Abstract
During the revision of the Neotropical Fomitiporia species with resupinate basidiomata, several collections from southern Brazil, central Argentina, and French Guiana were found to represent an undescribed species, on the basis of molecular (DNA sequence) and additional morphological and distributional data. This taxon is described and illustrated as Fomitiporia neotropica sp. nov. The species belongs to the Fomitiporia langloisii lineage, the lineage type within Fomitiporia that so far contains only species with resupinate basidiomata spanning exclusively over the Neotropics. Fomitiporia neotropica is morphologically variable regarding the presence/absence of hymenial setae, and secondarily, regarding the pore size. It also inhabits distinct ecosystems characterized by variable moisture regimes. The range of divergent positions in the DNA sequences used in this study (ITS, 28S, partial tef1-α, and rpb2), between specimens from distant origins, are of the same magnitude as those between specimens of other related species, such as F. langloisii, F. dryophila, F. maxonii, or F. mediterranea. A key to the species from the F. langloisii lineage is given.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fomitiporia (Hymenochaetales), typified by F. langloisii (Decock et al. 2007; Murrill 1907), is above all characterized by globose to subglobose, thick-walled, cyanophilous, and dextrinoid basidiospores, in addition to a dimitic (pseudodimitic) hyphal system. Its basidiomata are resupinate to pileate. Cystidioles and hymenial setae are variably present (Fischer 1996). The genus has been segregated into two morphological complexes based on the basidiomata habit: species with pileate basidiomata have been referred to as the F. robusta complex (e.g., F. robusta, F. erecta, F. hippophaeicola); species sharing resupinate basidiomata have been commonly referred to as the F. punctata complex (e.g., F. langloisii, F. punctata, F. pseudopunctata).
The genus has received much attention in the last 10 years, and an understanding of the phylogenetic structure of both morphological complexes has improved considerably. It is now evident that these two complexes have no phylogenetic grounds (Amalfi and Decock 2013; Amalfi et al. 2010, 2012), and that the resupinate and pileate habits are spread throughout the genus. Our understanding of the taxonomic diversity and species distribution range, in all biogeographical areas, has also greatly improved (Amalfi and Decock 2013; Amalfi et al. 2010, 2012; Dai et al. 2008; Decock et al. 2005, 2007; Fischer and Binder 2004; Fischer et al. 2005; Raymundo et al. 2012; Vlasák and Kout 2011; Zhou and Xue 2012). As far as the New World is concerned, the Fomitiporia robusta complex has been addressed by Fischer and Binder (2004), Vlasák and Kout (2011), Amalfi et al. (2012), and Amalfi and Decock (2013). As one of the consequences of these studies, the occurrence of F. robusta sensu stricto (s.s.) in the Americas was brought into question [in our opinion, F. robusta s.s. corresponds to Phellinus robustus var. robustus sensu Domański et al. (1967), or to Phellinus robustus sensu Ryvarden (1978), cf. Amalfi and Decock 2013)]. This species is more likely to be absent from the Americas, where multiple species occur, named or still unnamed, and pertain to various lineages; recent data strongly suggest that the New World supports the highest amount of diversity of species with pileate basidiomata (Amalfi et al. 2012; Amalfi and Decock 2013).
The Fomitiporia punctata complex in the New World has been comparatively less studied. Findings of Fomitiporia punctata sensu “North American auctores” (e.g., Gilbertson and Ryvarden 1987; Lowe 1966) have been reported all over North America. However, the concepts used at that time encompassed, in addition to F. punctata s.s. [e.g., sensu Ryvarden (1978) or Jahn (1967), both circumscriptions drawn on the basis of European specimens and not polluted by data taken from the materials of extra-European origins; in that sense, it corresponds to the F. punctata clade of Amalfi et al. (2010, 2012) and Amalfi and Decock 2013)], at least three other taxa, as demonstrated by Fischer and Binder (2004) and Decock et al (2007). In North America, F. punctata s.s. is more likely restricted to northern and northeastern temperate areas (Boulet 2003; Brazee et al. 2012; Fischer and Binder 2004). Its southern limit of distribution is still uncertain (see Decock et al. 2007), but in all probability the species is absent from the southern (southeastern, southwestern) USA, and a fortiori southward (Decock et al. 2007; Raymundo et al. 2012). Two other species, morphologically distinct and phylogenetically distant from F. punctata s.s., span over the southeastern subtropical belt of the USA or, in a biogeographical perspective, the southeastern and coastal plain, mixed-forest provinces of the subtropical division (Decock et al. 2007). Two historical names were unearthed for these species, viz. F. langloisii, re-instated as the genus type, and F. dryophila. Beyond the United States, F. langloisii and F. dryophila were also spotted southerly in (north) eastern Mexico (Raymundo et al. 2012), which constitutes to date their known southern limit of distribution.
Ryvarden (2004) reported F. punctata as rare in tropical America. Still, however, the literature is rich in reports of F. punctata in the Neotropics (e.g., Carranza-Morse 1992; David and Rajchenberg 1985; Loguercio-Leite and Wright 1991, 1995; Ryvarden 2004, Ryvarden and de Meijer 2002, Wright and Blumenfeld 1984). In light of recent data (Decock et al. 2007; Amalfi and Decock 2013; Amalfi et al. 2012), in the Neotropics, F. punctata are better described as sensu lato or sensu auctores. More likely, the concepts used will prove that this genus encompasses other species, of yet uncertain circumscriptions and perhaps belonging to distinct lineages, apart from F. punctata s.s.
Decock et al. (2007) also addressed the status of F. maxonii, a species poorly known at that time (Ryvarden 2004). This species is more distinctly "tropical" and is nowadays reportedly observed in southern Florida (Vlasák et al. 2011), the Greater Antilles, Mexico, Costa Rica, and southerly, down to Argentina (Decock et al. 2007; Raymundo et al. 2012).
In line with the above idea, pursuing the revision of Fomitiporia in the New World (Amalfi et al. 2012; Amalfi and Decock 2013; Decock et al. 2007; Raymundo et al. 2012), we applied a multilocus phylogenetic approach (based on DNA sequence data of the 5' end of the LSU, ITS-5.8S, partial tef1, and rpb2) to a set of Neotropical specimens with resupinate basidiomata. These specimens were resolved as two distinct clades, representing two distinct phylogenetic species, both within the F. langloisii lineage. One of them, F. neotropica sp. nov., is described below. The second clade is represented by only two specimens and we have chosen to refrain from naming it for the time being.
