Abstract
The Eurasian spruce bark beetle Ips typographus and their fungal associates can cause severe damage to Norway spruce forests. In this paper, by using both molecular and cultural methods, we compared fungal assemblages on bark beetles from different locations, characterized by different beetle population levels. Ips typographus was trapped in the western Alps in two outbreak and in two control areas. Sequencing of clone libraries of Internal Transcribed Spacer (ITS) identified 31 fungal Operational Taxonomic Units (OTUs), while fungal isolations yielded 55 OTUs. Only three OTUs were detected by both molecular and cultural methods indicating that both methods are necessary to adequately describe fungal richness. Fungal assemblages on insects from these four and from an additional 12 study sites differed among stands in response to varying ecological conditions and to the limited spreading ability of I. typographus. Ophiostomatoid fungi showed higher diversity in outbreak areas; the pathogenic Ophiostoma polonicum was relatively uncommon, while O. bicolor was the most abundant species. This result was not unexpected, as insects were trapped not at the peak but at the end of the outbreaks and supports the hypothesis of a temporal succession among Ophiostoma species. Ubiquitous endophytes of trees or common airborne fungi were present both in outbreak and in control areas. Wood decaying basidiomycetes were almost never detected on beetles. Yeasts were detected only by molecular analysis, and the OTUs detected matched those reported elsewhere in Europe and in the world, suggesting a very long association between some yeasts and bark beetles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Eurasian spruce bark beetle Ips typographus L. (Coleoptera, Curculionidae, Scolytinae) is one of the most destructive pests associated with Norway spruce [Picea abies (L.) Karst.] (Christiansen and Bakke 1988). This beetle normally breeds in weak or dead trees and in felled timber, but during outbreaks it may attack healthy trees causing severe damage (Sallé et al. 2005). In recent decades, numerous reports of significant damage caused by I. typographus have come from various regions of Europe (Solheim 1992a, b ; Viiri and Lieutier 2004; Jankowiak 2005; Sallé et al. 2005; Kirisits 2010), including the Italian Alps (Frigimelica et al. 2000; Stergulc et al. 2001; Faccoli and Stergulc 2004).
Ips typographus is commonly associated with ophiostomatoid fungal genera, including Ophiostoma, Grosmannia, Ceratocystiopsis, Ceratocystis, Leptographium and Pesotum (Mathiesen-Käärik 1953; Christiansen and Solheim 1990 ; Wingfield et al. 1993; Viiri and Lieutier 2004; Jankowiak 2005). Propagules of these fungi are carried both on the exoskeleton of beetles (pronotum and elytra) and in their digestive tract (Furniss et al. 1990; Solheim 1993a; Viiri and Lieutier 2004). Spruce bark beetles introduce spores or conidia of ophiostomatoid fungi mostly into the phloem of Norway spruce while digging galleries and breeding chambers. Ophiostomatoid fungi develop in the walls of larval galleries and in adjacent sections of bark and sapwood, causing blue-staining, a condition that generally lowers the quality of wood and may also reduce tree vigour (Kirisits and Offenthaler 2002).
Three ophiostomatoid species are commonly vectored by I. typographus: Ophiostoma polonicum Siemaszko, O. bicolor R.W. Davidson & D.E. Wells, and O. penicillatum (Grosm.) Siem. (Furniss et al. 1990; Paine et al. 1997; Kirisits et al. 2002; Linnakoski et al. 2012). Other species have been reported only as occasional associates (Solheim 1986; Solheim 1992b; Kirschner and Oberwinkler 1999; Viiri and Lieutier 2004; Jankowiak 2005 ; Jankowiak and Hilszczański 2005 ; Linnakoski et al. 2012). Among all ophiostomatoid species, O. polonicum is regarded as the most virulent vascular pathogen, and is believed to be associated with mortality of Norway spruce trees (Kirisits and Offenthaler 2002).
In addition to ophiostomatoid fungi, several Zygomycota, Ascomycota, Basidiomycota, anamorphic fungi, and yeasts have been reported as fungal associates of I. typographus (Siemaszko 1939; Leufvén and Nehls 1986; Solheim 1992a; Kirisits 2004; Jankowiak 2005; Persson et al. 2009), but very little is known about the overall fungal diversity of non-ophiostomatoid fungi present on this insect species.
Similarly, very little is known about the biogeographic diversity of fungal communities associated with bark beetles (Lieutier et al. 1991; Solheim 1993b; Roe et al. 2011).
Assemblages of fungi associated with bark beetles are usually investigated by isolations from bodies of individual insects and/or from gallery systems in the sapwood using various isolation methods (e.g., dilution plating, direct beetle streaking, indirect isolation through living beetle inoculation in sterilized logs, etc.) (Francke-Grosmann 1956; Juzwik and French 1983; Furniss et al. 1990; Klepzig et al. 1991; Six and Bentz 2003; Lee et al. 2006; Jankowiak and Rossa 2008; Kirisits 2010; Linnakoski et al. 2012). An array of DNA-based identification methods [e.g., amplified rDNA restriction and ribosomal DNA (rDNA) sequencing analyses] applied directly on insects may avoid the problem posed by fungi that are difficult to culture (Smit et al. 1999; Allen et al. 2003). At least in two cases, fungal diversity on beetles has been studied using both cultural and molecular techniques (Lim et al. 2005; Persson et al. 2009).
The main goals of this work were: i) to compare fungal assemblages associated with I. typographus from mountain and hill stands of Norway spruce, ii) to compare fungi from stands experiencing an outbreak with those present in control areas characterized by endemic beetle populations, and iii) to compare the diagnostic efficacy of molecular and cultural techniques in the detection of fungi on I. typographus. Based on previous studies (Solheim 1992a; Wichmann and Ravn 2001; Kirisits 2004; Lim et al. 2005; Persson et al. 2009; Kirisits 2010; Kautz et al. 2011), we expected to see diversity among fungal communities depending on environmental differences, levels of beetle populations, and detection approach. This is the first extensive study on fungi associated with I. typographus in the Alps using molecular and cultural methods.
