Abstract
Background
Nutritional supplementation is a potential adjunct in the conservative management of carpal tunnel syndrome (CTS). This study investigated whether astaxanthin (a beta-carotenoid) increased the effectiveness of splinting in managing CTS.
Methods
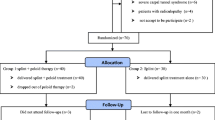
This is a triple-blinded randomized controlled trial where 63 patients with electrodiagnostically confirmed CTS were randomly allocated into either the experimental group (n = 32) (astaxanthin–4-mg capsules + splinting) or the control group (n = 31) (placebo + splinting). Medications were taken for 9 weeks followed by a 3-week washout. The primary outcome measure was the Symptom Severity Scale (SSS). Secondary outcome measures in the study included physical impairments, disability, and health status measures. Electrodiagnostic testing was performed before entry into the study and again at 12 weeks. All other outcomes were measured at baseline, 6, and 12 weeks.
Results
There was a reduction in symptoms as measured by the SSS over the course of treatment in both groups (p = 0.002), but no differences between the groups (p = 0.18). The Disability of Arm, Shoulder and Hand questionnaire and the Short Form 36-item Health Survey showed no effects over time or between treatment groups. The baseline difference between the groups in the level of total cholesterol and low-density lipoproteins remained constant over the course of the study. Impairment measures demonstrated no significant changes in grip, dexterity, or sensation.
Conclusion
At present, the role for astaxanthin as an adjunct in conservative management of CTS has not been established.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carpal tunnel syndrome (CTS) is the most common compression neuropathy with an incidence in the population estimated at 52/100,000 person-years for men and 149/100,000 person-years for women [42]. Non-operative management is the first line of treatment and generally includes wrist splinting of the affected extremity [13, 48]. Other conservative therapies that have been evaluated include non-steroidal anti-inflammatory drugs, injection of medications, rehabilitation modalities (therapeutic ultra-sound, stretching, and strengthening), and pyridoxine [38, 41, 49]. Specific factors are known to affect treatment outcome with upper extremity pathology. Patients on workers compensation are known to have a greater pain and disability with CTS. Severity of nerve compression is also known to affect the likelihood of success with conservative management [26, 31, 39].
Astaxanthin is a lipid-soluble carotenoid found in microalgae, yeast, salmon, trout, krill, shrimp, crayfish, and crustaceans. Astaxanthin, unlike some carotenoids, is not converted to vitamin A (retinol) in the human body. It has lower toxicity than vitamin A and may have a different antioxidant activity than other carotenoids. The US Food and Drug Administration has approved astaxanthin as a food coloring (or color additive) for specific uses in animal and fish foods. A patent awarded by the US Patents Department to Lorenz et al. in 2001 [30] claimed astaxanthin as a method of retarding and ameliorating CTS. Astaxanthin, whose scientific name is ketocarotenoid astaxanthin, 3,30-dihydroxy-b,b-carotene-4,40-dione, belongs to the family of xanthophylls, which are the oxygenated derivatives of carotenoid. Astaxanthin is ubiquitous in nature, especially in the marine environment [29]. It provides the red color of salmon meat and cooked shellfish and contributes to the pinkish-red color of their flesh [25]. Astaxanthin (and other carotenoids produced by Haematococcus algae) has a long history in the food chain and human diet because they occur naturally in foods commonly consumed by humans.
Previous studies have suggested that the bioactivities of carotenoids might be due to their prior conversion to vitamin A and focused on b-carotene. Subsequent studies showed that some carotenoids without pro-vitamin A activity were as active and at times more active than b-carotene [48]. Astaxanthin is one such carotenoid that does not possess a pro-vitamin A activity and has been reported to have potent bio-activities such as antioxidative, anticancer, antidiabetic, and anti-inflammatory activities, gastric, hepatic, neuro, cardiovascular, ocular, and skin-protective effects, and other activities, [50] which are distinctly different and, at least in some cases, more potent than that of other carotenoids [48].
