Abstract
Purpose
Mediastinal, hilar, and peripheral pulmonary lymphadenopathy is a hallmark sign of different benign and malignant diseases of the chest. Contrast-enhanced (CE) chest CT is a test frequently applied to examine thoracic lymph node zones. We attempted to find out whether mediastinal, hilar, and peripheral lymph nodes delineate equally in CE chest CT with reduced dose (CE-LDCT, about 1 mSv) when compared with accepted standard CE chest CT (CE-SDCT).
Materials and methods
In this ethics committee-approved, mono-institutional, retrospective (20 months) matched case–control study, two independent, blinded observers compared measurable lymph node delineation (yes–no) in six different International Association for the Study of Lung Cancer (IASLC) zones (upper mediastinal, aortopulmonary, subcarinal, lower mediastinal, hilar, peripheral) between 62 CE-LDCT cases and 124 CE-SDCT controls (respective tube charge, 100, 120 KVp, computed tomography dose index, 1.66 ± 0.51, 5.36 ± 2.24 mGy, automatic exposure control-modulated 64-row multi-detector chest CT with iterative image reconstruction). Individual matching for gender (53% female), age (53 ± 19 years), body height, weight, anterior–posterior and transverse diameters of chest and lung ruled out pre-test confounders. Lymph node size (cut-off value, 1 cm) was a potential post-test confounder. Two-tailed T test and Chi-square test were significant for p < 0.05.
Results
Measurable lymph nodes delineated equally in cases (261/372 IASLC zones, 70%; 280/372, 75%) and controls (528/744, 71%; 519/744, 70%; no significant differences, power 90%). One observer delineated significantly more peripheral zone lymph nodes in cases (35/62) than in controls (43/124); there were no significant differences otherwise. Lymph node size did not differ significantly; effective dose was 1.0 ± 0.3 mSv in cases and 3.4 ± 1.5 mSv in controls.
Conclusion
CE-LDCT with about 1 mSv demonstrated equal delineation of thoracic lymph nodes when compared with accepted standard CE-SDCT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Computed tomography (CT) of the chest comprises radiological examinations with different protocols serving different indications and is among the most commonly applied CT tests. Common indications include the investigation of benign and primary or metastatic malignant disease of the lung parenchyma and mediastinum and cardiovascular disease, particularly pulmonary embolism [1,2,3,4,5]. However, CT represents the single most contributor to diagnostic radiation exposure, with increasing numbers of per capita examinations since the early 2000s [2,3,4]. In turn, diagnostic radiation exposure is the leading contributor to non-natural radiation dose [6].
Improved CT technology, such as multi-detector row CT (MDCT), automatic exposure control (AEC), or iterative image reconstruction (IIR), increases dose efficiency, particularly in lung disease, due to the high intrinsic contrast between air and soft tissue [3, 7,8,9]. Improvements support recommendations to apply highly dose-saving chest CT (LDCT) in occupational medicine, e.g. to find asbestos-related pleural and pulmonary lesions [10]. For general medical purposes within the same purview, standard-dose chest CT (SDCT) ranges from 4 to 6 mSv (formerly, 4–7 mSv), as elsewhere in the European Union [1, 2, 4, 11,12,13].
LDCT provides diagnostic image quality, without significant loss of anatomic detail, in the lung and pleura [8,9,10] and in the thoracic skeleton [14, 15]. However, LDCT also depicts thoracic soft tissue structures, including hilar and mediastinal lymph nodes that play a role in both benign and malignant lung disease [16, 17]. Unenhanced LDCT in occupational medicine detects depositions of X-ray opaque matter in mediastinal, hilar, and pulmonary lymph nodes [10]. In principle, however, intravenous contrast enhancement (CE), which is frequently used to improve delineation of thoracic lymph nodes at SDCT, can also be applied at LDCT for the same purpose [18].
LDCT examinations, as recommended for occupational medicine, have been offered in our institution in conjunction with CE upon special request by chest physicians. We hypothesized that there was no significant difference in the delineation of thoracic lymph nodes between CE-LDCT and CE-SDCT.