Materials and methods
Survey in mountain and hill stands
A total of 12 Norway spruce stands were surveyed in 2005 in northwestern Italy. A group of six stands was located in hill areas around Alessandria (400 – 600 m a.s.l.), while a second group of six stands was selected in the mountain areas of the Vigezzo, Antigorio, Soana and Susa Valleys (800 – 1150 m a.s.l.). Norway spruce was the dominant species in all stands.
In late July, I. typographus were trapped by baiting the insects with the commercial pheromone Pheroprax® for 15 days. Beetles were collected weekly and individually transferred using sterile tweezers from the traps into sterile 1.5 ml microcentrifuge tubes and stored at −40 °C before processing.
DNA analysis
Ten beetles per trap were randomly selected and pooled together in 1.5 ml microcentrifuge tubes. Fungal DNA extraction was carried out according to method described by Schweigkofler et al. (2005). PCR amplification of the Internal Transcribed Spacer (ITS2) was performed with primers ITS3 (White et al. 1990) and TW13 (O'Donnell 1993). The PCR mix consisted of 6.25 μL template DNA, 10X PCR Buffer, 2 mM dNTPs, 5 U/μM Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA), 50 mM MgCl2, and 10 μM of forward and reverse primers in a 25 μL reaction volume. Amplifications were run in an iCycler thermocycler (Bio-Rad, Hercules, CA) with the following cycling parameters: initial denaturation at 95 °C for 1.25 min, then 34 cycles of denaturation at 93 °C for 35 s, annealing at 58 °C for 55 s, extension at 72 °C for 50 s, with an increase of 5 s after each cycle and a final extension at 72 °C for 10 min.
One clone library was generated for each of the 12 study sites as follows: PCR products were cloned into plasmids and transformed into Escherichia coli using the TOPO TA Cloning kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Positive colonies from each reaction were amplified using T7 forward and M13 reverse primers (Sambrook et al. 1989), visualized on 1.5 % agarose gel and subsequently sequenced on an ABI 3100 sequencer.
All sequences were compared with those deposited in the National Center for Biotechnology Information’s (NCBI) GenBank nucleotide BLAST search. The sequence homology for delimiting fungal species and genera was set at 98 – 100 % and 95 – 97 %, respectively (Persson et al. 2009).
Sequences that were of insect origin were excluded from the analysis.
Survey in stands with outbreak presence of I. typographus and control areas
Four areas, two in the Antigorio Valley and two in the Soana Valley, were selected for the study (Table 1). In each valley, one outbreak (i.e. epidemic) and one control (i.e. endemic) population of the beetle were identified on the basis of the extent of visible tree damage. Outbreak and control areas were about 4 and 2 km from each other, in the Antigorio and Soana Valleys, respectively. Pheromone traps were used to collect adult I. typographus beetles as previously described. Beetles were collected weekly and single individuals were transferred using sterile tweezers from the traps into sterile 1.5 ml microcentrifuge tubes. The tubes containing beetles for isolations were stored at 5 °C and those assigned for direct DNA extractions at −40 °C.
DNA-based and culture-based fungal diagnoses
For DNA-based diagnosis, ten specimens per trap of I. typographus were randomly selected and pooled together as an individual sample in 1.5 ml microcentrifuge tubes; DNA was extracted as described above. One clone library was constructed for each area, for a total of four libraries. Operational Taxonomic Units (OTUs) were designated using sequence similarity with fungal sequences deposited in GenBank, as described above.
For culture-based diagnosis, 15 insects per trap were randomly selected and used for isolations. Isolations were performed by rolling insects, without surface sterilization, across the medium with sterile tweezers (Aukema et al. 2005; Jankowiak and Rossa 2008). To minimize the selective bias linked to the use of a specific culture media, isolations were made on three different media: i) 2 % Malt Extract Agar (2 % MEA; 20 g malt extract, 20 g agar, 1000 mL distilled water) supplemented with 200 mg tetracycline to isolate the general fungal flora (Jankowiak 2005); ii) 2 % MEA supplemented with 4 mg benomyl and 100 mg ampicillin to isolate preferentially the Basidiomycota (Kim et al. 2005); iii) selective medium (CH-MA; 20 g malt, 16 g agar, 1000 mL distilled water) supplemented with 100 mg streptomycin sulphate and 200 mg cycloheximide for the isolation of Ophiostoma spp. and their anamorphs (Harrington et al. 1981; Kirisits et al. 2002).
Petri dishes were incubated in the light at room temperature for four weeks and were daily inspected for fungal growth and the occurrence of sexual and asexual fungal structures. Pure fungal cultures were obtained by transferring small pieces of agar containing mycelium onto fresh 2 % MEA plates for subsequent morphotyping. Fungal isolates were grouped into mycelial morphotypes based on growth morphology and macroscopic features. OTUs identification via traditional methods was achieved by macro- and micro-morphological analysis using taxonomic guides and standard procedures (Domsch et al. 1980; von Arx 1981; Kiffer and Morelet 1997). Non-sporulating fungi were grouped as sterile mycelia (sensu Lacap et al. 2003) and divided into different morphotypes based on similar cultural characters. Among these, sterile basidiomycetes were identified by scoring mycelia for presence of clamp connections. DNA sequence information was used to assist in identification of OTUs that were unresolved by morphological analysis or were sterile.
A representative sample of identified OTUs has been deposited as dried cultures at the Herbarium Patavinum (PAD), University Museum Centre (CAM), University of Padua, Italy.
Data interpretation and analyses
The abundance of each OTU identified by sequencing or culturing was expressed as percentage of sequences/cultures of that OTU on the total number of sequences for each library or of isolates for each area, respectively. The Sorensen similarity index (Ss) was used to compare fungal communities from different locations. The index was calculated as follows:
where a is the number of OTUs in one sampling, b is the number of OTUs in a second sampling and c is the number of OTUs shared by the two samplings (Magurran 2004).
