Abstract
The world of cardiac imaging is proposing to physicians an ever-increasing spectrum of options and tools with the disadvantages of patients presently submitted to multiple, sequential, time-consuming, and costly diagnostic procedures and tests, sometimes with contradicting results. In the last two decades, the CCTA has evolved into a valuable diagnostic test in today’s patient care, changing the official existing guidelines and clinical practice with a pivotal role to exclude significant CAD, in the referral of patients to the Cath-Lab, in the follow-up after coronary revascularization, and finally in the cardiovascular risk stratification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The cardiovascular diseases (CVD) are the principal cause of death and health expenditure in western countries, more than all cancers combined and with the coronary artery disease (CAD) still the leading killer [1]. The CAD is a long-term pathological process characterized by a progressive atherosclerotic plaque accumulation along the wall of the epicardial arteries, whether obstructive or non-obstructive and with a more or less long subclinical phase [2], which can be modified by lifestyle adjustments, pharmacological therapies, and invasive interventions aimed to reach disease stabilization or regression. The CAD is a chronic disease with long and stable periods but most often progressive, even in clinically apparently silent periods and can become unstable at any time, typically due to an acute atherothrombotic event caused by plaque rupture or erosion [2]. Not rarely, the fatal event may be the first clinical presentation [3]. Following the recent guidelines of the European Society of Cardiology (ESC), the dynamic nature of the CAD results in various clinical presentations, classifiable as acute coronary syndrome (ACS) or chronic coronary syndrome (CCS) [2]. In general, in stable patients, a high suspicion of CAD with a clinical indication to invasive coronary angiography (ICA), in order to pursue an invasive treatment, is placed by the pretest probability assessment (PTP) of disease based on appropriate clinical risk scores such as the Framingham Risk Score (FRS) [4] or the Diamond and Forrester Score modified and updated [5]. To date, in clinical practice, between one half and two-thirds of elective ICA are completed without intervention [6], which indicates that current diagnostic strategies for stable patients suspected of having CAD may overestimate disease [7]. Given the lower than expected real prevalence of CAD and the fact that not all patients with coronary stenosis ≥ 50% require invasive treatment, it is necessary to have a noninvasive diagnostic test that can hypothetically act as an effective gatekeeper for the cardiac Cath-Lab in patients with current indications for ICA and that allows patient management to be promptly addressed by the results of the noninvasive test without the need for additional diagnostic procedures.
Coronary CT angiography
Since its clinical introduction in the early 2000s, thanks to a continuous technological evolution in both hardware (HW) and software (SW) components, the coronary CT angiography (CCTA) has rapidly satisfied all the basic characteristics and the so-called perceived values of each new imaging diagnostic test: less invasiveness, more availability, less time-consuming, more user-friendly, greater accuracy, significant impact on patient management, and costs [8]. The explosion of a huge scientific literature over the past 10–15 years on the role not only diagnostic but also prognostic [9, 10] of CCTA has certified its evolution from mere innovation to established technology in the cardiological clinical arena through the achievement and overcoming of all the different phases relative to the definition and widespread of a new diagnostic technique (Fig. 1). Since the first CCTA multicenter/multivendor studies [11,12,13,14], the common denominator has been its high negative predictive value (NPV), identifying the CCTA as the only one noninvasive diagnostic method able to rule out significant CAD and the consequent need for further tests or revascularization procedures. Furthermore, its capability to differentiate healthy coronary arteries (i.e., free from any atherosclerotic plaques) from atherosclerotic coronary arteries (obstructive/non-obstructive) and to assess the coronary plaque burden [15] has progressively opened the door to its potential role in the stratification of the cardiovascular risk in the individual patient unlike what is offered by population-based clinical scores.