Materials and methods
Data were organized and presented in this manuscript in accordance with the STROBE statement [19].
Ethical considerations, study design, setting, and study population
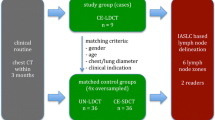
A prospective study design was dismissed and a retrospective case–control design favoured because CE-LDCT examinations had previously been performed in our institution upon special request. Based on the World Medical Association Declaration of Helsinki, the institutional ethics committee for human studies approved of our retrospective, mono-institutional, matched case–control study of CE-LDCT (cases) and CE-SDCT (controls) examinations performed within a 20-month time period (Fig. 1) in the city-centre division for internal medicine of our department under stable scan conditions, and waived individual patient consent. Patients whose chest CTs were retrospectively analysed were legally able to consent to the examination, had signed the respective forms, could follow breathing instructions, did not have contraindications for CE, were examined supine, in the arterial phase of CE, and had a body mass index (BMI) not exceeding 30 kg/m2. Exclusion criteria were deviations from those inclusion criteria, previous radiotherapy of the mediastinum, severe motion artefacts per the original CT report, or incomplete or interrupted CT scan. The study included 62 patients with CE-LDCT. Based on institutional radiology files, two control patients of the same gender with CE-SDCT during the study period were matched to each patient case, per body weight, body height, BMI, sagittal and lateral chest diameter, and sagittal and lateral lung diameter, such that there were 124 controls (Table 1), and a test power of 90% for all and 80% for each of six different thoracic lymph node zones was reached (see “Study Size” section).
Variables
Lymph node delineation in each of six different thoracic lymph node zones was the primary variable of interest in this study. The outcome was either positive, if at least one lymph node in an individual lymph node zone delineated such that its long and short axis could be reproducibly measured with electronic callipers, or negative, if such delineation was impossible. Secondary variables of interest included CT dose index (CTDI) and dose length product (DLP), which were individually provided for each patient by the CT scanner as a mandatory requirement. Effective dose (ED) was approximated according to EUR 16262 EN [20]. Exposures were either CE-LDCT (cases) or CE-SDCT (controls). Besides matching criteria, short-axis diameter of the largest lymph node delineated in an individual lymph node zone was a potentially confounding variable whose outcome was either “large”, if short-axis diameter exceeded 1 cm, or “small” otherwise. It was assumed that larger lymph nodes would be easier to detect than smaller lymph nodes. Two independent observers evaluated all CE-LDCT and CE-SDCT images to assess variability of perception as a potential effect modifier.
Data sources and measurements
CE-LDCT and CE-SDCT images were retrieved from the institutional picture archiving and communication system (PACS, Syngo Studio VB36C, Siemens Medical Solutions, Erlangen, Germany) and displayed in stack mode on a clinically certified 21-inch 5k greyscale monitor. Observers scrolled through images for visual evaluation of lymph node delineation and lymph node short-axis diameter at window/centre levels of 350HU/50HU. Observer 1 was a fifth-year radiology resident. Observer 2 was a cross-sectional imaging attending with more than 10 years of post-fellowship clinical work experience in chest CT.
Six different lymph node zones were evaluated, based on the seventh edition of the International Association for the Study of Lung Cancer (IASLC) map, including the upper zone (lymph node stations 2R, 2L, 3a, 3p, 4R, and 4L), aortopulmonary zone (lymph node stations 5 and 6), subcarinal zone (lymph node station 7), lower zone (lymph node stations 8 and 9), hilar/interlobar zone (lymph node stations 10 and 11), and peripheral zone (lymph node stations 12, 13, and 14), respectively [21,22,23]. The supraclavicular lymph node zone (lymph node station 1) was not evaluated, since it was not regularly covered completely by chest CT. The IASLC map was available to each independent observer [21,22,23].