In order to determine whether the sampling size was appropriate to describe the total fungal richness, a rarefaction analysis was performed on data from the two outbreak and two control areas by pooling OTUs defined by culturing and DNA analysis. Rarefaction analysis was performed according to the methods for individual-based data as described by Colwell et al. (2012).
An UPGMA (Unweighted Pair Group Method with Arithmetic Mean) cluster analysis was performed on OTUs defined by culturing and DNA analysis pooled together, to visualize patterns and determine number of discrete fungal assemblages in outbreak and control beetle populations of the Antigorio and Soana Valley.
Tests were performed with PASW Statistics 18 (2009) and rarefaction analysis was performed by using EstimateS 8.2.0 (2009).
Results
Survey in mountain and hill stands
A total of 114 ITS sequences representing 29 fungal OTUs using a 95 % similarity threshold were obtained from the libraries, including 55 from the mountain stands and 59 from the hill stands. The mycobiota was dominated by Ascomycota and anamorphic fungi (20 OTUs, 69 %), followed by Basidiomycota (7 OTUs, 24 %) and Zygomycota (1 OTU, 3 %). Fourteen OTUs of Ascomycota and Basidiomycota yeasts were identified (Table 2). In mountain stands, the number of OTUs per stand ranged from 2 to 6, while in hill stands that number ranged from 0 to 6.
The two groups of stands (i.e., mountain vs hill stands) were characterized by very different fungal assemblages (Ss = 0.18), with only three OTUs (Alternaria tenuissima, Cryptococcus oeirensis and Wickerhamomyces bisporus) found in both. Additionally, in both stand groups, the composition of fungal assemblages differed from stand to stand, and few fungal OTUs were found in at least two sites. A single ophiostomatoid fungus, Ophiostoma polonicum, was identified in two mountain stands of the Soana Valley.
Survey in stands with outbreak presence of I. typographus and control areas
To increase our ability to identify rare fungal OTUs, our second study was performed using a more intensive sampling approach in one outbreak and one control area in each of two valleys (Antigorio and Soana).
A total of 367 ITS sequences representing 31 fungal OTUs (using 95 % sequence similarity as a threshold) were obtained from the libraries, including 151 from outbreak and 216 from control areas.
A total of 313 fungal isolates, representing 55 fungal OTUs, were obtained from isolations. 69 % of 205 plated insects generated at least one fungal colony, while the remaining 31 % were either colonized by bacteria or appeared to be uncolonized. There was no effect of the three different culture media on number of OTUs recovered, but about 90 % of ophiostomatoid isolates were obtained from selective medium CH-MA (data not shown).
When comparing the two identification approaches, there were 28 OTUs detected exclusively by clone libraries, 52 OTUs detected exclusively by culturing, and only three OTUs were detected by both methods (Table 3). Community compositions revealed by each method were almost totally different (Ss = 0.07). To account for the potential effects of geographic location, the same analysis was performed independently for each valley, with identical results of insignificant overlap between diagnostic approaches (Ss = 0.03 for Antigorio Valley; Ss = 0.18 for Soana Valley).
Cultures of non-ophiostomatoid OTUs showing abundance > 10 % in at least one area were: Alternaria alternata, Cladosporium cladosporioides, Fusarium spp., Mucor hiemalis, Penicillium spp., Talaromyces trachyspermus and Trichoderma spp. OTUs defined by DNA sequencing showing abundance > 10 % in at least one area were: Beauveria bassiana, Candida ontarioensis, unidentified yeast BAF5 and unidentified yeast BAF22. Based on DNA analysis, Beauveria bassiana was the most abundant OTU (0 – 69 % of OTUs by site). All ascomycetous and basidiomycetous yeasts were detected only by the molecular method.
Except for Ophiostoma polonicum, all ophiostomatoid fungi were detected by culture isolations. Ceratocystis tetropii was the only one detected by both methods. Ophiostomatoid OTUs showing abundance > 10 % in at least one area included Ophiostoma bicolor and Ophiostoma sp. 1.
Combining the data of clone libraries with those obtained by culturing, the overall fungal community associated with I. typographus included 11 ophiostomatoid fungi; 28 other Ascomycota and anamorphic fungi, three Basidiomycota; 17 ascomycetous, basidiomycetous, and unidentified yeasts; and two Zygomycota. Twenty-one morphological types of fungi were dark, hyaline and pinkish sterile mycelia whose sterility persisted in colonies grown on 2 % MEA incubated at 20 – 25 °C for several months (Table 3). The trends of rarefaction curves suggest that the sampling effort was adequate and comparable for all four sites (Fig. 1).
There were 54 OTUs exclusive to the Antigorio Valley and 16 OTUs exclusive to the Soana Valley. Fungal communities from the two valleys were largely different from one another (Ss = 0.27). When pooling the two control and the two outbreak populations irrespective of the valley of origin, the resulting Ss was even lower than that obtained by comparing the overall fungal community between the two sites (Ss outbreak = 0.16; Ss control = 0.26).
In the Antigorio Valley, 41.8 % of OTUs were outbreak-specific while 40 % were only found in control areas; only 17.9 % were detected in both populations (Ss = 0.30). In the Soana Valley 48 % of OTUs were outbreak-specific, 24 % control-specific and 27.6 % were shared by two populations, thus resulting in a higher similarity value between outbreak and control areas (Ss = 0.43).
Beauveria bassiana, an entomopathogenic fungus, was the most abundant OTU and the only one with Epicoccum nigrum and Penicillium spp. to be detected in all four areas.
The assemblage of ophiostomatoid fungi consisted of Ceratocystis tetropii, Grosmannia cucullata, G. europhioides, Ophiostoma bicolor, O. breviusculum, O. piceae, O. polonicum, three other Ophiostoma OTUs and one Sporothrix OTU. Grosmannia europhioides, O. polonicum and O. piceae, were detected exclusively in control areas, all other species were present in outbreak areas. Finally, G. cucullata, O. bicolor and Ophiostoma sp. 2 were detected in both outbreak and control areas. Among them, O. bicolor was the most common OTU.