CCTA as the first test in patient with stable angina
In the last 15 years, many scientific guidelines, statements, or appropriateness criteria have proposed/indicated the diagnostic role of CCTA in different clinical scenarios, proposing this technique initially to support and subsequently to replace second- or higher-level diagnostic tests in the patient’s diagnostic workflow with suspected CAD. The assessment of CAD by CCTA was initially based on the exclusion or confirmation of stenosis using a binary approach (greater or lesser) with a significance threshold set at the 50% reduction in the vessel lumen diameter, but subsequently transformed into a multi-grade approach [16, 17] with different stenosis values (0, < 25%, 25–49%, 50–69%, 70–89%, ≥ 90%) in order to stratify at best the severity of disease and put stronger indications on further functional tests or treatment’s procedures [16]. Despite the availability of quantitative analysis SW from several years, the CAD assessment by CCTA is performed in clinical practice by means of a visual evaluation, which rarely differs from the quantitative analysis of coronary angiography (QCA) by more than one range of stenosis. Generally, CCTA stenoses < 50% rarely require consideration for revascularization, while intermediate stenoses (50–69%) are associated with QCA stenosis ≥ 70% in 15% of cases; so, additional assessment of myocardium ischemia may significantly impact patient management. A CCTA stenosis ≥ 70% corresponds to ICA criteria for revascularization at least 50% of the time, while stenoses ≥ 90% are virtually diagnostic of severe CAD/occlusion [16]. Anyway, it has been demonstrated that the visual evaluation during ICA (mostly performed in clinical practice in the majority of the Cath-Labs) yields higher stenosis degrees compared with QCA [18]. The state-of-art CT scanners (i.e., > 64-row CT) for optimal CCTA allow to evaluate not only the patency/stenosis of the coronary lumen but also an accurate evaluation of all the main plaques characteristics in terms of composition, attenuation pattern, and high-risk features useful to assess the so-called plaque burden (Table 1) [15] and with proved prognostic significance [10]. Several quantitative research tools, anyway with still limited application in the clinical routine, allow to discriminate the different components of atherosclerotic plaques on the basis of their density values (Hounsfield Units, HU) such as the low-density core (< 60HU or even < 30UH), considered nowadays an hallmark finding suggestive for unstable plaque, in particular, if associated with other findings as the positive remodeling (PR) [19] and the napkin-ring sign (NRS) [10, 20]. Even if interesting, these quantitative analyses are still time-consuming and strongly influenced by the overlapping HU-values of the different plaque components, with CT-attenuation values still depended by different scan and technical parameters (scan protocols, slice thickness, kVp, reconstruction algorithms/filters, noise, HU-threshold values), by the iodine concentration of the contrast agent administered intravenously and consequent adjacent vessel lumen contrast-enhancement [21]. The Scot-Heart study has shown that the addition of CCTA to standard of care (SOC) in patients with suspected angina due to CAD clarifies the diagnosis and enables targeting of interventions [22], resulting in a significantly lower rate of death from CAD or non-fatal myocardial infarction (MI) at 5 years than SOC alone, without resulting in a significantly higher rate of ICA or coronary revascularization (13.5% versus 12.9%) [23]. Several studies assessed CCTA for selective ICA [24,25,26]; in particular, the Conserve study [27], a multinational and randomized clinical trial with more than 1.600 patients either direct ICA or CCTA, showed that the absence of ≥ 50% stenosis on ICA was significantly lower in the CCTA-guided arm (25% vs 61%, p < 0.001), the major adverse cardiovascular events (MACE) rates were similar (4.6% vs. 4.6%) at median of 1-year follow-up, and that 57% of lower costs were observed in the CCTA-guided arm ($2755 vs $1183). Figure 2 shows the typical diagnostic workflow and the role of CCTA in symptomatic patient at low/intermediate pretest likelihood of CAD, underlining the well-recognized limitations of such noninvasive anatomical test in the evaluation of intermediate stenosis (60–70%), in which a functional test is often required. The recent 2019 ESC guidelines for the diagnosis and management of CCS [2] as well as the latest update of the National Institute for Health and Care Excellence (NICE) clinical guidelines [28] put an end to doubts and discussion about the CCTA, officially recognizing its diagnostic role in this clinical setting. In particular, CCTA is nowadays recommended as the initial test for diagnosing CAD in symptomatic patients in whom obstructive CAD cannot be excluded by clinical assessment alone (Class I), with functional imaging for myocardial ischemia recommended if CCTA has shown CAD of uncertain functional significance or is not diagnostic (Class I). Similarly, CCTA should be considered as an alternative to ICA if another noninvasive test is equivocal or non-diagnostic (Class IIa). Differently, CCTA is not recommended when extensive coronary calcification [15], irregular heart rate, significant obesity, reduced respiratory compliance, or any other conditions make good image quality unlikely (Class III) [2]. To this acknowledgment corresponds a significant limitation of the role of exercise-ECG, now recommended for the evaluation of exercise tolerance, symptoms, arrhythmias, blood pressure response, and event risk in selected patients (Class I) or as an alternative test to rule-in/rule-out CAD when other noninvasive or invasive imaging methods are not available (Class IIb) [2]. The continuous evolution of CT technology has also recently allowed the development and execution of functional analysis of coronary flow, in a sort of hybrid imaging, through the evaluation derived from CT of the fractional flow reserve (FFRct) [29,30,31] or myocardial perfusion (CT perfusion, CTP) [32], with the advantage of reducing the false positive (FP) test results, mainly in the assessment of the intermediate