Previously, all patients underwent chest CT from the apex to the base of the lungs in supine position, arms above the head, on the same clinical whole-body 64-detector row CT scanner (Optima 660, GE Healthcare Europe, Garching, Germany). CE-LDCT cases were examined at 100 KVp, with tube current ranges of 10–40 mA (BMI up to 25 kg/m2) or 10–60 mA (BMI more than 25 kg/m2). CE-SDCT controls were examined at 120 KVp, with a tube current range of 70–220 mA. Invariably, CE included 70 ml of iodinated contrast media (injection rate, 3 ml/s, 350 mg of iodine per ml, Iomeprol “Imeron” 350, Bracco, Konstanz, Germany), followed by 60 ml of normal saline solution at 3 ml/s, and a delay of 33 s from the start of CE injection to the start of the CT scan, 64 × 0.625 mm collimation, tube rotation time 0.7 s, pitch 0.984:1, AEC in x-, y-, and z-directions (auto-mA, reference noise index 7), and “large field-of-view” settings, which are optimized by the manufacturer for the CT scanner based on measurements in a 32-cm CT phantom. After primary reconstruction (slice thickness 0.625 mm), axial images were reformatted continuously with a slice thickness of 5 mm, without gaps or overlapping, and standard soft tissue kernel as provided by the manufacturer. Two-dimensional IIR (ASIR, GE Healthcare Europe, Garching, Germany) was applied at levels of 70% for cases and 40% for controls, respectively, per institutional protocols.
Bias
To clarify observer tasks prior to the study, the two observers jointly assessed three CE-SDCT scans which had been screened for but excluded from the controls. To rule out potential effects of learning or memory, all CT scans in the study were anonymized jointly, by randomly assigning a five-digit number to each, put in sequence from the lowest to the highest random number, and grouped into six different packages, which were evaluated in individually randomized order by each observer. Within each package, observers worked from the lowest random number up. Another researcher entered all study results in spreadsheet tables (Microsoft Excel, Microsoft Corporation). Individual indications for chest CT were retrieved from the radiology information system and summarized for each of the CE-LDCT cases and CE-SDCT controls as representing either non-neoplastic or neoplastic/presumably neoplastic disease.
Study size
To make a potential study result of “no statistically significant difference” in the delineation of thoracic lymph nodes clinically meaningful, a test power of 90% and an alpha error of p < 0.05 were aspired to for the joint evaluation of all six IASLC lymph node zones as the level of comparison between CE-LDCT cases and CE-SDCT controls [21,22,23].
The formula,
where “N” is the total sample size, “P1” the expected delineation rate among cases, “P2” the expected delineation rate among controls, “q1” the proportion of cases, “q2” the proportion of controls, “P” = q1*P1 + q2*P2, “za” the standard normal deviate for alpha (1.96 for an alpha error of 0.05), and “zb” the standard normal deviate for beta (1.284 for a test power of 90%), calculated sample size [24].
The number of eligible CE-LDCT cases was 62 (372 IASLC lymph node zones). Twofold oversampling with 124 CE-SDCT controls (744 lymph node zones) leads to N = 1116 IASLC lymph node zones. For an expected delineation rate of P1 = 0.750, P2 should be either 0.834 or higher (N > 1093) or 0.655 or smaller (N > 1103) to conclude that there was a significant difference [24]. Total sample size for individual IASLC lymph node zones was N = 186. For an expected delineation rate of P1 = 0.750, P2 should be either 0.909 or higher (N > 184) or 0.541 or smaller (N > 185) to conclude that there was a significant difference in an individual zone, while any other P2 would be insignificant, with zb = 0.840 (power 80%) [24].
Quantitative variables and statistical methods
Dichotomous variables were recorded in two-by-two contingency tables, separately for the two independent observers, and subjected to two-tailed Chi-square testing [25]. Respective proportions of agreement and associated kappa values were measures of inter-observer variability [26]. Averages ± standard deviation (SD) were calculated for quantitative measures of radiation exposure, including CTDI and DLP, and ED, as based on a conversion factor (EK) of 0.017 for adults [20]. Measures of radiation exposure and quantitative matching variables (potential confounders) were subjected to two-tailed Student’s t test for unpaired data and uneven variance [27]. p values < 0.05 were multiplied by the number of tests performed on the same data set, and results were called significant only if they remained < 0.05.