Studies have confirmed that up to 6 mg of astaxanthin per day from a Haematococcus pluvialis algal extract can be safely consumed by healthy adults [17]. Absorption is aided by including the dosage in a fat-rich meal. A dosage of 4 mg of astaxanthin per day from Haematococcus algae is a relatively low amount that would normally be consumed in the human diet from salmonids or shrimp and will not cause any adverse effects in humans.
Splinting has been accepted as a relatively risk-free method of treating CTS conservatively. It has demonstrated effectiveness, although the rate of success and the severity of the CTS amenable to splinting are debatable [38]. Astaxanthin has been claimed to have effect on CTS [30, 34] as it acts as an anti-inflammatory agent because of its inhibitory effects on the production of nitric oxide [51]. Therefore, a potential role for astaxanthin would be as an adjunct to increase the effectiveness of splinting in a conservative approach to CTS.
The purpose of this study is to evaluate the effectiveness of the food additive astaxanthin as an adjunct in the management of CTS.
Materials and Methods
Subjects
All patients presenting with symptoms of primary CTS were evaluated by surgeons at the Hand and Upper Limb Center, London, Ontario, Canada. The diagnosis of carpal tunnel syndrome was made first by the referring family physician and secondarily by experienced hand surgeon at the Hand Center, and all were verified by electrophysiology. Referral electrophysiology was performed by different physical medicine specialists depending on the referring family physician. Follow-up electrophysiology was performed in a single laboratory. All were hand surgeons, and physical medicine specialists were academically appointed at the same university and follow the criteria stated by Remphel et al. [40] for clinical and electrophysiological diagnosis of CTS which includes a recommendation for using the most current version of the American Association of Electrodiagnostic Medicine criteria for diagnosis of CTS. Patients were recruited into the study by their hand surgeon and referred to the Clinical Outcomes Lab for independent blinded assessment of study outcomes. The patients signed an informed consent form before they could participate in the study. Patients were included in the study if they satisfied the following inclusion and exclusion criteria:
Inclusion Criteria
-
1.
CTS clinically diagnosed by hand surgeons and supported by electrophysiological abnormality
-
2.
Competent to comply with treatment and complete study evaluations
-
3.
Aged 18–65 years
Exclusion Criteria
-
1.
Concurrent hand pathology including recent trauma, i.e., fracture, amputation, tumor, or nerve compression such as thoracic outlet syndrome [23];
-
2.
Pregnancy;
-
3.
Wrist arthritis, rheumatoid arthritis, diabetes mellitus, or thyroid disease;
-
4.
Urgent or severe CTS requiring early operative intervention;
-
5.
Inability to complete study forms/assessments; and
-
6.
Neurological disorders.
Outcomes and Measures
The primary outcome of this study was the severity of symptoms of CTS measured by the Symptom Severity Scale (SSS) [28] designed specifically for CTS outcome evaluation. The primary outcome was measured at baseline, 6 and 12 weeks. The SSS has 11 questions covering six different symptom areas. Each question has five responses ranging from 1 point (mildest) to 5 points (most severe). Previous research on a group of 38 patients with CTS has shown the SSS to be a highly reliable and responsive tool in capturing the symptom severity [28]. A reliability of 0.91 has been documented for the SSS [28]. At the conclusion of the study, patients also answered a global rating of change to say whether they had no improvement, mild moderate improvement in symptoms, or complete resolution.
Secondary outcomes in our study included physical impairments, disability, and health status measures. Secondary outcomes were measured at baseline, 6, and 12 weeks according to standardized methods described below, with the exception of electrophysiology which was performed only at baseline and 12 weeks.
-
1.
Motor nerve function: Motor nerve functional impairment was assessed by measuring pinch (tripod) and grip strength using the NK computerized hand evaluation system. The inter-instrument reliability of the NK pinch gauge was high (ICC > 0.90) [31]. The pinch strength scores obtained with NK pinch gauge and two other pinch gauges were consistent and can be compared with normative data obtained with the other pinch gauges [31]. The inter-rater reliability for NK pinch gauge was also very high (ICC > 0.87) [32].
-
2.