stenoses (50–70%) and thus increasing both the positive predictive value (PPV) and specificity [33, 34] (Tables 2 and 3). The rationale behind the assessment of ischemia is given by the fact that although the severity of the stenosis is one of the factors capable of predicting the presence/absence of ischemia, the association between anatomy and ischemia is poor. The FAME study [35] has shown that stenoses of 50–70% can cause ischemia (FFR < 0,8) in 35% of cases, while stenoses > 70% can result in no-ischemia (FFR > 0.8) in 20%. A recent meta-analysis on the diagnostic performance of different invasive and noninvasive cardiac imaging techniques compared to invasive FFR [36], considered the reference-standard for the functional assessment of CAD [37], showed higher specificity and diagnostic accuracy for the FFRct alone or even higher if associated with CCTA compared to cardiological (ICA and stress echocardiography) or nuclear medicine techniques. The first real-world experience of CCTA with FFRct as gatekeeper to the Cath-Lab in non-emergent symptomatic patients with intermediate CAD [38] showed that a conclusive FFRct result has obtainable in 98% of patients who had CT scans referred for FFRct testing (i.e., very low dropout rate) and that the implementation of FFRct for clinical decision making may influence the downstream diagnostic workflow. Actually, in case of FFRct < 0.75 the risk of a FP result using FFR as the reference-standard is very low (< 10%), while patients with FFRct > 0.80 may be deferred from ICA having a favorable short-term prognosis. Differently, in patients with FFRct ranging between 0.76 and 0.80, a non-negligible number of FP results may be still expected. The ADVANCE-Registry has shown that FFRct modifies the treatment recommendation in two-thirds of subjects as compared to CCTA alone, is associated with less negative ICA, predicts revascularization, and identifies subjects at low-risk of MACE [39]. A recent NXT-trial sub-study [40] has shown that a FFRct value ≤ 0.8 in individuals with stable CAD is a strong predictor of long-term clinical outcomes and superior to the anatomical detection of significant stenosis on CCTA, while a normal FFRct is associated with favorable clinical outcome and that the FFRct numeric value is an independent predictor of outcomes. The presence of gross parietal calcifications or calcified plaques extended circumferentially for more than 180° still constitute today, for the so-called beam-hardening artifacts, a limit of the method and the main cause of overestimation of stenoses [15]. It has even recently shown that FFRct provides high and superior diagnostic performance compared with CCTA alone in patients with high coronary artery calcium scores (CACS) [41] as well as at all levels of CACS [42]. Table 4 summarizes and compares the main strengths and limitations of CTP and FFRct. Further analysis from the DISCOVER-FLOW [43] and the NXT [44] trials also demonstrated how the analysis and implementation of the FFRct allow a cost reduction of 30–32% improving the clinical outcomes (12–19% less events) at 1-year follow-up (versus ICA visual strategy).
CCTA for risk stratification
The ICA is not an accurate predictor of the ACS. Most of the MI occur on coronary arteries affected by stenosis < 70% [45], while the angiographic severity of stenosis may be inadequate to predict time and location of a subsequent occlusion that will produce a MI [46]. The clinical risk scores evaluate simply and easily measurable risk factors (RFs) variables predicting (a priori) the 10-year risk of CAD for a population, dividing arbitrarily subjects into three categories of risk: low (< 10%), intermediate (10–20%) and high risk (> 20%) [4]. The NCEP ATP-III guidelines couple the treatment intensity to the magnitude of risk, with attention on lipid modification and LDL-cholesterol targets [47]. Anyway, these guidelines have never been validated through randomized controlled trials, but their simplicity, low cost, and reasonable prognostic accuracy have made this approach the standard template for prevention. In addition, the FRS underestimates the variability of the magnitude of atherosclerotic burden between subjects with similar levels/profiles of RFs, presumably related to other known/unknown genetic/environmental factors [48]. Most of the subjects with sudden cardiac death (SCD) or non-fatal MI do not experience prior chest pain or exertional dyspnea, emphasizing the importance of early detection and treatment of underlying subclinical atherosclerosis (ATS). More than 75% of all hard-coronary events occur in people misclassified at low/intermediate risk by the traditional RFs-based approach and, consequently, this not offers optimal preventive therapy [49, 50], while others are misclassified as high risk and advised to take drugs that reduce RFs they do not need and remain under medical treatment for the rest of their lives. To date, no guideline indicates CT as a screening tool for CAD prevention. In particular, the current guidelines in primary prevention provide an initial assessment and risk stratification (RS) based on the analysis of traditional RFs, followed by goal-directed therapy when necessary [51]. The cerebral/peripheral vascular diseases, diabetes mellitus, previous myocardial ischemia, or more than 2 traditional RFs are currently considered equivalent of the coronary heart disease (CHD) [52], but the existing guidelines do not recognize the non-obstructive CAD as an CHD-equivalent event, though most of the heart attack originate from non-obstructive plaques and frequently without any pre-monitory symptoms [53]. From histology and intravascular imaging (intravascular ultrasound, IVUS; optical coherence tomography, OCT) studies, distinct features of vulnerable plaques at higher risk of rupture have been identified [54]: large plaque volume, large necrotic core, thin fibrous-cap atheroma (TFCA), spotty calcification, and PR [55]. The currently emerging research is focusing on improving coronary RS tools using CCTA parameters based on what we learned from invasive imaging [56, 57], given the high correlation described between the histological and IVUS/OCT plaque features (TCFA, plaque burden) more frequently observed in patients