Results
Patients
Evaluation of primary and secondary variables and potential confounders was completed for all CE-LDCT cases and CE-SDCT controls and both independent observers.
Main results
Lymph node delineation
Overall, no significant difference was found in thoracic lymph node delineation between CE-LDCT cases and CE-SDCT controls, whether in all lymph node zones together or separately in each individual lymph node zone (Table 2, Figs. 2, 3, 4). Observer 1 delineated lymph nodes in approximately 70% of all lymph node zones together in both CE-LDCT cases and CE-SDCT controls, with ranges between individual lymph node zones of 40–90% in cases and 43–98% in controls, and no significant differences (Table 2, Figs. 2, 3, 4). Observer 2 delineated lymph nodes in approximately 75% of all IASLC lymph node zones together in CE-LDCT cases and 70% in CE-SDCT controls, with ranges between individual lymph node zones of 52–90% in cases and 35–93% in controls, statistically significant advantage after correction for multiple testing for CE-LDCT in the peripheral (pulmonary) zone (X2 = 7.1787, 0.005 < p < 0.010), and no significant differences in other zones, respectively.
Delineation of thoracic lymph nodes as perceived by two independent observers in six different zones per IASLC classification [21,22,23]. There were no statistically significant differences between CE-LDCT cases (n = 62, observer 1, dark grey bars, observer 2, light grey bars) and matched CE-SDCT controls (n = 124, observer 1, light grey hatched bars, observer 2, dark grey hatched bars) after correction for multiple testing, except for peripheral (pulmonary) zone lymph node delineation in observer 2
Other analyses
Measures of radiation exposure
Radiation exposure in CE-LDCT was only a fraction of that in CE-SDCT. When based on individual estimates of patient dose as provided by the CT scanner and documented in PACS as a mandatory requirement, average ED of CE-LDCT was approximately 30% of average ED of CE-SDCT per EUR-16262-EN calculation [20]. For CE-LDCT cases, CTDI was 1.66 ± 0.51 mGy, DLP was 58.96 ± 19.01 mGy*cm, and ED was 1.00 ± 0.32 mSv. For CE-SDCT controls, CTDI was 5.36 ± 2.24 mGy, DLP was 198.64 ± 88.29 mGy*cm, and ED was 3.38 ± 1.50 mSv.
Pre-test analysis of potential confounders
Matching of CE-LDCT cases and CE-SDCT controls resulted in no significant differences in any of the potential confounders (Table 1). Individual indications for CE-LDCT cases/CE-SDCT controls were unclear pleural effusion in 0/2, infectious disease in 15/18, interstitial lung disease in 9/5, granulomatous disease in 6/1, neoplasia screening in 16/20, follow-up on neoplasia in 11/73, preparation of or follow-up on intervention in 4/3, and other in 1/2, such that there was preponderance of non-neoplastic disease among cases (35/62, 56%) and neoplastic or presumably neoplastic disease among controls (93/124, 75%, Χ2 16.5128, p < 0.001).
Post-test analysis of lymph node size
Jointly for all IASLC lymph node zones, observer 1 found large lymph nodes in 31/372 zones among CE-LDCT cases (8.3%) and in 53/744 zones among CE-SDCT controls (7.1%; Χ2 0.3621, p > 0.5), with ranges between different lymph node zones of 2–16%/0–13% for cases/controls, and no significant differences. Observer 2 found large lymph nodes in 45/372 zones among CE-LDCT cases (12.1%) and in 77/744 zones among CE-SDCT-controls (10.4%; Χ2 0.6085, 0.25 < p < 0.5), with ranges between different lymph node zones of 2–23%/2–19% for cases/controls, and no significant differences.
Inter-observer agreement
Inter-observer agreement in CE-LDCT cases/CE-SDCT controls was 81%/80% (kappa scores 0.53/0.51) for overall lymph node delineation and 92%/94% (kappa scores 0.61/0.64) for the presence of large lymph nodes, with ranges between individual IASLC zones of 68–92%/60–91% and 87–98%/93–98%, respectively.