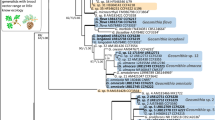
Cluster analyses based on Ss divided the fungal community of each valley into three clusters (Figs. 2 and 3). Two of these clusters are exclusively associated with outbreak or control beetle populations respectively, while the third includes taxa shared by both types of beetle populations.
Dendrogram of cluster analysis using UPGMA for Antigorio Valley. Numbers refer to taxa code, Table 3
Dendrogram of cluster analysis using UPGMA for Soana Valley. Numbers refer to taxa code, Table 3
Discussion
This is the first study using both molecular and cultural methods on fungi associated with I. typographus in mountain and hill stands of the Alps characterized both by outbreak and endemic populations of this beetle.
On the whole, the spectrum of fungi identified was consistent with that reported for I. typographus elsewhere in Europe (Mathiesen-Käärik 1953; Solheim 1986, 1992a , b, 1993a; Viiri and Lieutier 2004 ; Jankowiak 2005 ; Sallé et al. 2005; Persson et al. 2009 ; Kirisits 2010 ; Linnakoski et al. 2012).
The first survey identified two distinct and mostly non-overlapping assemblages of fungi in mountain and hill stands. The composition of fungal assemblages was different even when comparing sites within the same altitudinal range. Only Taphrina sp. and Phallus impudicus cannot be considered directly associated with I. typographus and they were deemed to be contaminant.
Environmental and climatic factors are likely to affect the composition of the fungal flora associated with I. typographus (Kirisits 2004); however, both ecological-environmental conditions and spatial segregation may have resulted in the differences reported in this study. The minimal distance among study sites in fact was 2 – 5 km, a distance that may exceed the normal range of dispersal of this beetle. On average in fact, 95 % of new outbreaks are reported to occur within 500 m from infestations that occurred in the previous year (Wichmann and Ravn 2001; Kautz et al. 2011). The fact that buffer zones between 100 m and 1500 m have prevented significant attacks in managed forests (Wermelinger 2004) also points to a limited dispersal range of this beetle species.
Data from our second survey clearly show that the cultural method identifies a greater number of fungal OTUs compared to the molecular method. This finding would support the belief that investigations on fungal diversity in environmental samples are probably incomplete when identifications rely exclusively on DNA analyses. Nevertheless, it should be noted that many fungal clones in our studies remained unidentified due to the small length of the sequences or to the low similarity with ITS sequences in the GenBank database, suggesting that our inability to identify these OTUs may be related to technical issues (e.g. quality of DNA, unavailability of sequences in GenBank database), rather than to an intrinsic limit of the molecular method. It appears that for a complete description of the fungal community vectored by I. typographus both molecular and cultural methodologies are necessary. This finding is supported by previous studies (Lim et al. 2005; Persson et al. 2009) showing that different methods tend to provide complementary information on fungal diversity.
Several fungi detected by the molecular method were never isolated in pure culture, indicating that some might be unculturable directly from environmental samples. In general, ophiostomatoid fungi and fast-growing fungi were more frequently detected by pure culture isolations. By contrast, and in agreement with previous reports (Lim et al. 2005; Persson et al. 2009), ascomycetous and basidiomycetous yeasts could be detected exclusively by using the molecular method. Since every method or medium of isolation may be selective for some species but not appropriate for others, we cannot exclude that yeasts could have been isolated by using other isolation techniques (e.g. dilution plating) or media, and the same is true for other groups of fungi. Three of the yeasts detected in this study on I. typographus had identical ITS sequences to those detected by Lim et al. (2005) on bark beetles in Canada, one on Dendroctonus ponderosae (BAF22), and two on Ips pini (BAF5 and BAF15). Recently, BAF22 and BAF5 were also detected by the direct molecular analysis (T-RFLP) on I. typographus beetles hibernated under the bark of standing trees and in forest litter (Persson et al. 2009). Whether the sequence identity of yeasts between this and other studies is the result of a true association of the same yeast species with different insect species from different continents, or whether it is the result of ITS transfers among species, remains to be determined. In either case, the data are suggestive of a very long association between some yeasts and bark beetles. Leufvén and Nehls (1986) have in the past successfully isolated other yeasts reported to be associates of I. typographus, e.g. Kuraishia capsulata, Nakazawaea holstii, and species in the genera Cryptococcus and Ogataea. The role of yeasts in the life cycle of I. typographus is yet unknown but the common association with the bark beetle suggests that they can play an important ecological role. There is experimental evidence that some yeasts and bacteria, living in the digestive tract of insects, might be involved in digestion, detoxification processes of plant chemicals to which the insects are exposed, and pheromone production (Vega and Dowd 2005; Rivera et al. 2007).
The ordination of fungal taxa identified cumulatively by two methods shows that there are three different fungal assemblages in both valleys: two of these are exclusively associated with outbreak or control beetle populations respectively, while the third includes taxa shared by both types of beetle populations. The variation in the spectrum of fungi can depend on various factors, including the levels of beetle populations (Kirisits 2004, 2010), the ecological differences among stands (Kirisits 2004), the stage of the infestation and tree damage (Solheim 1992a), and the distance among study sites in relation to the ability of the insects to spread (Wichmann and Ravn 2001; Kautz et al. 2011). However, very little is known how different factors may affect this variation (Linnakoski et al. 2012).
Penicillium spp. were among the most abundant OTUs ranging between 11 % and 12 % in the Antigorio Valley and between 23 % and 69 % in the Soana Valley. This finding is in accordance with reports of Jankowiak (2006) and Jankowiak and Rossa (2008) on fungi associated with two bark beetles Tomicus piniperda (L.) and Pityogenes bidentatus (Herbst) from Scots pine (Pinus sylvestris L.). Other Penicillium species were isolated from Dendroctonus sp. (Whitney 1982), Crypturgus cinereus (Hrbst.) and Ips sexdentatus (Börn.) (Kirschner 2001). Penicillium species are widely distributed in nature and are not associated with any specific species of bark beetles on coniferous trees (Jankowiak 2006); they are primarily wind dispersed and do not depend on beetle vectors for dissemination. Some other OTUs identified in this study (e.g. Ampelomyces humuli and Graphiopsis chlorocephala which are known to be obligate parasites of fungi and plants, respectively) cannot be considered associated with I. typographus, and were thus deemed to be contaminant.