Dexterity: Dexterity was measured using the NK Hand Dexterity Board (NKHDT). This provided an objective assessment of the patient’s ability to manipulate three subgroups of objects (small, medium, and large). NKHDT has demonstrated good concurrent validity (r = 0.47–0.87) with Jebson’s Hand Function Test [33] and has a good test–retest reliability 0.48–0.85 [43]. Its responsiveness has also been found to be satisfactory [1].
-
3.
Vibration sensory threshold: A vibrometer with a software-controlled protocol was used to determine vibration sensory threshold. Vibrometer measurements have been found to be consistent with electrodiagnostic findings of CTS [16]. Vibrometry at 50 Hz has demonstrated reliability (reliability coefficient = 0.86) [19].
-
4.
Upper extremity disability: It was measured using the Disability of Arm, Shoulder and Hand (DASH) Questionnaire [6, 20]. It is a 30-item self-report measure that evaluates impairments, activity limitations, as well as participation restrictions due to disorders of the upper limb [44, 45]. The total DASH score is calculated from the raw scores obtained and can range from 0 (no disability) to 100 (severe disability). The DASH has been validated for use in carpal tunnel syndrome [15, 27].
-
5.
Health status: Physical health status was measured using the physical component summary score of the Short Form 36-item Health Survey (SF-36). This is a 36-item questionnaire, yielding an eight-scale profile of scores, as well a physical component summary score and a mental component summary score [46, 47]. The SF-36 is a generic health status measure that has been used in multiple musculoskeletal disorders [2, 5, 8, 14, 24], including carpal tunnel syndrome [3, 4, 9].
-
6.
Touch perception sensory threshold: Touch threshold was measured using the NK pressure specified sensory device. It has demonstrated high test–retest reliability of r = 0.95 [12].
-
7.
Electrophysiology: Parameters including the distal latencies, conduction velocities, and amplitudes of the motor and sensory potentials were measured. At baseline, these were taken from the records provided by the referring family physician/physical medicine specialist; follow-up EMG was performed in a single lab according to a standardized protocol. In both cases, laboratories comply with the recommended guidelines proposed by the American Association of Electrodiagnostic Medicine as outlined by the consensus criteria for diagnosis of carpal tunnel [40]. Buch and Foucher [11] and others [18, 40] have described nerve conduction studies to be an indispensable part of the pre-operative evaluation of CTS. These tests have been reported to have very high sensitivity and specificity of 85% and 87%, respectively [18].
-
8.
Lipid profile: A high level of low-density lipoproteins (LDL) has been described as a risk factor for idiopathic CTS [7, 36]. Blood levels of LDL, high-density lipoproteins (HDL), triglycerides, and total cholesterol were measured in blinded samples by an independent laboratory.
Procedure
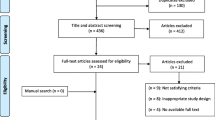
Sixty-three patients with electrodiagnostically confirmed CTS were recruited into the study over a 1-year interval (see Table 2). Astaxanthin capsules and identical placebos were provided to patients according to a computer-generated randomized permutated block design. Allocation was blinded to surgeons, assessors, and clinic staff. The characteristics of the recruited patients are described in Table 1. The recruited patients were randomly allocated into either the experimental group (n = 32) or the control group (n = 31).
Evaluation
All patients were evaluated by their surgeon for entry into the study, and the all study assessments were administered by a blinded research assistant. All patients had standardized tests performed upon entry into study and at 6 and 12 weeks following surgery. Components of these evaluations were:
-
1.
Baseline medical evaluation including history and clinical examination by experienced hand surgeon;
-
2.
Electrophysiology to establish severity of CTS;
-
3.
Screening blood work including CBC, electrolytes, BUN, creatine, ESR, CRP, LFT, and lipid profile.
-
4.
Radiography was performed as part of the clinical evaluation if indicated by history or physical examination, e.g., diagnosis of malalignment with a previous wrist fracture/wrist arthrosis. Results were used to exclude ineligible patients, but not recorded as a study outcome.
-
5.