Discussion
Key finding
The key finding of this study was that delineation at chest CT of thoracic lymph nodes as per the IASLC map did not differ significantly between CE-LDCT and CE-SDCT, although radiation exposure at CE-LDCT was 70% lower than at CE-SDCT.
Limitations
Several limitations apply to this study. First, it was mono-institutional and limited to one specific 64-detector row CT scanner, such that it remains unclear whether radiation dose could be similarly reduced (or even further) on other CT scanners. However, similar dose reduction options can be expected elsewhere, because the CT technology applied here is ubiquitously available and currently in widespread clinical use [1, 7, 8]. In fact, a small pilot study which used a different 64-detector row CT scanner limited radiation dose similarly [18]. It has been shown for pulmonary embolism CT protocols, though, that radiation doses are lower with 64-detector row CT scanners than with less detector rows [28]. Second, the study compared only two different CE chest CT protocols. However, the study addresses the principle of dose reduction at CE chest CT rather than a plethora of CT protocol options. The two CE chest CT protocols included were based on contemporary recommendations for occupational medicine and general medicine, respectively [4, 10]. Third, the study design was retrospective and case-controlled rather than prospective and randomized. However, the matching process effectively eliminated potential confounders, and lymph node size as another potential confounder could not be prospectively controlled anyway. Fourth, the inclusion criteria did not specify chest disease entities. In fact, there was a significant preponderance of benign disease among CE-LDCT cases and malignant disease among CE-SDCT controls. However, the study addressed thoracic lymphadenopathy as such, which is a common feature of many different benign and malignant diseases. Fifth, the study exclusively analysed mediastinal, hilar, and pulmonary lymph nodes, while it left out others, such as supra- and infra-clavicular, axillary, chest wall, retro-crural, or diaphragmatic lymph node stations. However, the lymph node stations analysed here are generally covered at chest CT and are thus representative of the quality of lymph node delineation. Also, they are independently defined by the IASLC map [21,22,23]. Sixth, the study did not attempt to distinguish between benign and malignant lymph nodes. However, in clinical practice, lymph node size, particularly with a short-axis diameter exceeding 1 cm, lymph node clustering, and uptake of contrast media into lymph nodes are criteria applied to detect potentially malignant lymphadenopathy [29]. In this study, lymph node size was treated as a potential confounder of lymph node delineation rather than as a secondary endpoint of analysis. Seventh, there was no comparison with unenhanced chest CT. However, it has previously been shown that lymph node delineation is significantly inferior at unenhanced chest CT [18]. Eighth, delineation of pulmonary and pleural structures was not analysed. However, it has previously been demonstrated that highly dose-saving chest CT protocols delineate pulmonary and pleural structures sufficiently in occupation-related disease [8,9,10]. Ninth, osseous structures were not analysed. However, it has previously been shown that highly dose-saving CT protocols provide diagnostic image quality, without significant loss of anatomic detail, in the thoracic skeleton [14, 15]. Tenth, due to inclusion criteria, inference from the study population to the general population is limited to patients with a BMI of 30 kg/m2 or less.
Interpretation
Within the limits of this study, the hypothesis that there was no significant difference in the delineation of thoracic lymph nodes per the IASLC map between CE-LDCT, at an average of 30% of standard dose, and CE-SDCT, should be accepted as true, with a test power of 90%, based on joint evaluation of six different lymph node zones, and with reproducible results between two independent observers [1, 2, 4, 11, 12, 21,22,23]. This study corroborates a recent retrospective pilot study which applied a different 64-detector row CT scanner [18]. The IASLC lymph node zones have been defined in both surgical and CT radiological anatomy [21,22,23]. Although originally developed for N-staging of lung cancer, the IASLC map can be applied to localize thoracic lymphadenopathy in any disease process [21]. Protocols for CE-LDCT and CE-SDCT followed recommendations by governing bodies rather than being implemented arbitrarily [4, 10, 30]. While thoracic lymph node delineation was similar between CT protocols in each and all of the six different IASLC lymph node zones, it was incomplete in some. It can be ruled out therefore neither that increasing radiation dose beyond CE-SDCT would improve lymph node delineation, nor that decreasing radiation dose below CE-LDCT would still yield acceptable lymph node delineation. In fact, a preliminary study in ten patients on sub-milli-sievert chest CT with different IIR techniques reported promising delineation of mediastinal structures [31]. Small-scale studies imply that custom-made CE-LDCT protocols might be suitable to follow-up patients with specific malignant diseases [32, 33]. In contrast to those studies, however, our study had a large sample size with high statistical power, included non-oncological patients, and applied chest CT protocols previously recommended by authorities [1, 2, 4, 10,11,12], and the IASLC map as an established and generally accepted localization tool [21].