Other frequent OTUs, such as Alternaria alternata, Cladosporium cladosporioides, Epicoccum nigrum and Trichoderma spp., were reported as ubiquitous fungi and endophytes of trees (Fisher et al. 1991; Lumley et al. 2001; Ragazzi et al. 2003 ; Lygis et al. 2005; Menkis et al. 2006; Wang and Guo 2007), and were often associated with different bark beetle species (Lieutier et al. 1989; Peverieri et al. 2006; Bueno et al. 2010). Noteworthy is also the common occurrence of some insect-pathogenic fungi, such as Beauveria bassiana and Isaria coleopterorum, and of one mycoparasite, Clonostachys rosea.
The ubiquitous nature of species that are commonly air-dispersed, including those that are endophytic or pathogenic to insects, may justify the presence of these OTUs both in outbreak and control areas. In the Antigorio Valley, with the exception of B. bassiana and Trichoderma spp. that were only found in the “outbreak cluster”, all other OTUs (i.e. A. alternata, C. cladosporioides, E. nigrum, I. coleopterorum and Penicillium spp.) were found in the “outbreak/control cluster”. In the Soana Valley, E. nigrum and Penicillium spp. belong to the “outbreak cluster”, while B. bassiana to the “outbreak/control cluster”, while all other OTUs are absent. However, it must be emphasized that B. bassiana and Trichoderma spp. were also present in the control area (4 % and 2 %, respectively) of Antigorio Valley and E. nigrum and Penicillium spp. were also present in the control area (0.6 % and 23 %, respectively) of Soana Valley, thus further confirming that the species mentioned in this section can all be considered ubiquitous associates of I. typographus.
A limited number of Basidiomycota was isolated, despite the use of a selective medium, and only Polyporus sp. was detected in clone libraries. Historically, Basidiomycota have only occasionally been found in association with bark beetles because traditional isolation techniques are likely to underestimate the actual occurrence of these fungi (Linnakoski et al. 2012). There is emerging evidence that Basidiomycota are more common associates of bark beetles than previously thought, and a clear association between a few taxa and I. typographus has been reported (Kirschner et al. 2001a, b; Kolařík et al. 2006; Oberwinkler et al. 2006; Persson et al. 2009).
More than 25 ophiostomatoid fungi have been previously reported as fungal associates of I. typographus in various parts of Europe (Kirisits 2004). Many of these taxa were encountered in only a few investigations or generally occurred at low frequency. In this study, ophiostomatoid fungi represented 13 % of the total number of taxa detected in all four areas. Ophiostoma penicillatum and O. ainoae H. Solheim, were previously reported to be consistently associated with I. typographus across Europe (Viiri and Lieutier 2004 ; Jankowiak 2005 ; Sallé et al. 2005; Persson et al. 2009; Kirisits 2010), but were not recorded in this study. This is the first report of O. breviusculum associated with I. typographus in Europe. Previously, this species within the O. picea-complex was isolated only from Ips subelongatus Motschulsky and Dryocoetes baikalicus Reitter from Larix kaempferi (Lamb.) Carr. in Japan (Chung et al. 2006; Yamaoka et al. 2009).
Ophiostomatoid fungi occurred with varying frequencies in the two investigated sites but, on the whole, outbreak areas showed higher diversity than control ones. However, the most pathogenic species, Ophiostoma polonicum, was unexpectedly overall rare. Reports on the abundance of O. polonicum vary, but it has often been reported as a dominant element of the mycobiota of I. typographus (Solheim 1986, 1992a, b; Krokene and Solheim 1996; Kirisits 2004). The sporadical presence of O. polonicum and the high frequency of O. bicolor in this study may be related to the population dynamics of I. typographus. Solheim (1993a) suggested that O. polonicum occurs at low frequencies during endemic periods, but its frequency increases during the outbreak phase when vigorous trees are attacked; this pathogen, in fact, tolerates oxygen-deficient conditions of wood of healthy trees (Solheim 1992a). Evidence for this hypothesis is, however, not conclusive, as O. polonicum has either not been reported at all (Grosmann 1931) or was rarely found also in areas with high damage levels of I. typographus (Mathiesen-Käärik 1953; Kirisits 2004; Jankowiak 2005 ; Jankowiak and Hilszczański 2005 ; Sallé et al. 2005). Solheim (1992a, b), Jankowiak and Hilszczański (2005) and Jankowiak (2005) have demonstrated that a temporal succession of fungi into phloem and sapwood could be responsible for the varying frequencies reported for ophiostomatoid fungi in Norway spruce. In this successional scenario, O. polonicum is the primary invader, occurring most frequently in the sapwood of Norway spruce trees during the early stages of brood development of I. typographus. Within two or three weeks though, new Ophiostoma species substitute O. polonicum. Ophiostoma bicolor is known to be one of the first species to follow O. polonicum, and its frequency increases rapidly during the first weeks after attack. In our study, the fact that I. typographus specimens were collected when Norway spruce trees were already dying could have greatly accounted for the low frequency of occurrence of O. polonicum. This interpretation is also supported by the occurrence in our study of G. europhioides [in many investigations referred to as Grosmannia piceaperda (Rumbold) Goid.] and C. tetropii, previously reported as tertiary and quaternary invaders, respectively, of Norway spruce (Solheim 1992a). Besides, and maybe in addition to the successional theory, vectoring of pathogenic Ophiostoma spp. may be explained by threshold effects, and infection may be associated with I. typographus outbreaks only when insect populations reach a certain size. In that case, even if frequency of detection is low for any given fungal species, that low frequency may be counterbalanced by large numbers of insect vectors. It is likely that our sampling occurred when beetles populations were already crashing, and thus it is possible our sampling occurred when populations of the beetle had gone under the threshold levels necessary for effective vectoring of O. polonicum (Wermelinger 2004).