with acute MI or ACS than stable angina [56], with those plaques showing low-attenuation core, PR, and NRS at CCTA [19, 57]. The technical evolution and the considerable radiation dose reduction (even < 1 mSv) offered by state-of-art CT scanners allow to scan subjects with less restricted inclusion criteria for CCTA, with a further compelling application in the field of CAD-detection in addition to the role of gatekeeper to ICA: the RS of asymptomatic individuals to target/personalize medical therapy to prevent CHD. The recent 2019 ACC/AHA guidelines on the Primary Prevention of Cardiovascular Disease [58] state that the assessment of CACS can be indicated (Class II) in case of uncertainty about the initiation of preventive interventions (e.g., statin therapy) in patients classified at intermediate risk for CAD (≥ 7.5% to < 20% 10-year) or in selected adults at borderline risk (5% to < 7.5% 10-year). But what if in these patients the CACS was 0 and the CCTA documented a non-calcific and non-obstructive plaque? The guidelines say nothing about it and while they recognize the CACS as a risk modifier in asymptomatic subjects (Class IIb; level B), they do not recognize this role to CCTA in individuals at borderline or intermediate risk. The CCTA is the only diagnostic technique able to noninvasively detect the subclinical ATS, with the advantage to go beyond the simple luminal stenosis assessment analyzing all other CAD features suggestive for high-risk plaque (HRP) like the low-density non-calcified plaque (LD-NCP), total plaque volume (TPV), spotty calcifications, PR, and the NRS both visually and with semiautomated coronary plaque quantification/characterization analysis [59]. All these plaques characteristics have been shown to be independent predictors [19,20,21] as well as the strongest MACE predictors, thus suggesting their integration into the coronary RS and intensification of individual preventive measures [13]. Two other recent studies from the NXT trial have shown that differences in plaque volumes and composition may explain the discordance between coronary stenosis severity and ischemia, being the plaque volumes inversely proportional to the FFR irrespective of stenosis severity and with a strong association between stenosis severity, plaque characteristics, FFRct and FFR, suggesting some threshold values of quantitative plaque analysis as predictors of FFR < 0.80 (LD-NCP volume > 30 mm3, NCP volume > 185 mm3, TPV > 195 mm3, and plaque length > 30 mm) [60] and that the adding total vessel HRP-volume to stenosis severity improves discrimination of ischemia in CCTA performed in patients with stable angina pectoris [61]. Recently, the development and first application of artificial intelligence (AI) with an integrated machine-learning (ML) ischemia risk score seem to improve the prediction of lesion-specific ischemia by invasive FFR, over stenosis, plaque measures, and pretest likelihood of CAD [62]. Summarizing, HRP-features can be easily determined during routine evaluation of CCTA and should be included in standardized reports in accordance with the current guideline recommendations [17], offering relevant prognostic information, with incremental value in comparison with clinical scores and other imaging modalities (CACS or myocardial perfusion imaging, MPI). The noninvasive assessment of ATS could offer a more patient-specific approach for the clinical management of disease, permitting risk reclassification. CCTA will have a pivotal role in guiding preventive therapeutic strategies in the next future, encouraging clinicians to careful management of the associated RFs, to intensify the prophylactic treatment, and carefully follow-up patients to prevent future MI.
CCTA in the emergency department
Acute chest pain (ACP) is one of the most common reasons for admission in the Emergency Department (ED); initial triage has on one hand to rapidly identify ACS for immediate treatment, on the other to identify very low-risk patients that can be safely discharged. However, clinical presentation, common RFs, and risk scores are not sufficient, and the MACE rate of very low-risk patients is about 2% [63]. Consequently, noninvasive tests are mandatory: the introduction of high-sensitive troponins (hs-Tn) sampling deeply modified and accelerated patients’ triage, increasing sensitivity; nevertheless, the specificity of a mild troponins increase is not so high, and in this scenario, CCTA can play a significant role, because of its well-known high NPV. Many guidelines and consensus documents analyzed the role of CCTA in ACP. Appropriateness criteria defined as appropriate the use of CCTA in low- to intermediate-risk patients presenting with ACP and non-conclusive ECG and serum biomarkers [64, 65]; ESC non-ST ACS guidelines suggested CT as test in patients with non-conclusive serum biomarkers [66]. Also, important clinical trials demonstrated the role of CT, not only in diagnosis but also in prognosis definitions. The first trial was the ROMICAT [67], whose results were published in 2009; 368 patients with ACP, normal serum markers, and negative or non-conclusive ECG underwent CCTA: 50% of patients showed normal coronary arteries, 31% non-obstructive CAD and 19% showed non-conclusive results or obstructive CAD. Conclusions were that due to the high number of negative studies in low- to intermediate-risk patients, CCTA significantly improves patient management in ED. In 2011, the CT-STAT trial [68] was the first published multicenter randomized trial comparing CCTA or MPI and SOC triage, in 699 patients, having as primary and secondary endpoints efficacy and safety and costs, respectively. CCTA-triage resulted in reduced time to diagnosis and lower costs. In 2012 the ACRIN trial [69] was published, enrolling 1370 low- to intermediate-risk patients, randomized 2:1 to CCTA and SOC strategies. Primary endpoint was safety at 30 days, while secondary endpoints were length of stay in ED (lower in CCTA group) and direct discharge (increased in CCTA group). In this trial, no increased access to ICA was observed, and patients of CCTA arm showed a lower rate of ICA findings, compared to SOC arm. In 2012, the ROMICAT II results were published [70]; 1000 patients with suspected ACS, without