Further analyses revealed that although inter-observer agreement was high on lymph node delineation, agreement beyond chance was moderate in both CE-LDCT cases and CE-SDCT controls, unlike previously observed [18]. There was wide inter-observer variability between different IASLC lymph node zones despite detailed instruction. This has previously been observed in delineation of lymph node compartments, gross target volume, and planning target volume in patients with non-small-cell lung cancer scheduled for radiotherapy [34]. While it appears that delineation of thoracic targets represents a difficult task, secondary findings in this study imply that decreasing radiation dose as investigated here decreases neither thoracic lymph node delineation nor inter-observer agreement.
Conclusion
In conclusion, it appears that CE chest CT can be performed at much lower radiation exposure to the individual patient than currently accepted, with unchanged thoracic lymph node delineation. This implies that large-scale reduction in radiation dose is possible in routine clinical care, since lymph node delineation is crucial for both diagnosis and follow-up of different benign and malignant diseases. Currently, inference to the general population is limited to dose-reduced chest CT as recommended by governing bodies for occupational medicine, to the IASLC lymph node zones investigated here, and to patients with a BMI of 30 kg/m2 or less. Further studies should demonstrate whether investigation of other thoracic soft tissue structures will be limited by dose reduction and whether radiation dose can be reduced in patients with higher BMI.
Abbreviations
- AEC:
-
Automatic exposure control
- BMI:
-
Body mass index
- CE:
-
Intravenous contrast enhancement or intravenously contrast-enhanced
- CE-LDCT:
-
Contrast-enhanced highly dose-saving computed tomography of the chest
- CE-SDCT:
-
Contrast-enhanced computed tomography of the chest with standard dose
- CT:
-
Computed tomography
- CTDI:
-
Computed tomography dose index
- DLP:
-
Dose length product
- ED:
-
Effective dose
- EK:
-
Conversion factor of EUR 16262 EN
- IASLC:
-
International Association for the Study of Lung Cancer
- IIR:
-
Iterative image reconstruction
- LDCT:
-
Highly dose-saving computed tomography of the chest
- MDCT:
-
Multi-detector row-computed tomography
- PACS:
-
Picture archiving and communication system
- SD:
-
Standard deviation
- SDCT:
-
Computed tomography of the chest with standard dose
References
Foley SJ, McEntee MF, Rainford LA (2012) Establishment of CT diagnostic reference levels in Ireland. Br J Radiol 85:1390–1397
Shrimpton PC, Hillier MC, Meeson S et al (2014) Doses from computed tomography (ct) examinations in the UK—2011 review. Public Health England, Centre for Radiation, Chemical and Environmental Hazards, September, 2014. PHE publications gateway number: 2014179. www.gov.uk/phe. Accessed 16 May 2016
Mayo JR, Aldrich J, Muller NL et al (2003) Radiation exposure at chest CT: a statement of the Fleischner Society. Radiology 228:15–21
Federal Office for Radiation Protection (2015) Radiation topics: X-ray diagnosis—harmful or useful? Salzgitter, Germany: Federal Office for Radiation Protection; September, 2015. https://www.bfs.de/SharedDocs/Downloads/BfS/DE/broschueren/ion/stth-roentgen.pdf. Accessed 16 May 2016 (in German)
Kanal KM, Butler PF, Sengupta D et al (2017) U.S. diagnostic reference levels and achievable doses for 10 adult CT examinations. Radiology 284:120–133
Bundesministerium fuer Umwelt, Naturschutz, Bau und Reaktorsicherheit (2015) Umweltradioaktivitaet und Strahlenbelastung Jahresbericht 2013. BMUB, Bonn, Germany (in German, Summary in English, French, and German)