Impairment and disability outcome measures
Interventions
All patients had been referred by a primary practitioner and received management prior to being seen by a hand surgeon. The exact nature of this varied according to primary care physician. Patient agreed to participate in a trial of splinting with random allocation to the adjunctive treatment.
Patients were randomized to receive either placebo or astaxanthin which were coded to maintain triple blinding (physician, research assistant, and patient). The astaxanthin group received 4-mg capsules of astaxanthin; the placebo group a visually identical capsule. All patients were asked to take the tablets after evening meals. The medications were taken for a 9-week period followed by a 3-week wash-out period. Phone checks were performed at 3-week intervals by the study assistant to check that patients were taking their medication, had enough medication, and had experienced no adverse reactions or unusual symptoms. Patients were asked to return medication bottles, and pill counts were performed to further monitor compliance. Compliance was also evaluated by questionnaire at each re-evaluation.
The wrist was splinted in a position of neutral wrist flexion, i.e., 0° at night, with supplemental splinting during the day for activities that placed the wrist in a position of risk. Patients were instructed not to request a change in treatment during the course of the study and to inform the research assistant if a change became necessary.
Statistical Analysis
Data was entered and verified by cross-checking the database with original records, performed by a second blinded research assistant. All statistical analysis was performed using SPSS statistical software (α = 0.05). Analysis of variance (generalized linear models with repeated measures on the time factor) was used to detect treatment differences over time and between the two treatment groups for the primary outcome measure (SSS) and all secondary outcomes. Number needed to treat (NNT) is the number of patients who need to be treated in order to achieve one successful additional improvement in symptoms (above control) and is the inverse of the absolute risk reduction. The absolute risk reduction is the difference in rate of patients achieving a positive improvement in symptoms in the control group versus the treatment group.
Results
Primary Outcome
Patients were similar on most descriptive factors with the exception of the fact that more people in the astaxanthin group had a previous episode of splinting for CTS (see Table 1) and they had higher baseline cholesterol levels. There was a significant improvement in symptom severity over time in both treatment groups (p = 0.002) (see Table 2). There was no significant difference in the functional scores over time. There was no significant difference between the groups for either symptoms or functional status. More patients responded to global rating that they had a moderate or better improvement with astaxanthin as compared with placebo (13 vs. 8), although this was not significantly different (p = 0.18). This translated into a NNT of 7 (i.e., seven patients would be needed to be treated with astaxanthin before one patient would gain benefit not achieved through the standard treatment). However, the extent of reduction in symptoms was relatively small; the severity of symptoms at the end of the study was still clinically significant. To explore the potential impact of clinical covariates, ANCOVA was performed with presence of hand arthritis (e.g., mild CMC was not an exclusion), previous episode of splinting, or baseline cholesterol as a covariates. These variables were not significant covariates neither did it alter the significance of the primary outcome.
Secondary Outcomes
Impairments in secondary outcomes including the DASH, SF-36, and cholesterol (Table 3); hand impairment measures (dexterity, grip strength, and sensory threshold; Table 4) and electrodiagnostic findings (Table 5) were not significantly different between treatment groups. The majority of these measures also did not show a significant improvement over time with the exception of vibration thresholds which improved over time in both treatment arms and motor amplitude which improved in the placebo arm. Mental health status approximated the US population norm of 50 whereas physical health status was significantly lower than the population mean (see Table 4).
We did a follow-up survey of all patients who had exceeded the normal range for blood levels, in case the family physician had changed medications as a result of these findings. None of the patients reported medication changes, even when specifically questioned about cholesterol-lowering medications.
Side Effects
A few “side-effects” were reported by patients. Our ethics board did not require formal reporting of minor symptomatic complaints such as stomach upset and diarrhea. These were reported with equal frequency between the group getting active astaxanthin and the placebo. No moderate or severe adverse events were reported in either treatment arm.
Discussion
This study did not demonstrate a significant adjunctive effect of astaxanthin when added to a splinting program for conservative management of patients with prolonged symptoms of CTS. This is in contradiction to the reports of Lorenz et al. [30] claiming the ameliorating effects of astaxanthin on CTS.
