Abstract
Purpose
Facet joint insertion is a common treatment of chronic pain in the back and spine. This procedure is often performed under fluoroscopic guidance, where the staff’s repetitive radiation exposure remains an unsolved problem. Robotic ultrasound (rUS) has the potential to reduce or even eliminate the use of radiation by using ultrasound with a robotic-guided needle insertion. This work presents first clinical data of rUS-based needle insertions extending previous work of our group.
Methods
Our system implements an automatic US acquisition protocol combined with a calibrated needle targeting system. This approach assists the physician by positioning the needle holder on a trajectory selected in a 3D US volume of the spine.
Results
By the time of submission, nine facets were treated with our approach as first data from an ongoing clinical study. The insertion success rate was shown to be comparable to current clinical practice. Furthermore, US imaging offers additional anatomical context for needle trajectory planning.
Conclusion
This work shows first clinical data for robotic ultrasound-assisted facet joint insertion as a promising solution that can easily be incorporated into the clinical workflow. Presented results show the clinical value of such a system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Like any spinal synovial joint degeneration, inflammation can lead to pain while moving, initiating a cycle of physical degradation, irritation of facet innervations and muscle spasms. Image-guided insertion of local anesthetics and steroids into or around the facet joint aims to break this vicious cycle and thereby provide pain relief. Facet joint injections are the reference treatment when dealing with facet-related spinal pain caused by joint degeneration, inflammation or injury [5]. Treatment is usually ambulatory and performed under fluoroscopic guidance [11]. X-ray images are used in order to both find the initial needle insertion point and correct the needle trajectory once it has been inserted. This leads to a high variability in accuracy, duration and radiation exposure depending on the physician’s level of experience [17] and the particular patient’s anatomy. The physician’s accumulated exposure motivates the search for alternatives to fluoroscopic guidance.
Ultrasound imaging (US) has been proven to be effective in facet joint insertion by providing complementary information to physical examination [14]. Compared to fluoroscopy, the reported success rate, analgesic effect and functional improvement showed no significant differences [8]. However, the coordination between the use of free-hand US and the simultaneous insertion of the needle creates difficulties. Furthermore, in clinical practice, the ultrasound plane is commonly acquired orthogonally to the x-ray projection, requiring the physicians to adjust their perception of the procedure accordingly. The inherent physics of US combined with complex bone structures in the spinal anatomy increase the difficulty of free-hand US needle insertions and therefore require extensive expertise [17] and special training [12]. Guidance system has shown to improve the results of the surgery [15]. In this document, the rUS solution shown in Fig. 1 is proposed, in which a robot assists the doctor; by performing a 3D US sweep and physically guiding the insertion. This allows the physician to view a 3D volume in which the main spine anatomy is visible [9]. The volumetric representation permits information to be rendered in multiple axes, as shown in Fig. 2, increasing the amount of information available to the physician. This improves their perception of the anatomy at hand and may help to improve the aforementioned difficulties. Furthermore, since the compounding is registered with the robot’s workspace, it also enables the desired needle path to be selected in the digital volume. During needle placement, the robotic assistant can then place the needle holder as dictated by the digitally planned trajectory with the help of a rigid needle guide calibrated to the US transducer.
This study extends our group’s previous efforts toward a 3D rUS needle guidance solution applied to facet joint insertions [18, 19] by presenting the first clinical evaluation. A total of nine insertions were performed in three discrete sessions as part of an ongoing clinical study. In accordance with the ethical approval, in this first study the robotic solution is only used to augment the procedure and does not interfere with the OR workflow, such that a comparison with the current standard is carried out. The adaptations presented in this work are thereby derived from an intensive study of the OR workflow and describe the main technical alterations compared to previous systems, including i) the implementation and validation of new US strategies to improve the image quality, ii) the integration of the US probe with a commercially available, sterile needle guide solution, as well as iii) all technical improvements required for the safe use of the system in the OR with real patients. To this end, the work also discusses challenges faced in order to achieve a transition into clinical practice with a solution developed on the foundation of the previous work of our group.