Materials and methods
Collection localities of the new taxa
Specimens from Argentina were collected in the provinces of Córdoba, Jujuy (Parque Nacional Calilegua) and Misiones. Specimens from Brazil were collected in Rio Grande do Sul, Morrinhos do Sul, Lajeadinho (approx. 29º21'54"S, 49º56'05"W), Itapuã, Parque Estadual de Itapuã (approx 30°21’ – 30°26’S × 50°54’– 51°03’W), Porto Alegre, Refúgio da Vida Silvestre, UFRGS (approx. 30°03’ S, 51°07’W), and Santa Catarina, Florianópolis, Unidade de Conservação Ambiental Desterro-UCAD (approx 27°31'50.8"S, 48°30'44.3"W). The specimen from French Guiana was collected in the CNRS “inselberg” research plots, Nouragues Natural Reserve (approx. 04°05.5'N, 52°40.6'W, http://www.nouragues.cnrs.fr/F-inselberg.html). Voucher herbarium specimens of the new species are preserved at CORD, ICN and MUCL, with a duplicate of type material deposited at NY (herbarium acronyms are according to Thiers, continuously updated). The authors isolated the strains during fieldwork, from fresh basidiomata tissues; they were then plated on malt extract agar supplemented by 2 ppm benomyl (benlate) and 50 ppm chloramphenicol (Untereiner et al. 1998). Cultures were later purified in the laboratory in case of persistent bacterial contamination. Living cultures of the new species are preserved at MUCL with a duplicate at ICN. A duplicate of the ex-type strain is preserved also at the CBS (The Netherlands) (culture collection acronym according to the World Federation for Culture Collections, http://www.wfcc.info/ccinfo/collection/by_country/b/).
Morphology and anatomy
Basidiomata colors are described according to Kornerup and Wanscher (1981). Basidiomata sections were examined in Melzer's reagent, lactic acid Cotton Blue (Kirk et al. 2001), and KOH 4 %. All microscopic measurements were done in Melzer's reagent. In presenting the size range of the microscopic elements, 5 % of the measurements were excluded from each end and are given in parentheses, ave = arithmetical mean, Q = ratio of length/width of basidiospores, and aveQ = arithmetical mean of the ratio Q. Thirty samples each of pores, basidiospores, and setae were measured from each specimen.
Sequencing
One hundred and nine specimens and cultures representing 40 species (or potential species clades) were included in the phylogenetic analysis. The materials and sequences used in this study are listed in Table 1. As a rule, DNA was extracted from pure culture, except when noted (Table 1). DNA extraction, amplification and sequencing of the 5' end of the nuclear ribosomal LSU rRNA gene, the ITS regions (including 5.8S), the partial tef1-α gene, and the region between domains 6 and 7 of the second largest subunit of the rpb2 (Frøslev et al. 2005; Matheny 2005) were as described in Decock et al. (2007), Amalfi et al. (2010, 2012), and Amalfi and Decock (2013).
Phylogenetic analysis
The nucleotide alignment deposited at TreeBASE under study accession number "http://purl.org/phylo/treebase/phylows/study/TB2:S12874" (Amalfi and Decock 2013) was used as a starting dataset to align the additional sequences. Ambiguously aligned segments were also detected with the Gblocks 0.91b program (Castresana 2000; http://molevol.cmima.csic.es/castresana/ Gblocks.html) with settings “ALLOW SMALLER FINAL BLOCKS”, “ALLOW GAPS WITHIN BLOCKS”. The alignment was screened visually to detect additional ambiguously aligned regions. Alignments are deposited at TreeBASE under study accession number XXXX (http://purl.org/phylo/treebase/phylows/study/TB2:S12298). Indels present within our datasets, especially in the ITS1 region (Decock et al. 2007), were recoded as binary characters with the simple indel coding method (SIC, Simmons and Ochoterena 2000), as implemented in SeqState software (Müller 2005).
Phylogenetic analyses were performed separately for each locus and concatenated with (1) maximum parsimony, as implemented in PAUP* 4.0b10 (Swofford 2003), (2) Bayesian inference, as implemented in MrBayes 3.1.2 (Ronquist and Huelsenbeck 2003), and (3) Maximum likelihood (ML), as implemented in RAxML 7.0.4 (Stamatakis et al. 2008). Phellinus uncisetus was designated as the outgroup (Decock et al. 2007). The most parsimonious trees for each dataset were identified with heuristic searches performed with 1,000 random addition sequences, further evaluated by bootstrap analysis, retaining clades that were compatible with the 50 % majority rule in the bootstrap consensus tree. Analysis conditions were tree bisection and reconnection and addition branch swapping (TBR), with the starting tree obtained via stepwise addition, and the steepest descent not in effect, with MULTREES conditions effective. Models of evolution for Bayesian inference were estimated with the Akaike information criterion (AIC), as implemented in Modeltest 3.7 (Posada and Crandall 1998). The dataset was subdivided into 10 data partitions: ITS1, 5.8S, ITS2, nucLSU, tef1 1st and 2nd codon position, tef13rd codon position, tef1 introns, rpb2 1st and 2nd codon position, rpb2 3rd codon position, and the recoded indels (Table 2). The best-fit models for each partition were implemented as partition-specific models within partitioned mixed-model analyses of the combined dataset. Three optimal models of nucleotide substitution were selected for the ITS regions, the GTR + G model was used for ITS1, and the HKY + G model was used for ITS2, while the K80 model was used for 5.8S. The GTR + I + G model was used for the nucLSU dataset, for the rpb2 region (for the first and second codon, and for the third codon position), and for the first and second codon position of the tef1 region, while the GTR + G model was used for the third codon position and HKY + I was used for the tef1 introns dataset. For analysis of SIC data under Bayesian inference, we used the MrBayes restriction site model (F81–like), as recommended by Ronquist et al. (2005). All parameters were unlinked across partitions. Bayesian analyses were implemented with two independent runs, each with four simultaneous independent chains for 8,000,000 generations, starting from random trees, and keeping one tree every 1,000th generation. To detect topological conflicts among data partitions, we compared the nodes between the majority rule consensus trees obtained in the parsimony analysis from the individual datasets. Paired trees were examined for conflicts only involving nodes with bootstrap support values (BS) ≥ 70 % (Lutzoni et al. 2004; Mason-Gamer and Kellogg 1996; Reeb et al. 2004). A conflict was assumed to be significant if two relationships for the same set of taxa (one being monophyletic and the other non-monophyletic) were observed between trees. For Bayesian inference and ML analyses, congruence was tested by inspecting internodes with posterior probabilities ≥ 95 % resulting from the separate Bayesian and ML analyses (as outlined in Miadlikowska and Lutzoni 2004; Moncalvo et al. 2006).
Maximum likelihood (ML) searches conducted with RAxML involved 1,000 replicates under the GTRGAMMAI model, with all model parameters estimated by the program. In addition, 1,000 bootstrap (ML BS) replicates were run with the same GTRGAMMAI model. We provided an additional alignment partition file to force the RAxML software to search for a separate evolution model for each dataset, including the recoded indels.