ECG ischemic changes and negative initial serum markers were randomized between CCTA and SOC; the ACS rate was 8%. Hospitalization length was lower and discharge rate higher in the CCTA group, without differences in the outcome at 28 days, but with increase in downstream exams and no costs reduction in the CCTA group. As CCTA can give information not only about stenosis severity, but also about plaque characteristics (Fig. 3), in 2015 a cohort observational study from the ROMICAT II population was performed [71], to compare hs-Tn and advanced analysis of CCTA (stenosis plus plaque assessment) versus standard troponins and conventional CCTA (stenosis only). Results showed higher risk stratification and better diagnostic accuracy for the first approach. (AUC: 0.84 vs 0.74). However, not all randomized clinical trials demonstrated similar results in terms of better outcome; on the other hand, quite all trials showed decreased length of stay in ED, decreased admission rates, and reduced costs in the CCTA groups. In 2015, the PROSPECT trial [72] compared CCTA and stress-MPI in 400 patients, randomized 1:1, having as primary endpoint the selection of patients to invasive management within 1 year. This trial did not demonstrate any significant statistical difference between the two arms, neither for the primary endpoint, nor for the secondary one (length of stay, costs, clinical events). In terms of outcome, the CATCH trial (median follow-up 18.7 months) [73], demonstrated that a CCTA-guided treatment strategy improves clinical outcome in patients with recent ACP and normal ECG and troponin values compared to SOC with a functional test (MPI, myocardial perfusion imaging). The introduction of hs-Tn [74], widely available in Europe, in the SOC made the early noninvasive imaging testing debatable. The BEACON trial, published in 2016 [75], was the first one that compared CCTA and SOC (including stress-ECG or MPI or direct discharge) with hs-Tn available in both arms (total population 500 patients). No significant differences were found in terms of primary endpoint (revascularization rate), and secondary endpoints as readmission to ED or MACE, while costs and further testing rate were reduced in the CCTA arm. However, further trials are needed to evaluate the impact of the association of hs-Tn and CCTA in outcome evaluation. Furtherly, the integration of new CT techniques, as CTP or FFRct, is still under investigation in ACS. We can conclude that nowadays the triage in ED is based on hs-Tn and in case of high clinical suspicion in patients with mildly elevated values and low to intermediate risk, CCTA should be performed.
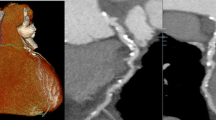
CCTA in patient with ACP: 3D VR a and curved MPR b well show non-calcified plaques in proximal and mid LAD with PR (b–d), suggesting for vulnerable plaques (ACP acute chest pain, LAD left anterior descending artery, MPR multiplanar reconstruction, 3D VR three-dimensional volume rendering, PR positive remodeling)
CCTA post-coronary revascularization
Timing of follow-up of revascularized patients has been object of debate for a long time. It is important to differentiate if the revascularized patient is symptomatic or asymptomatic, according to AHA-ACC-SCCT criteria [64]:
-
(a)
In presence of symptoms, CCTA is appropriate after CABGs, while is considered uncertain after stenting with stent diameter > 3 mm; for stents < 3 mm, CCTA is not indicated;
-
(b)
For asymptomatic subjects, CCTA is considered appropriate in case of left main stenting (stent > 3 mm) (Fig. 4), while is uncertain 2 years after stent > 3 mm implantation and 5 years after CABGs.
Fig. 4 Curved MPR a–b in patent stents in LMA and proximal LAD with homogeneous enhancement of the lumen. 3D VR c, curved d–e and stretched f MPR in mid-distal occlusion of LAD stent with hypodensity of the lumen, absent run-off proximal to the distal edge of the stent (arrowhead in d–f), and thin enhancement of distal LAD (arrow in d–f) due to collaterals (MPR multiplanar reconstruction, LMA left main artery, LAD left anterior descending artery, 3D VR three-dimensional volume rendering)
-
(c)
No indications exist for lower temporal intervals.
In case of coronary stent, the major limitation for the assessment of in-stent restenosis (ISR) is represented by the blooming artifact, caused by the high density stent structure that obscures the stent lumen; possible solution is the use of high convolution reconstruction algorithms; however, the final result depends on stent material and thickness of stent strut (better if 100 μm) and works better in case of higher diameter of the stent (> 3 mm) [76]. Other factors limiting stent imaging are bifurcation stenting and elevated heart rate; in the last case, the use of scanners > 64 slices and or higher temporal resolution can improve accuracy.
In case of CABGs, CCTA is able to clearly depict the patency or occlusion of the graft (Fig. 5), the origin or its anastomosis on the ascending aorta, the body of the graft, and the anastomosis to native coronary artery [77]; furthermore, native coronaries distal to grafts have to be assessed. CCTA in CABGs assessment is robust and effective, as demonstrated by many studies, without significant differences between 64-slice scanners and > 64 slice ones [78].
3D VR a–c and curved MPR d–f in multiple (5) CABGs: patent LIMA to LAD, patent RIMA to RI with a retro-aortic course, patent SVG to right-PL branch with a sequential anastomosis on OM branch (arrow in b), and 2 SVG occluded at the proximal anastomosis on ascending thoracic aorta (arrowheads in a) (3D VR three-dimensional volume rendering; MPR multiplanar reconstruction; LSA left subclavian artery; LIMA left internal mammary artery; LAD left anterior descending artery; RCA right coronary artery; RIMA right internal mammary artery; Ao aorta; SVG saphenous vein graft, RI ramus intermedius; OM obtuse marginal branch; PL posterior-lateral branch)
Going beyond current appropriateness criteria, CCTA in CABGs could be applied also:
-
In asymptomatic patients with a positive stress-test;
-
In patients with atypical chest pain and inconclusive stress-tests;
-
In preoperative planning for redo cardiac surgery (LIMA/RIMA in situ graft course; RV anatomy).