In conclusion, molecular and cultural methods provided a different picture of the fungal communities on beetles, emphasizing the need to use both approaches to provide a conclusive picture of mycobionts associated with I. typographus. As a result of the combined approach used in this study, our data may be among the first to provide a list of fungi inclusive of taxa from all of the most important functional groups associated with this bark beetle. Different fungal assemblages between hill and mountain stands were likely the consequence of different ecological features. Differences between outbreak and control areas, instead, were likely the result of different stages in the dynamics of beetle populations. Finally, based on results of rarefaction analysis, we surmise that the lack of overlap of fungal OTUs reported in this study may be explained by the limited ability of the insect to move at distances greater than 500 m rather than by an inadequate sampling resulting in the underestimation of the total fungal richness. Sampling of sites less than 500 m apart and when beetles populations are peeking during the outbreak should result in a significant larger overlap of fungal assemblages, because of the ability of beetles to move between sites at that distance and because of a greater match between the population dynamics of the beetles in nearby sites.
References
Allen TR, Millar T, Berch SM, Berbee ML (2003) Culturing and direct DNA extraction find different fungi from the same ericoid mycorrhizal roots. New Phytol 160:255–272
Aukema BH, Werner RA, Haberkern KE, Illman BL, Clayton MK, Raffa KF (2005) Quantifying sources of variation in the frequency of fungi associated with spruce beetles: implications for hypothesis testing and sampling methodology in bark beetle-symbiont relationships. For Ecol Manag 217:187–202
Bueno A, Diez JJ, Fernández MM (2010) Ophiostomatoid fungi transported by Ips sexdentatus (Coleoptera; Scolytidae) in Pinus pinaster in NW Spain. Silva Fenn 44:387–397
Christiansen E, Bakke A (1988) The spruce bark beetle of Eurasia. In: Berryman AA (ed) Dynamics of Forest Insect Populations. Pattern, Causes, Implications. Plenum Press, New York and London, pp 479–503
Christiansen E, Solheim H (1990) The bark beetle-associated blue-stain fungus Ophiostoma polonicum can kill various spruces and Douglas fir. Eur J For Pathol 20:436–446
Chung WH, Kim JJ, Yamaoka Y, Uzunovic A, Masuya H, Breuil C (2006) Ophiostoma breviusculum sp. nov. (Ophiostomatales, Ascomycota) is a new species in the Ophiostoma piceae complex associated with bark beetles infesting larch in Japan. Mycologia 98:801–814
Colwell RK, Chao A, Gotelli NJ, Lin S-Y, Mao CX, Chazdon RL, Longino JT (2012) Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J Plant Ecol-UK 5:3–21
Domsch KH, Gams W, Anderson TH (1980) Compendium of soil fungi. Academic Press, London
EstimateS 8.2.0 (2009) By Robert Colwell, University of Connecticut, Storrs, Connecticut
Faccoli M, Stergulc F (2004) Ips typographus (L.) pheromone trapping in south Alps: spring catches determine damage thresholds. J Appl Entomol 128:307–311
Fisher PJ, Petrini O, Petrini LE (1991) Endophytic ascomycetes and deuteromycetes in roots of Pinus sylvestris. Nova Hedwigia 52:11–15
Francke-Grosmann H (1956) Hautdrüsen als Trager der Pilzsymbiose bei Ambrosiakäfern. Z Morph u Ökol Tiere 45:275–308
Frigimelica G, Stergulc F, Faccoli M, Battisti A (2000) A comparison of damage caused by fungi and insects in pure and mixed stands of Picea abies in the south-eastern Italian Alps. In: Hasenauer H. (ed) Proceedings of the International Conference “Forest ecosystem restoration. ecological and economical impacts of restoration processes in secondary coniferous forests”. Vienna, Austria, pp 85–91
Furniss MM, Solheim H, Christiansen E (1990) Transmission of blue-stain fungi by Ips typographus (Coleoptera: Scolytidae) in Norway spruce. Ann Entomol Soc Am 83:712–716
Grosmann H (1931) Beiträge zur Kenntnis der Lebensgemeinschaft zwischen Borkenkäfern und Pilzen. Z Parasitenk 3:56–102
Harrington TC, Furniss MM, Shaw CG (1981) Dissemination of Hymenomycetes by Dendroctonus pseudotsugae (Coleoptera: Scolytidae). Phytopathology 71:551–554
Jankowiak R (2005) Fungi associated with Ips typographus on Picea abies in southern Poland and their succession into the phloem and sapwood of beetle-infested trees and logs. For Pathol 35:37–55
Jankowiak R (2006) Fungi associated with Tomicus piniperda in Poland and assessment of their virulence using Scots pine seedlings. Ann For Sci 63:801–808
Jankowiak R, Hilszczański J (2005) Ophiostomatoid fungi associated with Ips typographus (L.) on Picea abies (L.) H. Karst. and Pinus sylvestris L. in North-Eastern Poland. Acta Soc Bot Pol 74:345–350
Jankowiak R, Rossa R (2008) Associations between Pityogenes bidentatus and fungi in young managed Scots pine stands in Poland. For Pathol 38:169–177
Juzwik J, French DW (1983) Ceratocystis fagacearum and Ceratocystis piceae on the surface of free-flying and fungus-mat-inhabiting nitidulids. Phytopathology 73:1164–1168
Kautz M, Dworschak K, Gruppe A, Schopf R (2011) Quantifying spatio-temporal dispersion of bark beetle infestations in epidemic and non-epidemic conditions. For Ecol Manag 262:598–608
Kiffer E, Morelet M (1997) Les deutéromycètes. Classification et clés d’identification générique. INRA Editions, Paris
Kim JJ, Allen EA, Humble LM, Breuil C (2005) Ophiostomatoid and basidiomycetous fungi associated with green, red and grey lodgepole pines after mountain pine beetle (Dendroctonus ponderosae) infestation. Can J For Res 35:274–284