Results
DNA sequence comparisons
Sequence length and parsimony data for each dataset (length of aligned sequences, variable parsimony uninformative positions, parsimony informative positions, excluded characters) are summarized in Table 2. As already evidenced by Decock et al. (2007) and confirmed by Amalfi et al. (2012), the species forming the F. langloisii lineage, namely F. dryophila, F. langloisii, F. maxonii, F. sonorae, an unnamed Fomitiporia sp. MUCL 46181, and Fomitiporia sp. MUCL 53675, present a 31-bps-long deletion near the 5’ end of the ITS1 region. An identical deletion (in terms of length and position) is present in the ITS1 region of Fomitiporia sp. MUCL 53114, MUCL 51335, MUCL 51336, MUCL 54206, MUCL 54196, and MUCL 54212. This deletion seems to be a plesiomorphic character of the F. langloisii lineage.
Individual dataset comparisons
Sequence data and statistical analysis for each dataset and combined analysis have been provided (Table 2).
By comparing the tree topologies obtained for the individual datasets, no conflict involving significantly supported nodes was found using the reciprocal 70 % BP, 95 % PP, and ML BS criteria; the datasets were therefore combined.
Combined dataset analysis
Thirty-six characters in the ITS1 region were judged as too ambiguous to be aligned. Of the remaining 4,429 characters, 255 were variable but parsimony uninformative, and 1,352 were parsimony informative. The two Bayesian runs converged to stable likelihood values after 930,000 generations, and 7,070 stationary trees from each analysis were used to compute a 50 % majority-rule consensus tree in PAUP* to calculate posterior probabilities (PP). In the ML searches with RAxML, the combined dataset alignment had 1,980 distinct patterns, with a proportion of gaps and undetermined characters of 13.06 %.
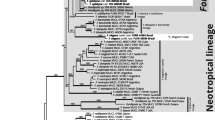
The heuristic search produced 1,280 equally-most parsimonious trees (4,024 steps long; CI 0.495, RI 0.849, RC 0.420), representing one main topology. This topology is congruent with published trees (Amalfi et al. 2010, 2012; Amalfi and Decock 2013) and is highly concordant with the topologies obtained by analyzing the individual dataset, and is almost identical to the Bayesian consensus tree and to the optimal tree inferred under the Maximum likelihood criterion (−lnL = 26969.634 , Fig. 1). The results of the phylogenetic inferences, independently of analyzing the datasets individually or combined, were highly congruent and resolve the same clades and lineages as previously reported (Amalfi and Decock 2013; Amalfi et al. 2010, 2012).
The 50 % majority-rule consensus tree from Bayesian inference of the combined ITS, LSU, tef1, and rpb2 sequences. Black dots on branches represent BPP, ML BS, and BS rates of 99 % or higher; grey dots on branches denote BPP greater than 95 % and ML BS greater than 85 %, but BS support lower than 50 %
Seven collections sharing resupinate basidiomata and originating from the Neotropics (viz. MUCL 53114, MUCL 51335, MUCL 51336, MUCL 54206, MUCL 54196, and MUCL 54212) clustered together in a new, well-supported terminal clade (Fig. 1, PS1). Two additional collections from French Guiana and Argentina formed a second well-supported terminal clade (Fig. 1, PS2). Both nest at the base of the F. langloisii lineage, as defined by Decock et al. (2007) and confirmed by Amalfi et al. (2012) and Amalfi and Decock (2013).
Morphological analysis
Morphological examinations of our collections belonging to this new Neotropical clade PS1 revealed no absolute combination of morphological features, thereby clearly defining one morphospecies. Some morphological parameters usually accounting as critical species descriptors were variable, or overlapped with those of other species. These included the number of pores / mm (and the pore size), the basidiospore size, and the presence / absence of hymenial setae.
The number of pores / mm ranges mostly from 6–9, with the extremes of 5 and 12. The pore diameter ranges from (70–) 75 to 145 (−150) μm, and the individual (specimen) averages range from 86 μm to 120 μm. Only 12 % of the pores are larger than 120 μm in diameter. The basidiospores are typical of Fomitiporia: subglobose to obovoid, slightly thick-walled, dextrinoid, and cyanophilous. Their size range is mainly 5.0–7.0 × 4.5–7.0 μm (ave = 5.9 × 5.7 μm). It overlaps considerably with the basidiospore size range of all other species of the F. langloisii lineage (Decock et al. 2007) except F. dryophila, for which the basidiospores are larger (6.2–8.0 (−8.5) × 5.7–7.3(−7.5) μm; ave = 7.0 × 6.5 μm, Decock et al. 2007).
Hymenial setae were observed in two specimens only, viz. MUCL 51335 and MUCL 51336 (cf. list of specimens examined below), both originating from Argentina. They are variably abundant, however: numerous in MUCL 51335 and scattered in MUCL 51336. In both specimens, they are mostly present in the dissepiment areas. They are also variable in shape, and are often apically rounded (Fig. 3).
Discussion and taxonomic conclusions
Within the Hymenochaetaceae, the presence/absence, shape, and size of setae (hymenial or extra-hymenial) have been regarded as confident taxonomic features to delimit species boundaries (e.g., David et al. 1982). In Fomitiporia, however, this might not be always so absolute. The presence of hymenial setae seems to be a very constant character in several species, such as F. tenuis (Decock et al. 2005, Cony Decock pers. obs.), F. bannaensis (Dai et al. 2001, Cony Decock pers. obs.), or F. spinescens (Coelho and Wright 1996; Coelho et al. 2009; Ryvarden 2004, Cony Decock pers. obs.). However, in others, the presence of setae, hymenial or extra-hymenial, may be variable and should not be considered as a critical parameter defining morphospecies. Such cases exist in the F. punctata and F. robusta species complexes. It concerns, for instance, F. aethiopica, F. pseudopunctata, or F. polymorpha, and F. robusta or F. erecta.
Decock et al. (2005) first segregated collections from the Ethiopian highlands into two species based on the presence/absence of hymenial setae: F. aethiopica, for the asetose specimens, and F. pseudopunctata for the setose specimens. Nevertheless, a subsequent multilocus phylogenetic approach revealed that asetose and setose Ethiopian specimens together formed a monophyletic clade (Amalfi et al. 2010). Both asetose and setose specimens were, in fact, conspecific and belonged to F. aethiopica (Amalfi et al. 2010). Therefore, the species concept had to be redefined to include variably present and variably shaped hymenial setae (Amalfi et al. 2010).