Related work
US guidance for needle insertions has been broadly used at different levels of complexity, from the most simple manual insertions using only an image as a guidance [14] to more advanced solutions to train physicians [12]. In general, different groups have reported a result comparable to the gold standard [8, 17]. Besides a manual guidance, several robotic needle placement solutions have been tested in phantoms and ex-vivo tissue. A robotic needle steering model was tested for lung biopsies based on X-ray images with accurate results in a phantom [4]. 3D US was also used in tests to guide a vibrating needle using US Doppler images and a steering system in ex-vivo bovine liver, showing errors comparable to manual insertions [3]. Furthermore, a 3D US-based automatic steering system which avoids moving obstacles based on 3D US images and a tracked needle tip for needle insertions in gelatin phantoms and biological tissue was developed in [2]. This work was expanded recently to employ an implicit force control system to improve contact of a transducer with curved surfaces [1]. In view of (semi-)autonomous robotic ultrasound (rUS) acquisitions on the human body, a system for MRI-based acquisitions showed the feasibility of force-compliant volumetric US acquisitions [7]. On this foundation, an image-based registration system for neurosurgical applications was presented [18, 19] which illustrates the starting point of this work. The work employed transversal ultrasound sweeps using a mechanically actuated ultrasound probe attached to the end effector of a robot, which were then registered to CT images. The needle path was determined pre-interventionally on the CT of a phantom. One US sweep was registered to the CT, and new US volumes were registered continuously to the original sweep, thus providing an online steering system.
To the best of our knowledge no rUS-guided solution for targeted insertions has ever been evaluated clinically for spinal needle insertion. This work aims at closing this gap, and discussing first results of our findings.
Methods
The proposed system is a step toward a rUS-based facet joint insertion system without any additional C-arm and is based on an autonomous acquisition of a panoramic US sweep compounded into a 3D volume. This 3D scan is used by the physician to plan the needle insertion trajectories for the robot to be reproduced. The two step process presented in previous work [18] is thereby modified such that the requirements with respect to pre-interventional data have been relieved. In the pre-interventional phase, the planning was previously performed in CT, which was then registered to interventional US images. However, by improving the quality of US images, CT planning can be omitted and the practitioner can perform intraoperative planning directly on the US data. This removes the uncertainty of the (deformable) registration between CT and US and removes the necessity for a pre-interventional CT. Finally, the previously introduced US–US registration is discarded for this study despite the promising results, as the movement of the patient is small enough, relative to the target area of the facet joint (ranging from \(14.61\pm 1.80\) to \(19.45\pm 2.3\) mm [6]), that registration is not considered necessary. However, it should be noted that axial movements of the system derived from the patient breathing are intrinsically compensated by the robot direct force control scheme, as described in [18].
a A visual representation of transformations: From the robot base to the EE \({^W}T_E\), from the EE to the US plane \({^E}T_U\), and from the US plane to both the tip of the needle \({^U}T_T\) and insertion point \({^U}T_I\). b Frame of reference used for the automatic US acquisition. a Robot transformations, b patient reference frames
System overview
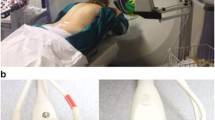
Hardware Setup The system is comprised of a KUKA LBR iiwa 7 R800 robot (KUKA Roboter GmbH, Augsburg, Germany), as well as a convex ultrasound probe which is rigidly affixed to the end effector with a dedicated needle guide. An overview of the setup is shown in Fig. 1.
The iiwa robot is a 7 joint arm with torque sensors in each of its joints and certified for human interaction. It is natively driven by the KUKA Sunrise.OS connected to a ROS client using a framework previously developed in our lab [7].
An Ultrasonix Sonix RP system with a C5-2/60 GPS Convex probe is used for B-mode acquisition (BK Ultrasound, Analogic Corp., Peabody, MA, USA). The frequency, depth and gain are set to 2.5 MHz, 100 mm, and 43%, respectively.
Probe mount with needle guide The US probe is attached to the end effector of the robot using a custom-designed mount, augmented with a detachable needle guide as shown in Fig. 1. The needle holder is coupled to the ultrasound transducer in order to guide the physician to the target during needle insertion. To allow for integration with a sterile setting, a commercially available needle guide (model Ultra-Pro II, Civco, Coralville, Iowa, USA ) is employed. The needle guide is composed of a two-part system: A reusable bracket attached to the US transducer and a disposable sterile snap-on needle guide. The reusable (unsterile) bracket is custom-designed for the system and adapted to the probe mount. The disposable kit features a quick-release function for the snap-on part and an opening mechanism to release the needle without removing it from the patient. The disposable sterile kit includes a telescopically-folded CIV-Flex cover, US gel and two rubber bands to fix the cover to the US probe.