Kirisits T (2004) Fungal associates of European bark beetles with special emphasis on the ophiostomatoid fungi. In: Lieutier F, Day KR, Battisti A, Grégoire J-C, Evans HF (eds) Bark and wood boring insects in living trees in Europe, a synthesis. Springer, Dordrecht, pp 181–236
Kirisits T (2010) Fungi isolated from Picea abies infested by the bark beetle Ips typographus in the Bialowieza forest in north-eastern Poland. For Pathol 40:100–110
Kirisits T, Offenthaler I (2002) Xylem sap flow of Norway spruce after inoculation with the blue-stain fungus Ceratocystis polonica. Plant Pathol 51:359–364
Kirisits T, Wingfield MJ, Chettri DB (2002) Studies on the association of blue-stain fungi with the Eastern Himalayan spruce bark beetle (Ips schmutzenhoferi) and with other bark beetles in Buthan. Yusipang Report, RNR RC Yusipang
Kirschner R (2001) Diversity of filamentous fungi in bark beetle galleries in central Europe. In: Misra JK, Horn BW, Robert W (eds) Trichomycetes and other fungal groups. Science Publishers, Inc., pp 175–196
Kirschner R, Oberwinkler F (1999) A new Ophiostoma species associated with bark beetles infesting Norway spruce. Can J Bot 77:247–252
Kirschner R, Begerow D, Oberwinkler F (2001a) A new Chionosphaera species associated with conifer inhabiting bark beetles. Mycol Res 105:1403–1408
Kirschner R, Sampaio JP, Gadanho M, Weiss M, Oberwinkler F (2001b) Cuniculitrema polymorpha (Tremellales, gen. nov. and sp. nov.), a heterobasidiomycete vectored by bark beetles, which is the teleomorph of Sterigmatosporidium polymorphum. Antoine van Leeuwenhoek 80:149–161
Klepzig KD, Raffa KF, Smalley EB (1991) Association of an insect-fungal complex with red pine decline in Wisconsin. Forest Sci 37:1119–1139
Kolařík M, Sláviková E, Pažoutová S (2006) The taxonomic and ecological characterization of the clinically important heterobasiodiomycete Fugomyces cyanescens and its association with bark beetles. Czech Mycol 58:81–98
Krokene P, Solheim H (1996) Fungal associates of five bark beetle species colonizing Norway spruce. Can J For Res 26:2115–2122
Lacap DC, Hyde KD, Liew ECY (2003) An evaluation of the fungal “morphotypes” concept based on ribosomial DNA sequences. Fungal Divers 12:53–66
Lee S, Kim JJ, Breuil C (2006) Diversity of fungi associated with mountain pine beetle, Dendroctonus ponderosae, and infested lodgepole pines in British Columbia. Fungal Divers 22:91–105
Leufvén A, Nehls L (1986) Quantification of different yeasts associated with the bark beetle, Ips typographus, during its attack on a spruce tree. Microb Ecol 12:237–243
Lieutier F, Yart A, Garcia J, Ham MC, Morelet M, Levieux J (1989) Champignons phytopathogènes associés à deux coléoptères scolytidae du pin sylvestres (Pinus sylvestris L.) et étude préliminaire de leur agressivité envers l’hôte. Ann For Sci 46:201–216
Lieutier F, Garcia J, Yart A, Vouland G, Pettinetti M, Morelet M (1991) Ophiostomatales (Ascomycètes) associées à Ips acuminatus Gyll (Coleoptera: Scolytitidae) sur le pin sylvestre (Pinus sylvestris L.) dans le Sud-Est de la France et comparison avec Ips sexdentatus Boern. Agronomie 11:807–817
Lim WY, Kim JJ, Lu M, Breuil C (2005) Determining fungal diversity on Dendroctonus ponderosae and Ips pini affecting lodgepole pine using cultural and molecular methods. Fungal Divers 19:79–94
Linnakoski R, Wilhelm de Beer Z, Niemelä P, Wingfield MJ (2012) Associations of conifer-infesting bark beetles and fungi in Fennoscandia. Insects 3:200–227
Lumley TC, Gignac LD, Currah RS (2001) Microfungus communities of white spruce and trembling aspen logs at different stages of decay in disturbed and undisturbed sites in the boreal mixedwood region of Alberta. Can J Bot 79:76–92
Lygis V, Vasiliauskas R, Larsson KH, Stenlid J (2005) Wood-inhabiting fungi in stems of Fraxinus excelsior in declining ash stands of northern Lithuania, with particular reference to Armillaria cepistipes. Scand J For Res 20:337–346
Magurran A (2004) Measuring biological diversity. Blackwell Publishing, Oxford
Mathiesen-Käärik A (1953) Eine Übersicht uber die gewöhnlichsten mit Borkenkäfern assoziierten Bläuepilze in Schweden einige für Sweden neue Bläuepilze. Med Stat Skogsf Inst Stockholm 43:1–74
Menkis A, Vasiliauskas R, Taylor AFS, Stenström E, Stenlid J, Finlay R (2006) Fungi in decayed roots of conifer seedlings in forest nurseries, afforested clear-cuts and abandoned farmland. Plant Pathol 55:117–129
Oberwinkler F, Kirschner R, Arenal F, Villarreal M, Rubio V, Begerow D, Bauer R (2006) Two new pycnidial members of the Atractiellales: Basidiopycnis hyaline and Proceropycnis pinicola. Mycologia 98:637–649
O'Donnell K (1993) Fusarium and its near relatives. In: Reynolds DR, Taylor JW (eds) The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, pp 225–233
Paine TD, Raffa KF, Harrington TC (1997) Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu Rev Entomol 42:179–206
PASW Statistics 18 (2009) Software version 18.0.0. SPSS Inc, Chicago
Persson Y, Vasaitis R, Långström B, Öhrn P, Ihrmark K, Stenlid J (2009) Fungi vectored by the bark beetle Ips typographus following hibernation under the bark of standing trees and in the litter. Microb Ecol 58:651–659
Peverieri GS, Capretti P, Tiberi R (2006) Associations between Tomicus destruens and Leptographium spp. in Pinus pinea and Pinus pinaster stands in Tuscany, central Italy. For Pathol 36:14–20
Ragazzi A, Moricca S, Capretti P, Dellavalle I, Turco E (2003) Differences in composition of endophytic mycobiota in twigs and leaves of healthy and declining Quercus species in Italy. For Pathol 33:31–38