The case of F. pseudopunctata / F. mediterranea is worth also discussing. Fomitiporia pseudopunctata was described and differentiated from F. punctata on the basis of the presence/absence of hymenial setae, respectively (David et al. 1982). Molecular data confirmed that F. pseudopunctata and F. punctata represent two distinct taxa (Amalfi et al. 2010, 2012). They also have globally-disjointed distribution ranges in Europe (though overlap is possible locally).
Fomitiporia mediterranea was originally described based on specimens mainly originating from the Mediterranean areas, found on Vitis vinifera, and related to Esca disease in Southern Europe (Fischer 2002). It was later shown to be a widespread species in southern Europe on multiple wild and cultivated hosts (e.g., Citrus, Corylus, Platanus, Vitis, Fischer 2002; Pilotti et al. 2005, 2010). Fomitiporia mediterranea was also distinguished from F. pseudopunctata based on the absence of setae (Fischer 2002).
However, our phylogenetic inferences, carried out from a multilocus dataset, showed that specimens identified as F. pseudopunctata (setose) and as F. mediterranea (asetose) from Southern France and Italy could be merged together with the reference material of F. mediterranea AFTOL ID688 into a single, monophyletic clade. Furthermore, analysis of a large set of ITS sequences of F. mediterranea (mostly retrieved from GenBank), originating from its known distribution areas and multiple hosts, and of F. pseudopunctata gathered from wild hosts in southern Europe, resulted in a single, monophyletic clade. The F. pseudopunctata entries are dispersed within the “F. mediterranea” entries (data not shown). These results strongly suggest that these two binomials in fact cover a single taxon, for which the nomenclaturally correct name is F. pseudopunctata. The presence of hymenial setae is also a non-critical character with which to morphologically define this species.
Fomitiporia polymorpha was described based on combined morphological and molecular data (Fischer and Binder 2004). The occurrence of hymenial setae was emphasized also as variable among specimens, ranging from absent to scattered.
Several species with pileate basidiomata also present such variability as far as hymenial or extra-hymenial setae are concerned. In Europe, they include F. robusta (David et al. 1982; Domański et al. 1967; Ryvarden 1978). Pieri and Rivoire (2000) also pointed out the presence/absence of both hymenial and extra-hymenial setae in F. erecta/Phellinus juniperinus. However, as stated by Amalfi et al. (2012), an integrating approach has never been applied to the European species, and would be necessary to tackle the F. robusta/F. erecta/Ph. juniperinus/Ph. rosmarini complex in Europe. In North America, F. texana shows a similar variable presence of setae (Raymundo et al. 2012).
In our case, two setose (MUCL 51335 and MUCL 51336) and five asetose specimens (MUCL 49549, MUCL 54206, MUCL 54196, MUCL 54212, and MUCL 54246) clustered within the same monophyletic terminal clade (PS1, Fig. 1). The range of divergent positions in the DNA sequences used in this study (ITS, 28S, partial tef1-a, and rpb2), among specimens from distant origins (from French Guiana to Argentina), are of the same magnitude as that among specimens of other species of this lineage, such as F. langloisii, F. dryophila, or still, F. maxonii. Furthermore, the two setose specimens did not form a two-specimen subclade within this clade PS1; MUCL 51335 is genetically more closely related to MUCL 54246 than to MUCL 51336.
Given that our collections, setose or asetose, form a single clade representing a phylogenetic unit, and notwithstanding the morphological variability, we interpret this as a single species. The search for a possible epithet within the synonyms of F. punctata (Decock et al. 2007; Ryvarden 1991; Robert et al. 2005) yielded no name that could apply to it. This species is therefore proposed below as F. neotropica sp. nov.
Taxonomy
Fomitiporia neotropica Campos-Santana, Amalfi, R.M. Silveira, Robledo et Decock, sp. nov. Figs. 2, 3 and 4
Fomitiporia neotropica, Basidiomata in situ. A, B. Actively growing, single-layered basidiomata in high moisture environment, French Guiana, MUCL 53114 (A, scale bar = 5 cm; B, scale bar = 2 cm). C. Argentina, older, drier basidiomata in environment with seasonal drought dryness, MUCL 49549 (C, scale bar = 5 cm)
Mycobank: MB805940
Etymology: neotropica, from the distribution areas in the Neotropics.
Basidiomata resupinata, effusa; pororum facies griseo-brunnea vel brunnea; pori circulari, 6–9 per mm, (70–) 75–125 (−150) μm diam; tubi stratosi, ad 3.5 mm longi, in series ad min. 2 singula strata, 1.0–3.5 mm crassa; systema hypharum dimiticum; hyphae generatoriae afibulatae, hyalinae ad pallido-luteae; hyphae skeletales flavo-brunneae, crassitunicatae, aseptatae; setae hymeniales presentes ad ausentes, ferrugineo-fuscae, subulatae vel leviter ventricosae, rectae, apice acutae vel retundatae, 10.0–30.0 × 3.5–6.5 (−7.0) μm; basidiosporae subglobosae vel globosae, leviter crassitunicatae, hyalinae, dextrinoideae, cyanophileae, 5.0–7.0 (−7.5) × 4.5–7.0 μm.
Holotypus hic designatus: Argentina, Provincia Jujuy, Calilegua Nationalis hortus, in mortuo stipite ignotae angiospermia, IV 2008, M. Amalfi, AR 7508, in herbarium MUCL 51335; isotypus in herbaria NY et CORD.
Basidiomata seasonal to at least bi-seasonal, resupinate, effused, following the substrate, adnate, extending up to 30 cm long, 14 cm wide, 1.5–5 mm in the thickest part, with a corky consistency when fresh, drying hard corky, with a densely fibrous texture; margin up to 0.5–2 mm wide, narrow, densely and very minutely velutinous, becoming slightly indurate on aging in multilayered specimens, from outside–inside white, pale yellow (3A[3–4], greyish to brownish orange (5[B–C][4–6], (5C[6–7], yellow ochre, caramel), light to dark brown on aging (6[D–E]6 cinnamon, 6 F(7–8); pore surface in brown shade, commonly yellowish brown (5E[4–5], greyish brown, dark blonde, 5D[ 3–4]) to darker brown (6E[4–6], greyish chocolate brown, or 6 F4, dark brown); pores small, round to ellipsoid at inclined parts, mostly 6–9 / mm (range: 5–12), (70–) 75–125 (−150) μm diam (ave = 119 μm); dissepiments entire, thin to thick, 20–110 μm diam (ave = 55 μm), commonly with free hyphal tips, sometimes with a greyish tint due to crystal deposit; subiculum 0.3–1.0 mm thick, densely fibrous, golden to light brown (5D[6–7], 5E8), homogeneous or with some denser, black, continuous or discontinuous thin lines; tube uni- or bi-layered, the layers indistinct or separated by a thin, slightly darker layer of sterile mycelium, 1.0–3.5 mm thick each, totalling up to 4 mm thick, concolorous with the pore surface.