Software infrastructure The system is connected using the mentioned custom ROS communication moduleFootnote 1 connected to a ROS client workstation for reporting the robot’s current state and executing new commands. Desired force and stiffness in tool direction during the panoramic sweep are set to \(F_{d} = {10}~{\mathrm{N}}\) and \(K_{d} = {0}~{\mathrm{N/m}}\), respectively. During the insertions of the needle stiffness is increased to \(K_{d} = {100}~{\mathrm{N/m}}\). The stiffness in all other directions is kept constant at \({1000}~{\mathrm{N/m}}\) in both cases. Both parameters were manually determined on former routine lab tests on healthy volunteers without insertions.
The publicly available PLUS library 2.2.0 [10], maintains a uni-directional connection via OpenIGTLink to the client workstation for streaming of incoming B-mode frames. The ImFusion Suite Version 1.5.16 (ImFusion GmbH, Munich, Germany) is used as the basis of the image processing system, running on a dedicated workstation (Intel(R) Core(TM) i7-7700HQ CPU 2.80 GHz, 16 GB RAM, NVIDIA GeForce GTX 1060 (6 GB) graphics card). The application integrates all the previously mentioned systems with our custom-developed software modules.
Ultrasound acquisition protocols
Previous to the US acquisition, the robot is placed at the side of the patient adjacent to the lumbar spine. The chosen protocol allows the physician to guide the robot by hand and select two points on the patient’s back, namely the center rostral (start) and caudal (end) of the sweep (Fig. 3).
We define: the sweep start and end points as S and E; and the spine direction vector as \(\vec {S}_{p} = \overrightarrow{SE} \). Thus, it is possible to define the vector \(\vec {H}\) as \(\vec {S}_{p} \times \vec {Z}_{\mathrm{up}}\), with \(\vec {Z}_{\mathrm{up}}\) the positive Z-axis of the local coordinate system. Finally, \(\vec {S}_{\mathrm{up}}\) is the rotation of the \(\vec {S}_{p}\) by \(\frac{\pi }{2}\) around \(\vec {H}\). For the US acquisition, \(\vec {S}_{\mathrm{up}}\) is the orientation vector of the US probe, as seen in Fig. 4. The robot then follows the selected path in force control mode along \(\vec {S}_{\mathrm{up}}\), automatically compensating for the changes in height of the patient’s surface.
In order to identify the best panoramic scanning strategy, different protocols, inspired from [13], for US acquisition are tested qualitativel y with respect to their influence on the robotic workspace, acquisition time, global image quality and the specific image quality of the facet joints (see Table 1). In a transversal sweep, the transducer is swept axially along the spine from the initial to the final position recording the transverse plane. The side-sweep consists of two parallel longitudinal sweeps of the transverse plane of the spine with a lateral offset of 2 centimeters and a roll angle of \(10^{\circ }\) to better visualize the facet joints. The zigzag protocol is a collection of arching sagittal sweeps, starting at \(-10^{\circ }\) and traveling from one 2 cm offset to the other while transitioning to an orientation of \(10^{\circ }\). In this way, the robot travels step-wise from the level of S to E. Finally, the complete protocol consists of a side-sweep augmented by a longitudinal and a zigzag sweep.
System calibration and targeting
The transformation from the end effector (EE) of the robot to the US plane \({^E}T_U\) can be extracted from the CAD model of the probe mount. The calibration between the US plane and the needle tip, represented \({^U}T_T\), can be performed in two different ways: i) by selecting the needle tip in an ultrasound frame, as shown in [18] or ii) by using the pivot calibration method, where the needle tip remains fixed at a point and the robot EE is rotated in different configurations [16]. The first method was used due to its simplicity. Finally, the transformation to the insertion point of the needle on the patient’s skin in relation to the US image plane \({^U}T_I\) is defined as the point in the intersection of the plane orthogonal to the US plane and the needle line, see Fig. 4. The complete transformations are defined as: \(Tip ={^W}T_E \cdot {^E}T_U \cdot {^U}T_T \) for the needle tip pose and \( Insertion Point = {^W}T_E \cdot {^E}T_U \cdot {^U}T_I \) for the insertion point pose. On the foundation of a complete system calibration, the needle target planning process can be carried out intraoperatively after the initial panoramic scan. A US-trained physician visualizes the volume and gives indications to the operator who directly selects the position of the needle trajectory by clicking in the US volume. Two points are selected: the entry on the patient skin and the target. The system then calculates the trajectory and moves to the patient while remaining in force mode.