Rivera FN, Gómez Z, González E, López N, Hernández Rodríguez CH, Zúňiga G (2007) Yeasts associated with bark beetles of the genus Dendroctonus Erichson (Coleoptera, Curculionidae, Scolytinae): molecular identification and biochemical characterization. In: Bentz B, Cognato A, Raffa K (eds) Proceedings from the Third Workshop on genetics of bark beetles and associated microorganisms. Fort Collins, U.S., pp 45–48
Roe AD, James PMA, Rice AV, Cooke JEK, Sperling FAH (2011) Spatial community structure of mountain pine beetle fungal symbionts across a latitudinal gradient. Microb Ecol 62:347–360
Sallé A, Monclus R, Yart A, Garcia J, Romary P, Lieutier F (2005) Fungal flora associated with Ips typographus: frequency, virulence, and ability to stimulate the host defence reaction in relation to insect population levels. Can J For Res 35:365–373
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Springs Harbor Laboratory Press, New York
Schweigkofler W, Otrosina WJ, Smith SL, Cluck DR, Maeda K, Peay KG, Garbelotto M (2005) Detection and quantification of Leptographium wageneri, the cause of black-stain root disease, from bark beetles (Coleoptera: Scolytidae) in Northern California using regular and real-time PCR. Can J For Res 35:1798–1808
Siemaszko W (1939) Zespoly grzbow towarzyszacych kornikom polskim. Planta Pol 7:1–54
Six DL, Bentz BJ (2003) Fungi associated with the North American spruce beetle, Dendroctonus rufipennis. Can J For Res 33:1815–1820
Smit E, Leeflang P, Glandorf B, van Elsas JD, Wernars K (1999) Analysis of fungal diversity in the wheat rhizosphere by sequencing of cloned PCR-amplified genes encoding 18S rRNA and temperature gradient gel electrophoresis. Appl Environ Microbiol 65:2614–2621
Solheim H (1986) Species of Ophiostomataceae isolated from Picea abies infested by the bark beetle Ips typographus. Nord J Bot 6:199–207
Solheim H (1992a) Fungal succession in sapwood of Norway spruce infested by the bark beetle Ips typographus. Eur J For Pathol 22:136–148
Solheim H (1992b) The early stages of fungal invasion in Norway spruce infested by the bark beetle Ips typographus. Can J Bot 70:1–5
Solheim H (1993a) Ecological aspects of fungi associated with the spruce bark beetle Ips typographus in Norway. In: Wingfield MJ, Seifert KA, Webber JF (eds) Ceratocystis and Ophiostoma. Taxonomy, ecology and pathogenicity. American Phytopathological Society, St. Paul, pp 235–242
Solheim H (1993b) Fungi associated with the spruce bark beetle Ips typographus in an endemic area in Norway. Scand J For Res 8:118–122
Stergulc F, Frigimelica G, Faccoli M, Battisti A (2001) Pest and disease damage in natural and secondary conifer stands of the Friuli – Venezia Giulia (North-eastern Italy). In: Forster B, Knízek M, Grodzki W (eds) Methodology of forest insect and disease survey in central Europe. Proceeding of the IUFRO working party Busteni, Romania: Forest Research and Management Institute (ICAS), pp 167–169
Vega EF, Dowd PF (2005) The role of yeast as insect endosymbionts. In: Vega FE, Blackwell M (eds) Insect-fungal associations: ecology and evolution. Oxford University Press, New York, pp 211–243
Viiri H, Lieutier F (2004) Ophiostomatoid fungi associated with the spruce bark beetle, Ips typographus, in three areas in France. Ann Sci For 61:215–219
von Arx JA (1981) The genera of fungi sporulating in pure culture. J. Cramer, Vaduz
Wang Y, Guo LD (2007) A comparative study of endophytic fungi in needles, bark, and xylem of Pinus tabulaeformis. Can J Bot 85:911–917
Wermelinger B (2004) Ecology and management of the spruce bark beetle Ips typographus – a review of recent research. For Ecol Manag 202:67–82
White TJ, Bruns TD, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Whitney HS (1982) Relationships between bark beetles and symbiotic organisms. In: Mitton JB, Sturgeon KB (eds) Bark beetles in North American Conifers: a system for study of evolutionary biology. University of Texas Press, Austin, pp 183–211
Wichmann L, Ravn HP (2001) The spread of Ips typographus (L.) (Coleoptera, Scolytidae) attacks following heavy windthrow in Denmark, analysed using GIS. For Ecol Manag 148:31–39
Wingfield MJ, Seifert KA, Webber JF (1993) Ceratocystis and Ophiostoma. Taxonomy, ecology, and pathogenicity. American Phytopathological Society, St. Paul
Yamaoka Y, Chung WH, Masuya H (2009) Constant association of ophiostomatoid fungi with the bark beetle Ips subelongatus invading Japanese larch logs. Mycoscience 50:165–172
Aknowledgments
This research was supported by Regione Piemonte (Direzione Opere pubbliche, Difesa del suolo, Economia Montana e Foreste).
We gratefully acknowledge Cristina Varese’s lab (Department of Plant Biology – University of Torino, Italy) for support in fungal identifications via classical methodology, G. Lione for assistance in statistical and rarefaction analyses, and the two anonymous Reviewers for helpful comments.
This work is dedicated to Prof. Giovanni Nicolotti.
Author information
Authors and Affiliations
Corresponding author
Additional information
Giovanni Nicolotti already deceased.
Rights and permissions
About this article
Cite this article
Giordano, L., Garbelotto, M., Nicolotti, G. et al. Characterization of fungal communities associated with the bark beetle Ips typographus varies depending on detection method, location, and beetle population levels. Mycol Progress 12, 127–140 (2013). https://doi.org/10.1007/s11557-012-0822-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-012-0822-1