Hyphal system dimitic, identical in the context of hymenophoral trama; generative hyphae hyaline to faintly yellow, thin-walled, sparsely branched, 1.5–2.5 (−3) μm wide (ave = 2.0 μm), skeletal hyphae pale yellow brown to golden brown, thick-walled, but with an open lumen, 2.5–4.0 μm wide (ave = 3.2 μm), with occasional local, intercalary or terminal swellings, tightly packed in the hymenophoral trama, with sub-parallel orientation.
Hymenium: basidia pyriform to subglobose, 7.5–9.5 × 7.0–9.5 μm, with four small sterigmata; basidioles identical in shape but slightly smaller; hymenial setae variably present, from absent to abundant, fusiform to slightly ventricose, slightly lageniform, apex pointed to rounded, 10.0–30.0 × 3.5–6.5 (−7.0) μm (ave = 16.8 × 4.6 μm), Q = 2.08–5.71 (aveQ = 3.7); basidiospores subglobose to broadly obovoid, 5.0–7.0 (−7.5) × 4.5–7.0 μm (ave = 5.9 × 5.7 μm), Q = 1–1.2 (aveQ = 1.05), thick-walled, the wall hyaline, cyanophilous, strongly dextrinoid; chlamydospore absent.
Type of rot: white rot;
Substrate and hosts: dead fallen branches, occasionally still attached to the tree, dead trunk, or living branches; Schinus sp. (Anacardiaceae), unidentified angiosperms.
Specimens examined: ARGENTINA: Córdoba, San Justo, Miramar, Mar Chiquita, approx 30°55'59.1"S, 62°42'17.1"O, elev. approx. 76 m, on a stem and dead branches attached to the tree, Schinus sp., Anacardiaceae, 29 Jul 2007, Robledo 1713 (MUCL 49549; culture ex-MUCL 49549); Jujuy province, Parque Nacional Calilegua, sendero La Junta, on a dead, standing trunk, unidentified angiosperm, Apr. 2008, M. Amalfi, AR 7508 (holotype, MUCL 51335; isotype NY, culture ex-holotype MUCL 51335, CBS); sendero La Junta, Apr 2008, M. Amalfi, AR 7608 (MUCL 51336, culture ex-MUCL 51336). BRAZIL: Rio Grande do Sul, Morrinhos do Sul, Lajeadinho, approx 29º21'54"S, 49º56'05"W, elev. approx. 180 m, on a dicotyledonous dead wood, unidentified angiosperm, 13 Mar 2010, Campos-Santana 030/10 (ICN 190598; culture ex-MUCL 54196); Santa Catarina, Florianópolis, Unidade de Conservação Ambiental Desterro-UCAD, approx 27°31'26.4"S, 48°30'31,7"W, elev. approx 198 m, on a dicotyledonous dead wood, unidentified angiosperm, 02 Oct 2010, Campos-Santana 253/10 (ICN 190599; culture ex-MUCL 54206); Rio Grande do Sul, Itapuã, Parque Estadual de Itapuã, approx. 30°27’S – 30°20’S; 51°03’S – 50°50’W, elev. approx. 192 m, on a stem and dead branches attached to the tree, unidentified angiosperm, 16 Oct 2010, Campos-Santana 319/10 (ICN 190600; culture ex-MUCL 54212); Rio Grande do Sul, Porto Alegre, Refúgio da Vida Silvestre da UFRGS, approx. 30°03’ S, 51°07’W, elev. approx. 130 m, on a dead wood, unidentified angiosperm, 16 Aug 2011, Campos-Santana 644/11 (ICN 190601; culture ex-MUCL 54246). FRENCH GUIANA, MUNICIPALITY OF REGINA: Nouragues Natural Reserve, CNRS "inselberg" research plots, “grand Plateau”, approx. 04°05.5' N, 52°40.6' W, elev. approx. 120 m, on a dead fallen branch, unidentified angiosperm, 04 Aug 2010, C. Decock, FG-10-263 (MUCL 53114, culture ex-MUCL 53114); same locality, on a dead fallen branch, unidentified angiosperm, 21 Jul 2013, C. Decock, FG-13-789 (MUCL 55071, culture ex-MUCL 55071).
Comments
Fomitiporia neotropica is characterized by the combination of a resupinate, effused, seasonal to at least bi-seasonal basidiomata, a white to yellow margin when fresh, a brown (light to dark brown) pore surface, small pores [mostly 6–9 / mm, (70–) 75–125 (−150) μm diam, ave = 119 μm], the occasional presence of irregularly shaped setae, mostly located in the dissepiments, 10.0–30.0 × 3.5–6.5 (−7.0) μm), and basidiospores whose average size ranges from 5.4–6.3 × 5.0–6.1 μm (arithmetic mean of the individual averages = 5.9 ± 0.25× 5.7 ± 0.3 μm, n = 7).
The most common phenotype within the specimens examined (six specimens, from distinct geographic origins) is characterized by the lack of setae; the setose phenotype (two specimens from the same forest area in Argentina) seems to be the exception. We should not exclude, however, cases of extreme paucity of setae, which makes them difficult to observe. This unbalanced ratio seems also to be the case for other species with variable presence of setae, such as F. aethiopica (Amalfi et al. 2010; Decock et al. 2005), F. polymorpha (Fischer and Binder 2004), or F. robusta (Ryvarden and Gilbertson 1994); the setose phenotype remains the exception for these taxa. It is unknown whether these phenotypes correspond to different genetic backgrounds, in which case this could not be evidenced by the genome loci examined, or if they represent differential expressions of a shared genotype, under different environmental circumstances. More data would be necessary to answer these questions.
Fomitiporia neotropica has been recorded from living and dead (then attached or fallen) branches. In central Argentina, it has been found growing on living stems and dead branches of Schinus sp. Its distribution range encompasses various ecological zones and extends from the (very) humid rainforest in French Guiana to the semi-deciduous Atlantic Forest in southern Brazil, and the subtropical, seasonally drier forests of NE Argentina. This suggests a wide ecological amplitude, and perhaps a more widespread distribution in South America. French Guiana represents, for the time being, the northern extreme of its known distribution. The southernmost known localities are in subtropical areas of NE Argentina.
From a phylogenetic perspective, the species belongs to the F. langloisii lineage (Decock et al. 2007; Amalfi et al. 2012; Amalfi and Decock 2013). This lineage contains, in addition to F. langloisii and F. neotropica, the taxa F. dryophila, F. maxonii, F. sonorae, and a still unnamed species that is observed in two collections originating from Argentina and French Guiana. This lineage more likely originates from and is endemic to the Neotropics. It is distributed from the subtropical belt of southeastern USA down to Argentina and southern Brazil. All the species from this lineage share an identical, apomorphic deletion of 31 bp near the 5’ end of the ITS1 region (Amalfi and Decock 2013; Amalfi et al. 2012; Decock et al. 2007), most likely inherited from a common ancestor.