Conclusions
World of cardiac imaging is proposing to physicians an ever-increasing spectrum of options and tools with the disadvantages of patients presently submitted to multiple, sequential, time-consuming, and costly diagnostic procedures and tests, sometimes with contradicting results. In the last years, CCTA has matured into a valuable diagnostic test in today’s patient care, changing the official existing guidelines and clinical practice with a pivotal role to exclude significant CAD, in the referral of patients to the Cath-Lab, and finally in the cardiovascular RS.
Availability of data and material
Not applicable
References
Virani SS, Alonso A, Benjamin EJ et al (2020) heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation 14:e139–e596
Knuuti J, Wijns W, Saraste A et al (2020) 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 41:407–477
La Russa R, Catalano C, Di Sanzo M et al (2019) Postmortem computed tomography angiography (PMCTA) and traditional autopsy in cases of sudden cardiac death due to coronary artery disease: a systematic review and meta-analysis. Radiol Med 124:109–117
Kannel WB, D’Agostino RB, Sullivan L, Wilson PW (2004) Concept and usefulness of cardiovascular risk profiles. Am Heart J 148:16–26
Lee UW, Ahn S, Shin YS et al (2020) Comparison of the CAD consortium and updated Diamond-Forrester scores for predicting obstructive coronary artery disease. Am J Emerg Med [Epub ahead of print]
Patel MR, Peterson ED, Dai D et al (2010) Low diagnostic yield of elective coronary angiography. N Engl J Med 362:886–895
Bittencourt MS, Hulten E, Polonsky TS et al (2016) European society of cardiology-recommended coronary artery disease consortium pretest probability scores more accurately predict obstructive coronary disease and cardiovascular events than the diamond and Forrester score: the partners registry. CAD Consortium Scores. Circulation 134:201–211
Agliata G, Schicchi N, Agostini A et al (2019) Radiation exposure related to cardiovascular CT examination: comparison between conventional 64-MDCT and third-generation dual-source MDCT. Radiol Med 124:753–761
Pundziute G, Schuijf JD, Jukema JW et al (2007) Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J Am Coll Cardiol 49:62–70
Feuchtner G, Kerber J, Burghard P et al (2017) The high-risk criteria low-attenuation plaque < 60 HU and the napkin-ring sign are the most powerful predictors of MACE: a long-term follow-up study. Eur Heart J Cardiovasc Imaging 18:772–779
Miller JM, Rochitte CE, Dewey M et al (2008) Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 359:2324–2336
Budoff MJ, Dowe D, Jollis JG et al (2008) Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 52:1724–1732
Meijboom WB, Meijs MF, Schuijf JD et al (2008) Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 52:2135–2144
Marano R, De Cobelli F, Floriani I et al (2009) Italian multicenter, prospective study to evaluate the negative predictive value of 16- and 64-slice MDCT imaging in patients scheduled for coronary angiography (NIMISCAD-Non Invasive Multicenter Italian Study for Coronary Artery Disease). Eur Radiol 19:1114–1123
Marano R, Savino G, Merlino B et al (2013) MDCT coronary angiography- postprocessing, reading, and reporting: last but not least. Acta Radiol 54:249–258
Cheng V, Gutstein A, Wolak A et al (2008) Moving beyond binary grading of coronary arterial stenoses on coronary computed tomographic angiography: insights for the imager and referring clinician. JACC Cardiovasc Imaging 1:460–471
Cury RC, Abbara S, Achenbach S et al (2016) CAD-RADS coronary artery disease e reporting and data system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr 10:269–281
Nallamothu BK, Spertus JA, Lansky AJ et al (2013) Comparison of clinical interpretation with visual assessment and quantitative coronary angiography in patients undergoing percutaneous coronary intervention in contemporary practice: the Assessing Angiography (A2) project. Circulation 127:1793–1800
Motoyama S, Sarai M, Harigaya H et al (2009) Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 54:49–57
Tanaka A, Shimada K, Yoshida K et al (2008) Non-invasive assessment of plaque rupture by 64-slice multidetector computed tomography–comparison with intravascular ultrasound. Circ J 72:1276–1281
Dalager MG, Bttcher M, Andersen G et al (2011) Impact of luminal density on plaque classification by CT coronary angiography. Int J Cardiovasc Imaging 27:593–600
SCOT-HEART investigators (2015) CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 385:2383–2391
Investigators SCOT-HEART (2018) Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 379:924–933
Shaw LJ, Hausleiter J, Achenbach S et al (2012) Coronary computed tomographic angiography as a gatekeeper to invasive diagnostic and surgical procedures: results from the multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multicenter) registry. J Am Coll Cardiol 60:2103–2114
Douglas PS, Pontone G, Hlatky MA et al (2015) Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies versus usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFR(CT): outcome and resource impacts study. Eur Heart J 36:3359–3367