System evaluation
In preparation of the clinical evaluation described in “Clinical evaluation” section, the employed technical components were altered with respect to previous work [18]. A different ultrasound probe was used to improve image quality, and new ultrasound protocols were implemented. In order to achieve clinical accuracy, new calibrations of the system were required and subsequent calibration tests were performed.
3D ultrasound acquisitions
In order to select the best strategy for our application, the overall visibility of the spine, facets visibility, the use of robot workspace and time required were evaluated prior to the clinical test on healthy volunteers. The different strategies were tested and qualitatively evaluated against the mentioned factors. The sub-parameters of offset to the spine [1–4 cm], and probe inclinations [\(5\hbox {--}20^{\circ }\)], which mainly affect the workspace of the robot, were also modulated for each sweep strategy to ensure the acquisition feasibility. Our results are listed in Table 1. Our evaluation led to the selection of the best strategy: use of the side-sweep protocol with an offset of 2 cm and a tilt of \(10^{\circ }\). Mean acquisition time is \(\sim 65\) s with the speed of the robot limited to 5% speed, both for safety and quality reasons.
Needle targeting accuracy
The two major error sources of the US system are i) the mechanical error from the kinematics of the cart on which the manipulator is mounted, the robot itself and needle guide calibration and ii) the calibration of the correspondence between the target in the 3D US volume and real-world robot target pose. The calibration of the needle w.r.t. to the robot EE was validated by targeting the same point on a flat surface 10 times from random initial robot poses. The lateral targeting error was found to be \(\pm {4,5}\,\mathrm{{mm}}\) in x axis and \(\pm {2,3}\,\mathrm{{mm}}\) in the y axis. During clinical practice, the error in the z direction is determined solely by the correspondence between the rendered 3D volume and the robot position since the robot’s vertical position is adapted online, as the robot attempts to maintain a constant contact force onto the patient. The correspondence between robot position and 3D volume was measured with two crossing plastic wires submerged in a bath of warm water (\(38.2\,^{\circ }\)C). A total of 16 US sweeps of the wire intersection were collected with random trajectories and the crossing points were manually segmented in the 3D volume. A total reconstruction error of \(4.78\pm {2.28}\,\mathrm{{mm}}\) was measured. Targeting error can be considered acceptable for a first clinical application on lumbar facet joints which are in the range of \(14.61\pm 1.80\) to \(19.45\pm 2.3\) mm depending on the level [6].
Clinical evaluation
The clinical evaluation focuses on performing the facet joint infiltration with less radiation than the required by the current practice. In close cooperation with the ethics committee, the study was designed to fit not only the Declaration of Helsinki and EU legislation, but also the standards of all physicians and technicians involved.
Study selection and workflow
Inclusion and exclusion criteria are illustrated in Table 2. A total number of nine facets, in three discrete sessions, from two different patients, were treated during this first phase of the clinical study. As part of the ongoing study, a total of 20 patients are planned to be treated.
The traditional workflow in the OR consists of an ambulatory procedure which is carried out by the doctor and at least one assistant. The patient has to lie prone on the interventional bed. The entire back of the patient with the exception of the lumbar area is covered with operating banquets. Next, the exposed area is sterilized. Several X-ray shots are taken, while a pointy metallic stylus, highly visible on the X-ray, is positioned on the back of the patient in order to find the entry point of the needle. Then the needle is inserted and a verification X-ray taken. If the needle is not exactly on the desired point, corrections are done by bending and partially reinserting the needle while taking more verification X-rays.
The proposed workflow, as stated in Fig. 5, establishes an additional layer on top of the normal procedure, independent of the standard procedure which can be completely removed at any time without alterations. The ultrasound transducer is draped with a sterile US cover previously filled with gel. The cover is then fixed with the sterile rubber bands, also included in the kit. The robot arm is finally covered as the last part of this preparation step. This process then continues following the standard clinical procedure; the material is prepared, the patient positioned and the back covered and treated with an antiseptic agent. The robotic control mode is then changed to hand guiding mode, and the physician, already with sterile equipment, moves the ultrasound transducer to the initial and final position of the sweep. In this way, the physician can guide the robot using the US images to properly center the sweep. Both positions are recorded by the robot operator. The robot then begins with the acquisition sweep. Once the sweep is finished, the volume is automatically compounded, while the physician takes verification X-rays for each facet to be injected and marks the desired entry point on the back of the patient. Then the physician selects the target and entry point in the compounded volume, using the available graphic information provided by the system on the three views (axial, sagittal and coronal), as well as the 3D rendering of the volume. The robot then moves to the desired position. Subsequently, the physician validates the insertion point by comparing the one suggested by the robot to the marks selected with the fluoroscope. If the targeting is correct, the doctor proceeds with the insertion and once the needle is slightly inserted, the needle holder is released, and the robot moves away from the patient. In the scope of this clinical trial, and as stated in the ethics declaration, the insertion is carried on and verified under X-ray supervision (Figs. 6, 7).