The results of this study augment and confirm the findings of Nir et al. [37]. In their study, they followed 20 subjects, randomly allocated to either to the astaxanthin group or the placebo group, for 8 weeks. They reported a trend of decreased pain rate and duration; but their results did not reach statistical significance. They concluded that there is insufficient evidence to recommend for or against the use of astaxanthin for CTS and recommended a further large-scale study. Our study in contrast included 63 subjects, and we analyzed multiple outcomes and still found non-significant between-group differences. We observed small differences over time to suggest that there was an improvement in symptoms in patient’s splinted with or without active astaxanthin. Since this analysis is dependent on repeated measures of individuals over time, it had higher power for detecting differences than did the between-group comparisons. Our study was powered to detect a 20% improvement. Both treatment arms experienced less than 10% improvement overall and thus did not reach a level considered clinically significant. If small differences of 10% were considered clinically significant, then sample size would need to be substantially larger.
Since we recruited patients with persistent symptoms, many of whom had a previous episode of conservative management, we might view this as a resistant population. In light of this, these results should not be considered generalizable to a first attempt at conservative management.
In Canada, patients are often managed at the primary care level for substantial amounts of time as hand surgeons have a waitlist. It is known that patients with more prolonged or severe carpal tunnel are less likely to respond to traditional conservative management [10, 35]. A number of these groups may have failed a second attempt at conservative management which may explain the relatively low overall response across different outcome measures. We selected a resistant population, with the view that, if astaxanthin was beneficial in a more difficult population, then it would be an important clinical contribution. However, this effectiveness was not demonstrated. A global rating of moderate improvement was more commonly reported in the astaxanthin group (13/32 versus 8/31) and was associated with a number needed to treat of 7 which might be considered a reasonable treatment threshold. Therefore, we cannot preclude the possibility that astaxanthin has a role if clear indications for patients who could potentially benefit could be defined. Any future study that wishes to do so should focus on patients earlier in their natural history or progression of carpal tunnel syndrome and who have not had a previous trial of conservative management. Since our study had relatively small numbers in subgroups, it was not possible for us to investigate whether specific subgroups were more likely to respond.
Patients were interviewed every 3 weeks with careful attention to the occurrence of self-reported side effects. It is interesting to note that patients in both groups felt that they experienced medication-related side effects. However, the nature and frequency of these were similar between the placebo and astaxanthin groups. As these primarily related to digestive problems or exacerbation of current problems (such as acne), it is highly likely these reported side-effects reflected the current health status of the patients rather than medication-induced changes. The fact that the placebo group reported side effects illustrates how patients can interpret routine symptoms as attributable to an inert medication. A similar attribution occurs in recall bias. This illustrates the importance of prospective, blinded placebo-controlled trials to provide unbiased assessment of treatment effects and side effects/complications.
Systematic reviews have confirmed that conservative management is effective [21, 35] and that surgery has a larger overall benefit [22, 41], in particular, for those who fail conservative management. Our findings of a small improvement over time in a group with chronic carpal tunnel syndrome are in agreement with these evidence syntheses. This study has not identified astaxanthin to be an effective adjunct to standard conservative management.
References
Amadio PC, Silverstein MD, Ilstrup DM, et al. Outcome after Colles fracture: the relative responsiveness of three questionnaires and physical examination measures. J Hand Surg. 1996;21:781–7.
Atroshi I, Andersson IH, Gummesson C, et al. Primary care patients with musculoskeletal pain. Value of health-status and sense-of-coherence measures in predicting long-term work disability. Scand J Rheumatol. 2002;31:239–44.
Atroshi I, Gummesson C, Johnsson R, et al. Symptoms, disability, and quality of life in patients with carpal tunnel syndrome. J Hand Surg Am. 1999;24:398–404.
Atroshi I, Johnsson R, Sprinchorn A. Self-administered outcome instrument in carpal tunnel syndrome. Reliability, validity and responsiveness evaluated in 102 patients. Acta Orthop Scand. 1998;69:82–8.
Beaton DE, Hogg-Johnson S, Bombardier C. Evaluating changes in health status: reliability and responsiveness of five generic health status measures in workers with musculoskeletal disorders. J Clin Epidemiol. 1997;50:79–93.