Fomitiporia neotropica should be compared to the species of the F. langloisii lineage, and to a lesser degree to F. punctata. In a morphological and biogeographical perspective, F. maxonii should be compared to F. neotropica. Both species are very similar except for the presence of setae, which have never been reported in F. maxonii (Decock et al. 2007; Ryvarden 2004; Vlasák et al. 2011). However, the distinction among asetose specimens of F. neotropica and F. maxonii proved challenging; for the time being, we have been unable to detect any unequivocal, classical morphological characters that could be used confidently to differentiate the two species in the absence of setae. Subtle differences may include free hyphal tips in the dissepiments (non-agglutinated), occasionally covered with crystals in F. neotropica, while the hyphal tips are agglutinated in F. maxonii. This should be ascertained by examining more specimens in fresh conditions; the drying process may modify the aspect of the dissepiments. Both species also share some ecological parameters, including the substrate (attached or fallen, dead branches, or living stems). Their distribution ranges overlap in South America; both species have at least been reported in Argentina [An Argentinean specimen of F. maxonii has been examined, the identity of which has been confirmed by DNA sequence comparisons: ARGENTINA: Iguazú national park, 25°41.12’ S – 54°26.8’ W, on a dead branch attached to a living tree, approx. 2 m above ground, unidentified angiosperm, Apr 2008, M. Amalfi Ar 3008, MUCL 51331, culture ex.- MUCL51331]. Fomitiporia maxonii jumps to exotic, cultivated hosts such as Citrus sp. (Decock et al. 2007), a feature not yet registered for F. neotropica.
Fomitiporia sonorae is a little-known species with hymenial setae, reported in southern USA and northern Mexico (Gilbertson and Ryvarden 1987; Raymundo et al. 2012). It is distinguished from F. neotropica by larger pores (5–6/mm against 6–9/mm) and longer setae (20–44 μm long against 10.0–30.0 μm, Gilbertson and Ryvarden 1987; Raymundo et al. 2012). Fomitiporia sonorae and F. neotropica also have different ecological requirements and geographic distributions. Fomitiporia sonorae is known in the distinctly drier ecosystems of Arizona (southern USA), where it was collected on hop bushes (Dodonaea viscosa, Sapindaceae) (Gilbertson and Ryvarden 1987). It has been recorded from two localities in northeastern Mexico (Raymundo et al. 2012), but this finding still needs molecular confirmation.
Fomitiporia dryophila differs from F. neotropica in having typically cushion-shaped to pseudopileate basidiomata, and distinctly larger basidiospores: (5.5–) 6.2–8.0 (−8.5) × (5.0–) 5.7–7.3(−7.5) μm (ave = 7.0 × 6.5 μm) (Decock et al. 2007). Fomitiporia langloisii shares with F. neotropica the basidiospore size, mostly 5.3–6.7 × 4.8–6.0 μm (ave = 6.0 × 5.5 μm) and 5.0–7.0 × 4.5–7.0 μm (ave = 5.9 × 5.7 μm), respectively (Decock et al. 2007). Fomitiporia langloisii has a much paler pore surface, greyish corky and honey-colored, sometimes with a faint pinkish tint in young specimens (Decock et al. 2007; Raymundo et al. 2012); in this feature it differs from F. neotropica. The distribution ranges of F. langloisii, F. dryophila, and F. neotropica are incompletely known. However, considering their current known distribution (Decock et al. 2007; Raymundo et al. 2012, Vlasák continuously updated http://mykoweb.prf.jcu.cz/polypores/index.html), habitats, and related ecological parameters, it is unlikely that the distribution range of F. langloisii and F. dryophila would overlap with that of F. neotropica. Fomitiporia langloisii and F. dryophila are sympatric in the subtropical, southeastern belt of the USA (Decock et al. 2007), or in biogeographical terms, the southeastern, coastal plain, mixed-forest provinces of the subtropical division. Both species were also recorded southerly, in northeastern Mexico (Raymundo et al. 2012). Fomitiporia dryophila seems to grow preferably on Quercus sp., but other hosts are reported (Decock et al. 2007; Raymundo et al. 2012, Vlasák continuously updated http://mykoweb.prf.jcu.cz/polypores/index.html), whereas F. langloisii has a wider host range.
Fomitiporia punctata differs morphologically from F. neotropica in having distinctly larger basidiospores. The basidiospore size range of F. punctata, as usually reported in northern-central Europe, North American, or temperate Asia, is 6.5–8.5 x 5.5–7.0 μm (Bernicchia 1990, 2005; Boulet 2003; Dai 1999; Domański 1972; Fischer and Binder 2004; Gilbertson and Ryvarden 1987; Núñez and Ryvarden 2000; Pieri and Rivoire 2000; Ryvarden and Gilbertson 1994). Ecologically, F. punctata inhabits distinct, temperate ecosystems of the northern hemisphere. Furthermore, F. punctata belongs to the Holartic lineage, which is distant from the F. langloisii lineage (Amalfi and Decock 2013; Amalfi et al. 2012).