Results
At the time of submission, the study was successfully performed on nine facet joints treated in three independent sessions; one session with five insertions for Patient 1 [70 y/o] and two sessions, each with two insertions for Patient 2 [72 y/o]. Of the nine facets treated, the five corresponding to Patient 1 were performed as a prescribed secondary prevention treatment and those of Patient 2 as diagnostic.
The guidance of the robot was successful in all the insertions, showing the correct entry point and inclination according to the physician verification on X-ray. Both the US images used for the planning and the verification X-rays can be observed on Fig. 8. Corrections were needed in five of the nine infiltrations and were performed under X-ray as required by the ethics committee. According to the experience within the medical department of the consortium, these preliminary results seem promising and the number of needle placements without corrections appears to be equal or even better to the normal procedure. Patients reported no pain or discomfort coming from the robotic part of the procedure and showed pain relief or no pain after the procedure. The duration of the procedure for the first patient was 48 min including both planning on US and the normal X-ray workflow. US acquisition and planning summed up to 11 min against the 8 required on X-ray. Duration of both sessions for the second patient was 27 and 19 min respectively, with a total of 15 and 6 min used on US acquisition and planning plus 8 min on X-ray. Time per insertion can be appreciated in Table 3. Positive qualitative evaluation about the usability was received from the physician and the assistant team, remarking the easy integration of the robotic solution without negatively affecting the normal workflow and the expected decrease on the learning curve for non-experienced doctors in comparison to the traditional X-ray based solution. Furthermore, in Patient 1, who had undergone three multilevel decompression surgeries, the visualization on X-ray was limited. However, the physician identified the scar tissue on US (Fig. 8) and used it as a landmark for the US planing.
Discussion
In this work, we have performed the first clinical trial using an ultrasound-based robotic guiding system to perform facet joint insertions. The entry point and inclination offered by the robot were successful compared to the verification X-rays. This suggest that, at least, the proposed solution would save the initial X-rays taken to find the right vertebral level and the exact entry point. However, radiation could not be completely removed as corrections under fluoroscopy were still needed. Further developments should focus on their minimization. Possible identified causes are: (i) non-optimal US image quality which hardens the target identification and selection, (ii) overall system accuracy and calibration, (iii) needle bending, (iv) difficulties for X-ray verification due to the challenging anatomy on the selected patients. In addition to the potential reduction of fluoroscopy, further benefits of our approach were discovered during our clinical trial. It was observed that the X-ray modality has its own shortcomings. In Patient 1, who had undergone previous invasive procedures in the target area, finding the facet joint with X-ray projections was shown to be harder or almost unfeasible due to the lack of visible anatomies. In theses cases, the proposed solution can visualize soft-tissue landmarks such as scarring and connective tissue, which can be used for guidance to the target anatomy. This benefit, and the reduction of radiation, constitutes major advantages of robotic US imaging for spinal insertions compared to traditional approaches. The interaction between the robot, the medical personnel and the patients, as well as the their evaluation of the system was highly satisfactory. Based on the short clinical experience, the overall planning time using US seem to be comparable to the time used by an expert on the traditional X-ray based approach. Building on these promising findings, the current system could be enhanced with new features such as bone detection or spine model registration to improve the usability of spinal rUS. In conclusion, our novel approach allows for easy integration to the clinical workflow while assisting the physician.