Beaton DE, Katz JN, Fossel AH, et al. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14:128–46.
Becker J, Nora DB, Gomes I, et al. An evaluation of gender, obesity, age and diabetes mellitus as risk factors for carpal tunnel syndrome. Clin Neurophysiol. 2002;113:1429–34.
Bergman S, Jacobsson LT, Herrstrom P, et al. Health status as measured by SF-36 reflects changes and predicts outcome in chronic musculoskeletal pain: a 3-year follow up study in the general population. Pain. 2004;108:115–23.
Bessette L, Sangha O, Kuntz KM, et al. Comparative responsiveness of generic versus disease-specific and weighted versus unweighted health status measures in carpal tunnel syndrome. Med Care. 1998;36:491–502.
Boyd KU, Gan BS, Ross DC, et al. Outcomes in carpal tunnel syndrome: symptom severity, conservative management and progression to surgery. Clin Invest Med. 2005;28:254–60.
Buch-Jaeger N, Foucher G. Correlation of clinical signs with nerve conduction tests in the diagnosis of carpal tunnel syndrome. J Hand Surg J British Soc Surg Hand. 1994;19:720–4.
Dellon AL, Keller K. Computer-assisted quantitative sensorimotor testing in patients with carpal and cubital tunnel syndromes. Ann Plast Surg. 1997;38:493–502.
Falkenburg SA. Choosing hand splints to aid carpal tunnel syndrome recovery. Occup Health Saf. 1987;56(60):63–4.
Gartsman GM, Brinker MR, Khan M, et al. Self-assessment of general health status in patients with five common shoulder conditions. J Shoulder Elbow Surg. 1998;7:228–37.
Gay RE, Amadio PC, Johnson JC. Comparative responsiveness of the disabilities of the arm, shoulder, and hand, the carpal tunnel questionnaire, and the SF-36 to clinical change after carpal tunnel release. J Hand Surg Am. 2003;28:250–4.
Gelberman RH, Szabo RM, Williamson RV, et al. Sensibility testing in peripheral-nerve compression syndromes. An experimental study in humans. J Bone Joint Surg Am. 1983;65:632–8.
Gene AS, Antonella D. Safety of an astaxanthin-rich Haematococcus pluvialis algal extract: a randomized clinical trial. J Med Food. 2003;6:51–6.
Gunnarsson L, Amilon A, Hellstrand P, et al. The diagnosis of carpal tunnel syndrome: sensitivity and specificity of some clinical and electrophysiological tests. J Hand Surg J British Soc Surg Hand. 1997;22:34–7.
Hubbard MC, MacDermid JC, Kramer JF, et al. Quantitative vibration threshold testing in carpal tunnel syndrome: analysis strategies for optimizing reliability. J Hand Ther. 2004;17:24.
Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29:602–8.
Huisstede BM, Hoogvliet P, Randsdorp MS, et al. Carpal tunnel syndrome. Part I: effectiveness of nonsurgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:981–1004.
Huisstede BM, Randsdorp MS, Coert JH, et al. Carpal tunnel syndrome. Part II: effectiveness of surgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:1005–24.
Hurst LC, Weissberg D, Carroll RE. The relationship of the double crush to carpal tunnel syndrome (an analysis of 1,000 cases of carpal tunnel syndrome). J Hand Surg J British Soc Surg Hand. 1985;10:202–4.
Jain R, Hudak PL, Bowen CV. Validity of health status measures in patients with ulnar wrist disorders. J Hand Ther. 2001;14:147–53.
Johnson EA, An G. Astaxanthin from microbial sources. Crit Rev Biotechnol. 1991;11:297–326.
Kaplan SJ, Glickel SZ, Eaton RG. Predictive factors in the non-surgical treatment of carpal tunnel syndrome. J Hand Surg J British Soc Surg Hand. 1990;15:106–8.
Kotsis SV, Chung KC. Responsiveness of the Michigan Hand Outcomes Questionnaire and the Disabilities of the Arm, Shoulder and Hand questionnaire in carpal tunnel surgery. J Hand Surg Am. 2005;30:81–6.