Dewey M, Rief M, Martus P et al (2016) Evaluation of computed tomography in patients with atypical angina or chest pain clinically referred for invasive coronary angiography: randomised controlled trial. BMJ 355:i5441
Chang HJ, Lin FY, Gebow D et al (2019) Selective referral using CCTA versus direct referral for individuals referred to invasive coronary angiography for suspected CAD: a randomized, controlled, open-label trial. JACC Cardiovas Imaging 12:1303–1312
Koo BK, Erglis A, Doh JH et al (2011) Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol 58:1989–1997
Min JK, Leipsic J, Pencina MJ et al (2012) Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 308:1237–1245
Nørgaard BL, Leipsic J, Gaur S et al (2014) Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: next Steps). J Am Coll Cardiol 63:1145–1155
Bamberg F, Becker A, Schwarz F et al (2011) Detection of hemodynamically significant coronary artery stenosis: incremental diagnostic value of dynamic CT-based myocardial perfusion imaging. Radiology 260:689–698
Gonzalez JA, Lipinski MJ, Flors L, Shaw PW, Kramer CM, Salerno M (2015) Meta-analysis of diagnostic performance of coronary computed tomography angiography, computed tomography perfusion, and computed tomography-fractional flow reserve in functional myocardial ischemia assessment versus invasive fractional flow reserve. Am J Cardiol 116:1469–1478
Li S, Tang X, Peng L, Luo Y, Dong R, Liu J (2015) The diagnostic performance of CT-derived fractional flow reserve for evaluation of myocardial ischaemia confirmed by invasive fractional flow reserve: a meta-analysis. Clin Radiol 70:476–486
Tonino PA, Fearon WF, De Bruyne B et al (2010) Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 55:2816–2821
Danad I, Szymonifka J, Twisk JWR et al (2017) Diagnostic performance of cardiac imaging methods to diagnose ischaemia-causing coronary artery disease when directly compared with fractional flow reserve as a reference standard: a meta-analysis. Eur Heart J 38:991–998
Pijls NH, Sels JW (2012) Functional measurement of coronary stenosis. J Am Coll Cardiol 59:1045–1057
Nørgaard BL, Hjort J, Gaur S, Hansson N et al (2017) Clinical use of coronary CTA-derived FFR for decision-making in stable CAD. JACC Cardiovasc Imaging 10:541–550
Fairbairn TA, Nieman K, Akasaka T et al (2018) Real-world clinical utility and impact on clinical decision-making of coronary computed tomography angiography-derived fractional flow reserve: lessons from the ADVANCE Registry. Eur Heart J 39:3701–3711
Ihdayhid AR, Norgaard BL, Gaur S et al (2019) Prognostic value and risk continuum of noninvasive fractional flow reserve derived from coronary CT angiography. Radiology 292:343–351
Nørgaard BL, Gaur S, Leipsic J et al (2015) Influence of coronary calcification on the diagnostic performance of CT angiography derived FFR in coronary artery disease: a substudy of the NXT trial. JACC Cardiovasc Imaging 8:1045–1055
Packard RR, Li D, Budoff MJ, Karlsberg RP et al (2017) Fractional flow reserve by computerized tomography and subsequent coronary revascularization. Eur Heart J Cardiovasc Imaging 18:145–152
Hlatky MA, Saxena A, Koo BK, Erglis A, Zarins CK, Min JK et al (2013) Projected costs and consequences of computed tomography-determined fractional flow reserve. Clin Cardiol 36:743–748
Kimura T, Shiomi H, Kuribayashi S et al (2015) Cost analysis of non-invasive fractional flow reserve derived from coronary computed tomographic angiography in Japan. Cardiovasc Interv Ther 30:38–44
Falk E, Shah PK, Fuster V (1995) Coronary plaque disruption. Circulation 92:657–671
Little WC, Constantinescu M, Applegate RJ et al (1988) Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation 78:1157–1166
Grundy SM, Cleeman JI, Merz CN et al (2004) Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 110:227–239
Karim R, Hodis HN, Detrano R, Liu CR, Liu CH, Mack WJ (2008) Relation of Framingham risk score to subclinical atherosclerosis evaluated across three arterial sites. Am J Cardiol 102:825–830
Ridker PM, Buring JE, Rifai N, Cook NR (2007) Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA 297:611–619
Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR (2008) C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation 118:2243–2251
De Backer G, Ambrosioni E, Borch-Johnsen K et al (2004) European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of eight societies and by invited experts). Atherosclerosis 173:381–391
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001) Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285:2486–2497
Adabag AS, Luepker RV, Roger VL, Gersh BJ (2010) Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol 7:216–225
Narula J, Garg P, Achenbach S, Motoyama S, Virmani R, Strauss HW (2008) Arithmetic of vulnerable plaques for noninvasive imaging. Nat Clin Pract Cardiovasc Med 5(Suppl 2):S2–S10
Naghavi M, Libby P, Falk E, Casscells SW et al (2003) From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation 108:1664–1672
Jang IK, Tearney GJ, MacNeill B et al (2005) In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation 111:1551–1555