Söderberg M, Gunnarsson M (2010) Automatic exposure control in computed tomography–an evaluation of systems from different manufacturers. Acta Radiol 51:625–634
Mueck FG, Michael L, Deak Z et al (2013) Upgrade to iterative image reconstruction (IR) in MDCT imaging: a clinical study for detailed parameter optimization beyond vendor recommendations using the adaptive statistical iterative reconstruction environment (ASIR) part 2: the chest. Rofo 185:644–654
Coppenrath E, Mueller-Lisse UG, Lechel U et al (2004) Low-dose spiral CT of the lung in the follow-up of non-malignant lung disease. RoFo Fortschr auf dem Gebiete der Rontgenstrahlen und der Nukl 176:522–528
Deutsche Gesetzliche Unfallversicherung (DGUV) (2011) Falkensteiner Empfehlung - Empfehlung für die Begutachtung asbestbedingter Berufskrankheiten. Berlin, Germany. http://publikationen.dguv.de/dguv/udt_dguv_main.aspx?FDOCUID=25466. Accessed 16 May 2016 (in German)
Bongartz G, Golding SJ, Jurik AG et al (2004) Appendix B—European field survey on MSCT. In: European guidelines for multislice computed tomography. Funded by the European Commission, Contract number FIGM-CT2000-20078-CT-TIP. http://www.msct.eu/CT_Quality_Criteria.htm. Accessed 16 May 2016
Friberg EG, Widmark A, Ryste Hauge ICH (2009) National collection of local diagnostic reference levels in Norway and their role in optimization of X-ray examinations. Norwegian Radiation Protection Authority, Østerås
Federal Office for Radiation Protection (2015) Notice of diagnostic reference levels for radiology and nuclear medicine examinations. Federal Office for Radiation Protection, Salzgitter, Germany. https://www.bfs.de/SharedDocs/Downloads/BfS/DE/fachinfo/ion/drw-roentgen.pdf. Accessed 16 May 2016 (in German)
Buty M, Xu Z, Wu A et al (2017) Quantitative image quality comparison of reduced- and standard- dose dual-energy multiphase chest, abdomen, and pelvis CT. Tomography 3:114–122
Ebner L, Knobloch F, Huber A et al (2014) Feasible dose reduction in routine chest computed tomography maintaining constant image quality using the last three scanner generations: from filtered back projection to sinogram-affirmed iterative reconstruction and impact of the novel fully integrated detector design minimizing electronic noise. J Clin Imaging Sci 4:38. https://doi.org/10.4103/2156-7514.137826
Suwatanapongched T, Gierada DS (2006) CT of thoracic lymph nodes. Part II: diseases and pitfalls. Br J Radiol 79:999–1000
Sharma A, Fidias P, Hayman LA et al (2004) Patterns of lymphadenopathy in thoracic malignancies. Radiographics 24:419–434
Paolini M, Wirth K, Tufman A et al (2016) Thoracic lymph node delineation at dose-reduced (1 mSv) dose-modulated contrast enhanced MDCT: a retrospective pilot study. Radiol Med 121:644–651
von Elm E, Altman DG, Egger M et al (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 4:e296. https://doi.org/10.1371/journal.pmed.0040296
Menzel HG, Schibilla H, Teunen D (eds.). European guidelines on quality criteria for computed tomography. EUR 16262 EN. http://www.drs.dk/guidelines/ct/quality/mainindex.htm. Accessed 16 May 2016
Rusch VW, Asamura H, Watanabe H et al (2009) The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 4:568–577
Kim JH, van Beek EJR, Murchinson JT et al (2015) The international association for the study of lung cancer lymph node map: a radiologic atlas and review. Tuberc Respir Dis 78:180–189
