nervous system and blood pressure in humans individualized patterns of regulation and their implications. Hypertension 56:10–16. https://doi.org/10.1161/hypertensionaha.109.140186
Kachuee M, Kiani MM, Mohammadzade H, Shabany M (2017) Cuffless blood pressure estimation algorithms for continuous health-care monitoring. IEEE Trans Biomed Eng 64:859–869. https://doi.org/10.1109/tbme.2016.2580904
Kavsaoglu AR, Polat K, Bozkurt MR (2014) A novel feature ranking algorithm for biometric recognition with PPG signals. Comput Biol Med 49:1–14. https://doi.org/10.1016/j.compbiomed.2014.03.005
Kelly R, Hayward C, Avolio A, Orourke M (1989) Noninvasive determination of age-related-changes in the human arterial pulse. Circulation 80:1652–1659. https://doi.org/10.1161/01.Cir.80.6.1652
Lu S, Zhao H, Ju K, Shin K, Lee M, Shelley K, Chon KH (2008) Can photoplethysmography variability serve as an alternative approach to obtain heart rate variability information? J Clin Monit Comput 22:23–29
Lyu Y et al (2015) Measuring photoplethysmogram-based stress-induced vascular response index to assess cognitive load and stress. In: Chi 2015: proceedings of the 33rd annual Chi conference on human factors in computing systems. https://doi.org/10.1145/2702123.2702399
Malpas SC et al (2001) The sympathetic nervous system’s role in regulating blood pressure variability. IEEE Eng Med Biol Mag 20:17–24. https://doi.org/10.1109/51.917720
McDuff D, Gontarek S, Picard RW (2014) Remote detection of photoplethysmographic systolic and diastolic peaks using a digital camera. IEEE Trans Biomed Eng 61:2948–2954. https://doi.org/10.1109/tbme.2014.2340991
Meshkati N (1988) Toward development of a cohesive model of workload. Advances in Psychology 52:305–314
Millasseau SC, Kelly RP, Ritter JM, Chowienczyk PJ (2002) Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin Sci 103:371–377. https://doi.org/10.1042/cs1030371
Mosby (2016) Mosby’s medical dictionary. Elsevier, Amsterdam
Mueller SC, Cromheeke S, Siugzdaite R, Boehler CN (2017) Evidence for the triadic model of adolescent brain development: cognitive load and task-relevance of emotion differentially affect adolescents and adults. Dev Cogn Neurosci 26:91–100. https://doi.org/10.1016/j.dcn.2017.06.004
Murray WB, Foster PA (1996) The peripheral pulse wave: information overlooked. J Clin Monit 12:365–377. https://doi.org/10.1007/bf02077634
Otsuka T, Kawada T, Katsumata M, Ibuki C (2006) Utility of second derivative of the finger photoplethysmogram for the estimation of the risk of coronary heart disease in the general population. Circ J 70:304–310. https://doi.org/10.1253/circj.70.304
Oviatt S (2006) Human-centered design meets cognitive load theory: designing interfaces that help people think. In: Proceedings of the 14th ACM international conference on multimedia, pp 871–880
Paas FG, Van Merrienboer JJ (1994) Instructional control of cognitive load in the training of complex cognitive tasks. Educ Psychol Rev 6:351–371
Paas F, Tuovinen JE, Tabbers H, Van Gerven PWM (2003) Cognitive load measurement as a means to advance cognitive load theory. Educ Psychol 38:63–71. https://doi.org/10.1207/s15326985ep3801_8
Padilla JM, Berjano EJ, Saiz J, Facila L, Diaz P, Merce S (2008) Assessment of relationships between blood pressure, pulse wave velocity and digital volume pulse. In: 2006 computers in cardiology
Peter L, Noury N, Cerny M (2014) A review of methods for non-invasive and continuous blood pressure monitoring: pulse transit time method is promising? IRBM 35:271–282. https://doi.org/10.1016/j.irbm.2014.07.002