Tentative keys to the Fomitiporia species of the F. langloisii lineage, including F. punctata
1a Basidiospores average 6.9–7.2 μm long; upper range to 8.5 μm; basidiomata cushion-shaped……….........................2
1b Basidiospores average < 6.5 μm (5.0–6.2 μm); upper range < 7 μm; basidiomata effused……………...............................3
2a Pore surface brown; temperate areas of North America…………………………………........….F. punctata
2b Pore surface pale-colored; margins indurate, blackish with age; subtropical, southeastern USA, eastern Mexico……………………...…………………..F. dryophila
3a Hymenial setae present…………………….....…………4
3b Hymenial setae absent……………………………………5
4a Pores 5–6 / mm; occurring in semi-desert area of southern USA / eastern Mexico……….............................…F. sonorae
4b Pores 6–11 / mm; known from eastern South America, humid forest……….......……................……….F. neotropica
5a Pore surface pale, cork-colored, honey; southeastern USA, eastern Mexico..………................................……F. langloisii
5b Pore surface darker, greyish brown to chocolate brown…………………………………………...........…….6
6a Dissepiments agglutinated...................................F. maxonii
6b Dissepiments with free hyphal tips (occasionally with a greyish tint due to crystal deposit)..................…F. neotropica
References
Amalfi M, Decock C (2013) Fomitiporia castilloi sp. nov. and evidence for multiples clades around F. apiahyna in Meso- and South America, representing potential species. Mycologia 105:873–887. doi:10.3852/11-423
Amalfi M, Yombiyeni P, Decock C (2010) Fomitiporia in sub-Saharan Africa: morphology and multigene phylogenetic analysis support three new species from the Guineo-Congolian rainforest. Mycologia 102:1303–1317. doi:10.3852/09-083
Amalfi M, Raymundo T, Valenzuela R, Decock C (2012) Fomitiporia cupressicola sp. nov., a parasite on Cupressus arizonica, and additional unnamed clades in the southern USA and northern Mexico, determined by multilocus phylogenetic analyses. Mycologia 104:880–893. doi:10.3852/11-196
Bernicchia A (1990) Polyporaceae s.l. in Italia. Istituto di Patologia Vegetale, Bologna, pp 1–594
Bernicchia A (2005) Polyporaceae s.l. Fungi Europaei 10, ed. Candusso, Italy: pp 1–808
Boulet B (2003) Les champignons des arbres de l'est de l'Amérique du Nord. Les publications du Québec, Québec, pp 1–727
Brazee NJ, Lindner DL, Fraver S, D’Amato AW, Milo AM (2012) Wood-inhabiting, polyporoid fungi in aspen-dominated forests managed for biomass in the U.S. Lake States. Fung Ecol 5:600–609. doi:10.1016/j.funeco.2012.03.002
Carranza-Morse J (1992) Pore fungi of Costa Rica. II. Mycotaxon 43:351–369
Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analyses. Mol Biol Evol 17:540–552
Coelho G, Wright JE (1996) Phellinus spinescens sp. nov. on bamboo from South America. Mycotaxon 59:383–387
Coelho G, da Silveira RMB, Guerrero RT, Rajchenberg M (2009) On poroid Hymenochaetales growing on bamboos in Southern Brazil and NE Argentina. Fungal Divers 36:1–8
Dai Y-C (1999) Phellinus sensu lato (Aphyllophorales, Hymenochaetaceae) in East Asia. Acta Bot Fenn 166:1–115
Dai Y-C, Zhang X-Q, Zhou T-S (2001) New and noteworthy species of Hymenochaetaceae from China. Mycosystema 20:16–21
Dai Y-C, Cui BK, Decock C (2008) A new species of Fomitiporia (Hymenochaetaceae, Basidiomycota) from China based on morphological and molecular characters. Mycol Res 112:375–380. doi:10.1016/j.mycres.2007.11.020
David A, Rajchenberg M (1985) Pore fungi from French Antilles and Guiana. Mycotaxon 22:285–325
David A, Dequatre B, Fiasson JL (1982) Two new Phellinus with globose, cyanophilous spores. Mycotaxon 14:160–174
Decock C, Bitew A, Castillo G (2005) Fomitiporia tenuis and Fomitiporia aethiopica (Basidiomycetes, Hymenochaetales), two undescribed species from the Ethiopian Highlands: taxonomy and phylogeny. Mycologia 97:124–132. doi:10.3852/mycologia.97.1.121
Decock C, Herrera Figueroa S, Robledo G, Castillo G (2007) Fomitiporia punctata (Basidiomycota, Hymenochaetales) and its presumed taxonomic synonyms in America: taxonomy and phylogeny of some species from tropical/subtropical areas. Mycologia 99:733–752. doi:10.3852/mycologia.99.5.733
Domański S (1972) Basidiomycetes: Aphyllophorales. Polyporaceae I. (resupinatae), Mucronoporaceae I (resupinatae). US Department of Agriculture Foreign Scientific Publication, Washington DC
Domański S, Orlos SH, Skirgiello A (1967) Grzyby (Zagwiowate II, Szczeciniakowate II). - Panstwowe Wydawnictwo Naukowe, Poland: pp 1–395 (english translation: 1973 - Fungi: Polyporaceae II (pileatae), Mucronoporaceae II (pilaetae) - U.S. Dep. Commerce, Springfield, USA: pp 1–332)
Fischer M (1996) On the species complexes within Phellinus: Fomitiporia revisited. Mycol Res 100:1459–1467
Fischer M (2002) A new wood-decaying Basidiomycete species associated with Esca of grapevine: Fomitiporia mediterranea (Hymenochaetales). Mycol Prog 1:315–324
Fischer M, Binder M (2004) Species recognition, geographic distribution, and host-pathogen relationships: a case study in a group of lignicolous Basidiomycetes, Phellinus s.l. Mycologia 96:799–811
Fischer M, Edwards J, Cunnington JH, Pascoe IG (2005) Basidiomycetous pathogens on grapevine: a new species from Australia - Fomitiporia australiensis. Mycotaxon 92:85–96
Frøslev TG, Matheny PB, Hibbett DS (2005) Lower level relationships in the mushroom genus Cortinarius (Basidiomycota, Agaricales): a comparison of RPB1, RPB2 and ITS phylogenies. Mol Phylogenet Evol 37:602–618. doi:10.1016/j.ympev.2005.06.016
Gilbertson RL, Ryvarden L (1987) North American Polypores. Part 2. Megasporoporia – Wrightoporia. Fungiflora, Oslo, pp 433–885
Jahn H (1967) Die resupinaten Phellinus-Arten in Mitteleuropa. Westf Pilzbr 6:37–124
Kirk PM, Cannon PF, David JC, Stalpers JA (2001) Ainsworth & Bisby’s Dictionary of the Fungi, 9th edn. CABI Publishing, Wallingford, pp 1–655
Kornerup A, Wanscher JH (1981) Methuen handbook of color, 3rd Edition, Fletcher & Son Ltd, Great Britain: pp 1–282
Loguercio-Leite C, Wright JE (1991) Contribution to a biogeographical study of the Austro-American xylophilous polypores (Aphyllophorales) from the Santa Catarina Island, SC., Brazil. Mycotaxon 61:161–166
Loguercio-Leite C, Wright JE (1995) The genus Phellinus (Hymenochaetaceae) on the island of Santa Catarina, Brazil. Mycotaxon 54:361–388
Lowe JL (1966) Polyporaceae of North America. The Genus Poria. State University College of Forestry, Syracuse University. Tech Pub 90:1–183