Jawad H, Sirajuddin A, Chung JH (2013) Review of the international association for the study of lung cancer lymph node classification system: localization of lymph node stations on CT imaging. Clin Chest Med 34:353–363
Hulley SB, Cummings SR, Browner WS et al (2007) Estimating sample size and power: applications and examples. In: Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB (eds) Designing clinical research, chapter 6, 3rd edn. Lippincott Williams and Wilkins, Philadelphia, pp 65–96
Glantz SA (1997) How to analyze rates and proportions. In: Glantz SA (ed) Primer of biostatistics, chapter 5, 4th edn. McGraw-Hill Health Professions Division, New York, St. Louis, San Francisco, Auckland, Bogota, Caracas, Lisbon, London, Madrid, Mexico City, Milan, Montreal, New Delhi, San Juan, Sydney, Tokyo, Toronto, pp 108–150
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Glantz SA (2012) The special case of two groups: the t-test. In: Glantz SA (eds) Primer of biostatistics, chapter 4, . 7th edn. McGraw-Hill Medical, New York Chicago San Francisco Lisbon London Madrid Mexico City Milan New Delhi San Juan Seoul Singapore Sydney Toronto, pp 49–72
Jaffe TA, Yoshizumi TT, Toncheva G et al (2009) Radiation dose for body CT protocols: variability of scanners at one institution. AJR Am J Roentgenol 193:1141–1147
Nambu A, Kato S, Motosugi U et al (2010) Thin-section CT of the mediastinum in preoperative N-staging of non-small cell lung cancer: comparison with FDG PET. Eur J Radiol 73:510–517
Bundesamt für Strahlenschutz (2016) Diagnostic reference values for diagnostic and interventional X-ray applications. Salzgitter, Germany, BAnz AT 15.07.2016 B8. https://www.bfs.de/SharedDocs/Downloads/BfS/DE/rsh/rsh/A25-Roentgen-DRW.pdf;jsessionid=17BC3192738EDB6864E5A4D1BFB14504.1_cid391?__blob=publicationFile&v=1. Accessed 11 October 2016 (in German)
Khawaja RD, Singh S, Gilman M et al (2014) Computed tomography (CT) of the chest at less than 1 mSv: an ongoing prospective clinical trial of chest CT at submillisievert radiation doses with iterative model image reconstruction and iDose4 technique. J Comput Assist Tomogr 38:613–619
Yamada T, Ono S, Tsuboi M et al (2004) Low-dose CT of the thorax in cancer follow-up. Eur J Radiol 51:169–174
Dinkel HP, Sonnenschein M, Hoppe H et al (2003) Low-dose multislice CT of the thorax in follow-up of malignant lymphoma and extrapulmonary primary tumors. Eur Radiol 13:1241–1249
Vorwerk H, Beckmann G, Bremer M et al (2009) The delineation of target volumes for radiotherapy of lung cancer patients. Radiother Oncol 91:455–460
Acknowledgements
This manuscript includes results of doctoral thesis work in preparation by Larissa Marwitz at the Faculty of Medicine of the University of Munich (“Ludwig-Maximilians-Universität”, LMU), Germany. Authors acknowledge the kind support of their research activities by Professors Maximilian F. Reiser and Jens Ricke, Directors of the Department of Radiology of the Faculty of Medicine of the University of Munich (“Ludwig-Maximilians-Universität”, LMU), Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Ethical standards
This was a retrospective study. It was performed in accordance with the Declaration of Helsinki and approved by the local ethics committee. This article does not contain any studies with animals.
Rights and permissions
About this article
Cite this article
Mueller-Lisse, U.G., Marwitz, L., Tufman, A. et al. Less radiation, same quality: contrast-enhanced multi-detector computed tomography investigation of thoracic lymph nodes with one milli-sievert. Radiol med 123, 818–826 (2018). https://doi.org/10.1007/s11547-018-0915-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-018-0915-2