Nakazato R, Otake H, Konishi A et al (2015) Atherosclerotic plaque characterization by CT angiography for identification of high-risk coronary artery lesions: a comparison to optical coherence tomography. Eur Heart J Cardiovasc Imaging 16:373–379
Arnett DK, Blumenthal RS, Albert MA et al (2019) 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 140:e563–e595
Di Cesare E, Patriarca L, Panebianco L et al (2018) Coronary computed tomography angiography in the evaluation of intermediate risk asymptomatic individuals. Radiol Med 123:686–694
Gaur S, Øvrehus KA, Dey D et al (2016) Coronary plaque quantification and fractional flow reserve by coronary computed tomography angiography identify ischaemia-causing lesions. Eur Heart J 37:1220–1227
Øvrehus KA, Gaur S, Leipsic J et al (2018) CT-based total vessel plaque analyses improves prediction of hemodynamic significance lesions as assessed by fractional flow reserve in patients with stable angina pectoris. J Cardiovasc Comput Tomogr 12:344–349
Dey D, Gaur S, Ovrehus KA et al (2018) Integrated prediction of lesion-specific ischaemia from quantitative coronary CT angiography using machine learning: a multicentre study. Eur Radiol 28:2655–2664
Kontos MC, Jesse RL (2000) Evaluation of the emergency department chest pain patient. Am J Cardiol 85:32B–39B
Taylor AJ, Cerqueira M, Hodgson JM et al (2010) ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriateuse criteria for cardiac computed tomography. a report of the American college of cardiology foundation appropriate use criteria task force, the society of cardiovascular computed tomography, the American college of radiology, the American heart association, the American society of echocardiography, the American society of nuclear cardiology, the north American society for cardiovascular imaging, the society for Cardiovascular Angiography and interventions, and the society for cardiovascular magnetic resonance. Circulation 122:e525–e555
Rybicki FJ, Udelson JE, Peacock WF et al (2016) 2015 ACR/ACC/AHA/AATS/ACEP/ASNC/NASCI/SAEM/SCCT/SCMR/SCPC/SNMMI/STR/STS appropriate utilization of cardiovascular imaging in emergency department patients with chest pain: a joint document of the American College of Radiology Appropriateness Criteria Committee and the American College of Cardiology Appropriate Use Criteria TaskForce. J Am Coll Cardiol 67:853–879
Roffi M, Patrono C, Collet JP et al (2016) 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 37:267–315
Hoffmann U, Bamberg F, Chae CU et al (2009) Coronary Computed Tomography Angiography For Early Triage of Patients with Acute Chest Pain - The Rule Out Myocardial Infarction Using Computer Assisted Tomography (ROMICAT) Trial. J Am Coll Cardiol 53:1642–1650
Goldstein JA, Chinnaiyan KM, Abidov A et al (2011) The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol 58:1414–1422
Litt HI, Gatsonis C, Snyder B et al (2012) CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med 366:1393–1403
Hoffmann U, Truong QA, Schoenfeld DA et al (2012) Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 367:299–308
Ferencik M, Liu T, Mayrhofer T et al (2015) highly sensitive troponin I followed by advanced coronary artery disease assessment using computed tomography angiography improves acute coronary syndrome risk stratification accuracy and work-up in acute chest pain patients: results from ROMICAT II trial. JACC Cardiovasc Imaging 8:1272–1281
Levsky JM, Spevack DM, Travin MI et al (2015) Coronary computed tomography angiography versus radionuclide myocardial perfusion imaging in patients with chest pain admitted to telemetry: a randomized trial. Ann Intern Med 163:174–183
Linde JJ, Hove JD, Sørgaard M et al (2015) Long-term clinical impact of coronary CT angiography in patients with recent acute-onset chest pain: the randomized controlled CATCH trial. JACC Cardiovasc Imaging 8:1404–1413
Haaf P, Reichlin T, Twerenbold R et al (2014) Risk stratification in patients with acute chest pain using three high-sensitivity cardiac troponin assays. Eur Heart J 35:365–375
Dedic A, Lubbers MM, Schaap J et al (2016) Coronary CT angiography for suspected ACS in the era of high-sensitivity troponins: randomized multicenter study. J Am Coll Cardiol 67:16–26
Dai T, Wang HuPF (2018) Diagnostic performance of computed tomography angiography in the detection of coronary artery in-stent restenosis: evidence from an updated meta-analysis. Eur Radiol 28:1373–1382
Marano R, Liguori C, Rinaldi P et al (2007) Coronary artery bypass grafts and MDCT imaging: what to know and what to look for. Eur Radiol 17:3166–3178
Jungmann F, Emrich T, Mildenberger P et al (2018) Multidetector computed tomography angiography (MD-CTA) of coronary artery bypass grafts—update 2017. Rofo 190:237–249
Funding
Not applicable
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marano, R., Rovere, G., Savino, G. et al. CCTA in the diagnosis of coronary artery disease. Radiol med 125, 1102–1113 (2020). https://doi.org/10.1007/s11547-020-01283-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-020-01283-y