Lutzoni F, Kauff F, Cox CJ, McLaughlin D, Celio G, Dentinger B, Padamsee M, Hibbett D, James TY, Baloch E, Grube M, Reeb V, Hofstetter V, Schoch C, Arnold AE, Miadlikowska J, Spatafora J, Johnson D, Hambleton S, Crockett M, Shoemaker R, Sung G-H, Lücking R, Lumbsch T, O’Donnell K, Binder M, Diederich P, Ertz D, Gueidan C, Hansen K, Harris RC, Hosada K, Lim Y-W, Matheny B, Nishida H, Pfister D, Rogers J, Rossman A, Schmitt I, Sipman H, Stone J, Sugiyama J, Yahr R, Vilgalys R (2004) Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. Am J Bot 91:1446–1480
Mason-Gamer RJ, Kellogg EA (1996) Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae). Syst Biol 45:524–545. doi:10.1093/ sysbio/45.4.524
Matheny PB (2005) Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe, Agaricales). Mol Phylogenet Evol 35:1–20. doi:10.1016/j.ympev.2004.11.014
Miadlikowska J, Lutzoni F (2004) Phylogenetic classification of peltigeralean fungi (Peltigerales, Ascomycota) based on ribosomal RNA small and large subunits. Am J Bot 91:449–464. doi:10.3732/ajb.91.3.449
Moncalvo JM, Nilsson RH, Koster B, Dunham SM, Bernauer T, Matheny PB, Porter TM, Margaritescu S, Weiß M, Garnica S, Danell E, Langer G, Langer E, Larsson E, Larsson KH, Vilgalys R (2006) The cantharelloid clade: dealing with incongruent gene trees and phylogenetic reconstruction methods. Mycologia 98:937–948
Müller K (2005) SeqState—primer design and sequence statistics for phylogenetic DNA data sets. Appl Bioinform 4:65–69. doi:10.2165/00822942-200504010-00008
Murrill W (1907) Polyporaceae. N Am Flora 9:1–72
Núñez M, Ryvarden L (2000) East Asian Polypores. Volume I. Synopsis Fungorum 13. Fungiflora, Oslo, pp 1–168
Pieri M, Rivoire B (2000) Le genre Phellinus. Quelques espèces rares ou critiques en France, avec une clé des espèces du genre Phellinus s.l. signalées en Europe occidentale. Bull Soc Mycol Fr 116:305–331
Pilotti M, Gervasi F, Brunetti A (2005) Molecular Identification of Fomitiporia mediterranea and Eutypa lata/Libertella blepharis in Platanus × acerifolia. J Phytopath 153:193–202
Pilotti M, Tizzani L, Brunetti A, Gervasi F, Di Lernia G, Lumia V (2010) Molecular identification of Fomitiporia mediterranea on declining and decayed hazelnut. J Pl Path 92:115–129
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818. doi:10.1093/bioinformatics/14.9.817
Raymundo T, Decock C, Valenzuela R, Amalfi M, Cifuentes J, Pacheco-Mota L (2012) New records of the genus Fomitiporia (Hymenochaetales, Basidiomycota) in Mexico. Rev Mex Biodivers 83:313–328
Reeb V, Lutzoni F, Roux C (2004) Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Mol Phyl Evol 32:1036–1060. doi:10.1016/ j.ympev.2004.04.012
Robert V, Stegehuis G, Stalpers J (2005) The MycoBank engine and related databases. http://www.mycobank.org
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi:10.1093/bioinformatics/ btg180
Ronquist F, Huelsenbeck JP, van der Mark P (2005) MrBayes 3.1 manual. School of Computational Science, Florida State University, Tallahassee
Ryvarden L (1978) The Polyporaceae of North Europe. Volume 2 (Inonotus – Tyromyces), vol 2. Fungiflora, Oslo, pp 219–507
Ryvarden L (1991) Genera of Polypores. Nomenclature and taxonomy. Synopsis Fungorum 5. Fungiflora, Oslo, pp 1–363
Ryvarden L (2004) Neotropical polypores 1. Synopsis Fungorum 19. Fungiflora, Oslo, pp 1–229
Ryvarden L, Gilbertson RL (1994) European polypores 2. Meripilus–Tyromyces. Synopsis Fungorum 7. Fungiflora, Oslo, pp 392–743
Ryvarden L, de Meijer AAR (2002) Studies in Neotropical Polypores 14. New species from the State of Paraná, Brazil. Synopsis Fungorum 15. Fungiflora, Oslo, pp 34–69
Simmons MP, Ochoterena H (2000) Gaps as characters in sequence-based phylogenetic analyses. Syst Biol 49:369–381. doi:10.1093/sysbio/49.2.369
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML Web-Servers. Syst Biol 75:758–771
Swofford DL (2003) PAUP*. Phylogenetic analysis using parsimony (*and Other Methods). Version 4. Sinauer Associates, Sunderland
Untereiner WA, Bonjean B, Decock C, Evrard P, de Frahan MH, Jamin N, Massart L, Nélissen L, Robert V, Bosschaerts M, Guissart F, De Brabandere J (1998) MUCL Catalogue of strains (Fungi-Yeast), 3eth edn. Published by the Belgian Office for Scientific, Technical and Cultural Affairs, Brussels
Vlasák J, Kout J (2011) Pileate Fomitiporia species in the USA. New combinations Fomitiporia calkinsii and F. bakeri. Mycol Prog 10:445–452
Vlasák J, Kout J, Vlasák J Jr, Ryvarden L (2011) New records of polypores from southern Florida. Mycotaxon 118:159–176
Wright JE, Blumenfeld SN (1984) New South American species of Phellinus (Hymenochaetaceae). Mycotaxon 21:413–425
Zhou LW, Xue HJ (2012) Fomitiporia pentaphylacis and F. tenuitubus spp. nov. (Hymenochaetales, Basidiomycota) from Guangxi, southern China. Mycol Prog 11:907–913
Acknowledgments
Marisa de Campos Santana acknowledges financial support received from CAPES (process 8296/11-1) and CNPq (Brazil) that enabled her one-year research stay at MUCL, Université catholique de Louvain, Belgium. Mario Amalfi acknowledges financial support received from UCL through a Fond Spécial de la Recherche scholarship and from the Wallonie–Bruxelles Federation through a travel grant to the Royal Ontario Museum (ROM), Canada. Cony Decock gratefully acknowledges the financial support received from the FNRS / FRFC (convention FRFC 2.4544.10) that enabled fieldwork in French Guiana, and from the Belgian State–Belgian Federal Science Policy through the BCCMTM research program. Cony Decock also thanks Dr. Anne Corval, Director of the "CNRS Guyane", for granting authorization and facilities for field research at the Nouragues “Inselberg” CNRS forest plots, and CNRS staff members in Cayenne and at the Nouragues “Inselberg” camp (namely, Mrs. Dorothée Deslignes, and Mr. Philippe Gaucher, Patrick Châtelet, Gilles Peroz, and Wemo Betian). Thanks are extended also to Stéphanie Huret for her help with the sequencing program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Marisa de Campos Santana and Mario Amalfi contributed equally to the research and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
de Campos Santana, M., Amalfi, M., Robledo, G. et al. Fomitiporia neotropica, a new species from South America evidenced by multilocus phylogenetic analyses. Mycol Progress 13, 601–615 (2014). https://doi.org/10.1007/s11557-013-0943-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-013-0943-1