Abstract
Purpose
To determine the clinical effectiveness and long-term outcomes of endovascular treatment for hepatic vein (HV)-type Budd–Chiari syndrome (BCS).
Materials and methods
From June 2011 to August 2016, 68 consecutive patients with symptomatic HV-type BCS underwent endovascular treatment in our center. Data on the baseline characteristics, technical success, clinical success, and long-term outcomes were collected and analyzed retrospectively.
Results
The technical success rate of endovascular treatment was 100%. Fifty patients underwent HV recanalization, and 18 underwent accessory HV (AHV) recanalization. The clinical success rate was 95.6% (65/68). During a mean follow-up period of 29.4 ± 13.6 months, 19 patients experienced re-obstruction of either the HV (n = 18) or the AHV (n = 1). The cumulative 1-, 2-, and 5-year primary patency rates were 80.0, 72.8, and 67.9%, respectively. The cumulative 1-, 2-, and 5-year secondary patency rates were 93.8, 90.3, and 82.9%, respectively. Univariate and multivariate analyses revealed that the independent predictor of a prolonged primary patency duration was recanalization of the AHV. Five patients died 1–28 months (median, 15 months) after treatment. The cumulative 1-, 2-, and 5-year survival rates were 96.9, 93.4, and 91.2%, respectively. There was no significant difference in survival between the HV and AHV recanalization groups.
Conclusion
Endovascular treatment is effective for patients with HV-type BCS. It can result in excellent long-term patency and survival rates. If it is applicable, AHV recanalization should be considered prior to treatment in order to achieve a longer patency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Budd–Chiari syndrome (BCS) is a rare disorder defined by hepatic venous outflow obstruction at any level of the hepatic vein (HV) and/or the inferior vena cava (IVC) that results in portal hypertension [1,2,3,4]. The goals of treatment for BCS include relief of hepatic congestion, resolution of symptoms, and improvements in liver function [1,2,3,4]. At present, BCS can be divided into three types according to the different sites of obstruction [1]:
-
HV-type BCS is defined as obstruction of three HVs (left, middle, and right HVs);
-
IVC-type BCS is defined as IVC obstruction with at least one patent HV; and combined-type BCS is defined as obstruction of both IVC and three HVs.
Different types of BCS require different treatment strategies. IVC-type BCS can easily be treated by IVC recanalization [1]. IVC recanalization is also well suited for most combined-type BCS disorders, since approximately 86–89% of patients with combined-type BCS have the benefit of the compensatory and patent accessory HV (AHV) [1, 4]. However, treatment for the HV-type BCS is relatively complex. Endovascular recanalization should be performed first, followed by trans-jugular intrahepatic portosystemic shunt (TIPS) if endovascular recanalization fails [5,6,7,8,9,10,11,12,13,14,15]. Most studies to date have focused on TIPS as the primary management of HV-type BCS [9,10,11,12,13,14,15], and some recommend that TIPS should be its first-line treatment [15]. As a result, studies on endovascular treatment for HV-type BCS are scarce. In addition, the long-term outcomes following endovascular treatment for HV-type BCS are not clear. Previous studies have demonstrated that the cumulative 5-year primary patency rates after endovascular treatment for HV-type BCS ranged from 60 to 90% [5,6,7,8].
In this study, we aimed to determine the clinical effectiveness and long-term outcomes of endovascular treatment for HV-type BCS.
Materials and methods
The present single-center, retrospective study was approved by our institutional review board. Informed consent for the procedure and clinical data management was obtained from each study patient.
Study design
From June 2011 to August 2016, 68 consecutive patients with symptomatic HV-type BCS underwent endovascular treatment in our center. Patients were excluded if they had any of the following conditions: diffuse obstruction of three HVs without a compensatory AHV, asymptomatic BCS due to well-established intrahepatic collateral vessels, or BCS secondary to a malignant tumor.