Pfeifer MA, Weinberg CR, Cook D, Best JD, Reenan A, Halter JB (1983) Differential changes of autonomic nervous-system function with age in man. Am J Med 75:249–258. https://doi.org/10.1016/0002-9343(83)91201-9
Ritter FE, Reifers AL, Klein LC, Schoelles MJ (2007) Lessons from defining theories of stress for cognitive architectures. Integr Mod Cogn Syst 1:254
Romeo RD (2013) The teenage brain: the stress response and the adolescent brain. Curr Dir Psychol Sci 22:140–145. https://doi.org/10.1177/0963721413475445
Shabanah EH, Toth A, Maughan GB (1964) Role autonomic nervous system in uterine contractility + blood flow. 2. Role of parasympathetic neurohormone acetylcholine in uterine motility + blood flow. Am J Obstet Gynecol 89:860. https://doi.org/10.1016/0002-9378(64)90056-0
Sherwood A, Steffen PR, Blumenthal JA, Kuhn C, Hinderliter AL (2002) Nighttime blood pressure dipping: the role of the sympathetic nervous system. Am J Hypertens 15:111–118. https://doi.org/10.1016/s0895-7061(01)02251-8
Shimazu H, Yamakoshi K-I, Kamiya A (1986) Noninvasive measurement of the volume elastic modulus in finger arteries using photoelectric plethysmography. IEEE Trans Biomed Eng 8:795–798
Sweller J, van Merrienboer JJG, Paas F (1998) Cognitive architecture and instructional design. Educ Psychol Rev 10:251–296. https://doi.org/10.1023/a:1022193728205
Takazawa K et al (1998) Assessment of vasoactive agents and vascular aging by the second derivative of photoplethysmogram waveform. Hypertension 32:365–370. https://doi.org/10.1161/01.Hyp.32.2.365
van de Vosse FN, Stergiopulos N (2011) Pulse wave propagation in the arterial tree. Ann Rev Fluid Mech 43:467–499. https://doi.org/10.1146/annurev-fluid-122109-160730
Wang L, Pickwell-MacPherson E, Liang YP, Zhang YT, IEEE (2009) Noninvasive cardiac output estimation using a novel photoplethysmogram index. In: 2009 annual international conference of the IEEE engineering in medicine and biology society, vol 1–20. IEEE engineering in medicine and biology society conference proceedings, pp 1746–1749. https://doi.org/10.1109/iembs.2009.5333091
Wolf S (1997) Primer on the autonomic nervous system—Robertson, D, Low, PA, Polinsky, RJ. Integr Physiol Behav Sci 32:171
Yeh YY, Wickens CD (1988) Dissociation of performance and subjective measures of workload. Hum Factors 30:111–120
Young MS, Brookhuis KA, Wickens CD, Hancock PA (2015) State of science: mental workload in ergonomics. Ergonomics 58:1–17. https://doi.org/10.1080/00140139.2014.956151
Zhang X, Lyu Y, Luo X, Zhang J, Yu C, Yin H, Shi Y (2018) Touch sense: touch screen based mental stress sense. In: Proceedings of the ACM on interactive, mobile, wearable and ubiquitous technologies, vol 2, p 87. https://doi.org/10.1145/3214290
Acknowledgements
This work was supported by Institute for Future Education and Evaluation Tsinghua University (2019IFEE003). We thank all the volunteers for their participation in our experiment. We thank all the reviewers for their insightful comments and helpful suggestions for improving the initial draft of this manuscript.
Author information
Authors and Affiliations
Contributions
XQ, JW contributed equally as first coauthors. YL, YZ contributed conception and design of the study; XQ and JS conducted experiments and organized the database; JW performed the statistical analysis; JW and QX wrote the first draft of the manuscript; JW and QX wrote sections of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xuan, Q., Wu, J., Shen, J. et al. Assessing cognitive load in adolescent and adult students using photoplethysmogram morphometrics. Cogn Neurodyn 14, 709–721 (2020). https://doi.org/10.1007/s11571-020-09617-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11571-020-09617-2