References
Abayazid M, Moreira P, Shahriari N, Patil S, Alterovitz R, Misra S (2015) Ultrasound-guided three-dimensional needle steering in biological tissue with curved surfaces. Med Eng Phys 37(1):145–150
Abayazid M, Vrooijink GJ, Patil S, Alterovitz R, Misra S (2014) Experimental evaluation of ultrasound-guided 3D needle steering in biological tissue. Int J Comput Assist Radiol Surg 9(6):931–939
Adebar TK, Fletcher AE, Okamura AM (2014) 3-D ultrasound-guided robotic needle steering in biological tissue. IEEE Trans Biomed Eng 61(12):2899–2910
Ding J, Stoianovici D, Petrisor D, Mozer P, Avila R, Ibanez L, Turner W, Yankelvitz D, Wilson E, Banovac F, Cleary K (2008) Medical needle steering for lung biopsy: Experimental results in tissue phantoms using a robotic needle driver. In: 8th IEEE international conference on bioinformatics and bioengineering, pp 1–5
Gopinathan A, Peh WC (2011) Image-guided facet joint injection. Biomed Imaging Interv J 7(2):e4
Gorniak G, Conrad W (2015) Lower lumbar facet joint complex anatomy. Austin J Anat 2(1):1–8
Hennersperger C, Fuerst B, Virga S, Zettinig O, Frisch B, Neff T, Navab N (2017) Towards MRI-based autonomous robotic us acquisitions: a first feasibility study. IEEE Trans Med Imaging 36(2):538–548
Hoon Han S, Park K, Rai Cho K, Park Y (2017) Ultrasound versus fluoroscopy-guided medial branch block for the treatment of lower lumbar facet joint pain: a retrospective comparative study. Medicine 96(16):e6655
Khallaghi S, Mousavi P, Gong RH, Gill S, Boisvert J, Fichtinger G, Pichora D, Borschneck D, Abolmaesumi P (2010) Registration of a statistical shape model of the lumbar spine to 3D ultrasound images. Med Image Comput Comput Assist Interv MICCAI 2010:68–75
Lasso A, Heffter T, Rankin A, Pinter C, Ungi T, Fichtinger G (2014) PLUS: open-source toolkit for ultrasound-guided intervention systems. IEEE Trans Biomed Eng 61(10):2527–2537
Mohammed Motawea A, Ramadan El Kholy M, Mohamed Ebied O, El Fattah Abd, Mousa W, Shawky Abdullah M (2015) Imaging-guided facet joint injection technique for lower back pain management. Am J Health Res 3(2):52–56
Moult E, Ungi T, Welch M, Lu J, McGraw RC, Fichtinger G (2013) Ultrasound-guided facet joint injection training using perk tutor. Int J Comput Assist Radiol Surg 8(5):831–836
Rasoulian A, Seitel A, Osborn J, Sojoudi S, Nouranian S, Lessoway VA, Rohling RN, Abolmaesumi P (2015) Ultrasound-guided spinal injections: a feasibility study of a guidance system. Int J Comput Assist Radiol Surg 10(9):1417–1425
Soni NJ, Franco-Sadud R, Schnobrich D, Dancel R, Tierney DM, Salame G, Restrepo MI, McHardy P (2016) Ultrasound guidance for lumbar puncture. Neurol Clin Pract 6(4):358–368
Ungi T, Abolmaesumi P, Jalal R, Welch M, Ayukawa I, Nagpal S, Lasso A, Jaeger M, Borschneck DP, Fichtinger G, Mousavi P (2012) Spinal needle navigation by tracked ultrasound snapshots. IEEE Trans Biomed Eng 59(10):2766–2772
Yaniv Z (2015) Which pivot calibration? Image-Guided Proced Robot Interv Model 9415:941527
Yoon SH, O’Brien SL, Tran M (2013) Ultrasound guided spine injections: advancement over fluoroscopic guidance? Curr Phys Med Rehabil Rep 1(2):104–113
Zettinig O, Frisch B, Virga S, Esposito M, Rienmüller A, Meyer B, Hennersperger C, Ryang YM, Navab N (2017) 3D ultrasound registration-based visual servoing for neurosurgical navigation. Int J Comput Assist Radiol Surg 12(9):1607–1619
Zettinig O, Fuerst B, Kojcev R, Esposito M, Salehi M, Wein W, Rackerseder J, Sinibaldi E, Frisch B, Navab N (2016) Toward real-time 3d ultrasound registration-based visual servoing for interventional navigation. In: IEEE international conference on robotics and automation (ICRA), pp 945–950
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (mp4 160435 KB)
Rights and permissions
About this article
Cite this article
Esteban, J., Simson, W., Requena Witzig, S. et al. Robotic ultrasound-guided facet joint insertion. Int J CARS 13, 895–904 (2018). https://doi.org/10.1007/s11548-018-1759-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-018-1759-x