Levine DW, Simmons BP, Koris MJ, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993;75:1585–92.
Lorenz RT, Cysewski GR. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000;18:160–7.
Lorenz RT, Cysewski GR. Method of retarding and ameliorating carpal tunnel syndrome United States patent US 6258855 B1, 2000.
MacDermid JC, Evenhuis W, Louzon M. Inter-instrument reliability of pinch strength scores. J Hand Ther. 2001;14:36–42.
MacDermid JC, Kramer JF, Woodbury MG, et al. Interrater reliability of pinch and grip strength measurements in patients with cumulative trauma disorders. J Hand Ther Official J Amer Soc Hand Ther. 1994;7:10–4.
MacDermid JC, Mule M. Concurrent validity of the NK hand dexterity test. Physiother Res Int. 2001;6:83–93.
Moore A. Blooming prospects? Eur Mole Biol Organ Rep. 2001;2:462–4.
Muller M, Tsui D, Schnurr R, et al. Effectiveness of hand therapy interventions in primary management of carpal tunnel syndrome: a systematic review. J Hand Ther. 2004;17:210–28.
Nakamichi K, Tachibana S. Hypercholesterolemia as a risk factor for idiopathic carpal tunnel syndrome. Muscle Nerve. 2005;32:364–7.
Nir Y, Spiller G, Multz C. Effect of an astaxanthin-containing product on carpal tunnel syndrome. J Am Coll Nutr. 2002;21:489.
O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003;(1):CD003219.
Phalen GS. The carpal-tunnel syndrome: clinical evaluation of 598 hands. Clin Orthop. 1972;83:29–40.
Rempel D, Evanoff B, Amadio PC, de Krom M, Franklin G, Franzblau A, et al. Consensus criteria for the classification of carpal tunnel syndrome in epidemiologic studies. Am J Public Health. 1998;88:1447–51.
Shi Q, Macdermid JC. Is surgical intervention more effective than non-surgical treatment for carpal tunnel syndrome? A systematic review. J Orthop Surg Res. 2011;6:17.
Stevens JC, Sun S, Beard CM, et al. Carpal tunnel syndrome in Rochester, Minnesota, 1961 to 1980. Neurology. 1988;38:134.
Turgeon TR, MacDermid JC, Roth JH. Reliability of the NK dexterity board. J Hand Ther. 1999;12:7–15.
Upper Extremity Collaborative Group. Measuring disability and symptoms of the upper limb: a validation study of the DASH questionnaire. Arth Rheum. 1996;39:S112.
Upper Extremity Collaborative Group. The Dash outcome measure: user’s manual. Toronto: Institute for Work and Health; 1999.
Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: a user’s manual. Boston, MA: The Health Institute, New England Medical Center; 1994. p. 1.1–10.12.
Ware JE, Snow KK, Kosinski M, et al. SF-36 health survey manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993.
Weiss D, Gordon, Blooms, et al. Position of the wrist associated with the lowest carpal-tunnel pressure: implications for splint design. J Bone Joint Surg 1995;77.
Wilson JK, Sevier TL. A review of treatment for carpal tunnel syndrome. Disabil Rehabil. 2003;25:113–9.
Yuan J, Chen F, Liu X, et al. Carotenoid composition in the green microalga Chlorococcum. Food Chem. 2002;76:319–25.
Yuan J-P, Peng J, Yin K, et al. Potential health-promoting effects of astaxanthin: a high-value carotenoid mostly from microalgae. Mol Nutr Food Res. 2011;55:150–65.
Acknowledgments
We would like to acknowledge support of Anthony Almada for contributing to study design/oversight of astaxanthin formulation and of IMAGINutrition/Meta Response Sciences for funding of the study.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
MacDermid, J.C., Vincent, J.I., Gan, B.S. et al. A blinded placebo-controlled randomized trial on the use of astaxanthin as an adjunct to splinting in the treatment of carpal tunnel syndrome. HAND 7, 1–9 (2012). https://doi.org/10.1007/s11552-011-9381-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11552-011-9381-1