Target vein selection
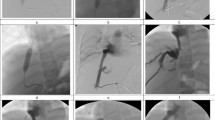
Diagnosis of HV-type BCS was established by reviewing the patient’s history and findings from abdominal ultrasound and abdominal magnetic resonance angiography (MRA). Selection of the target vein was made according to the abdominal MRA results. We usually selected one HV as the target vein for recanalization. The target vein chosen was the one HV with the shortest obstruction length. We also observed the MRA imaging to confirm whether the patient had the compensatory AHV (diameter ≥ 5 mm, Fig. 1). If the patient had the compensatory AHV and its obstruction length was shorter than that of all of the three main HVs (left, middle, and right HVs), then the AHV was chosen as the target vein.
Endovascular treatment of accessory hepatic vein (AHV) obstruction. a Preoperative magnetic resonance angiography (MRA) demonstrated a compensatory AHV (arrow). b Venography demonstrated ostial obstruction of the AHV. c Balloon dilation of the AHV obstruction. d The AHV was patent after endovascular treatment
Endovascular treatment
All patients were placed in the supine position. The blood pressure, heart rate, arterial oxygen saturation, and respiratory rate were monitored throughout treatment. All procedures were performed by two interventional radiologists under fluoroscopic guidance.
Recanalization of the HV was performed using a right jugular vein approach. The approach used for AHV recanalization depended on the angle between the ostium of the AHV and the distal side of the IVC. A femoral vein approach was used if the angle was either obtuse or right; otherwise, the jugular vein approach was used. A 0.035-inch guide wire (Terumo, Tokyo, Japan) and a 4 F VER catheter (Cordis, Miami Lake, FL, USA) were advanced to the ostium of the target HV or AHV, and then a guide wire was passed through the obstructed site. The catheter was then advanced into the target vein via the guide wire, and venography was performed to determine the extent of the obstruction. Finally, HV/AHV recanalization was performed by either balloon dilation or stent insertion (Fig. 1).
The balloon was manually dilated twice, with each dilation procedure lasting approximately 40 s. A stent was inserted if there was > 30% residual stenosis on venography after balloon dilation. The distal and proximal margins of the stent or the balloon needed to be at least 10 mm longer than the distal and proximal margins of obstruction, respectively. The diameter of the stent or balloon needed to be 2 mm larger than the HV/AHV diameter.
If HV/AHV recanalization failed via the IVC approach, then an ultrasound-guided percutaneous trans-hepatic route was used to access the HV/AHV. A 21G Chiba needle (Cook, Bloomington, IN, USA) was punctured into the target HV/AHV, a 6F sheath and a 4F VER catheter were placed into the target HV/AHV, and a guide wire was sent through the catheter to detect the obstructed site. When the guide wire passed through the obstructed site, the distal tip of the guide wire was pulled out of the right jugular/femoral vein using a vascular snare (Atrieve™ Vascular Snare Kit, Medical Device Technologies, Inc., Gainesville, FL, USA). Then, HV/AHV recanalization was performed using a combination of trans-hepatic and trans-jugular/femoral approaches (Fig. 2).
HV/AHV pressure was measured by a piezometer tube before and after endovascular recanalization. After treatment, all patients received subcutaneous low molecular weight heparin (5000 IU, twice a day) for 3 days, followed by oral warfarin for 12 months. The dose of warfarin was adjusted to maintain the international normal ratio (INR) of 2–3. Patients were monitored for INR every week until a stable INR was achieved in the therapeutic range, and further monitoring was done once a month.
Assessment of treatment
Technical success of endovascular treatment was defined when venography revealed that the target vein was restored with disappearance of the collateral vessels [1]. Clinical success was defined when symptoms and liver function tests improved after technical success of percutaneous recanalization [1]. Re-obstruction was suspected if BCS-related symptoms reappeared.
Follow-up was performed at 7 days, 1, 3, 6 months, and every 6 months after treatment. Content of follow-up included abdominal ultrasound and clinical examinations. The follow-up ended if the patient died, underwent TIPS, surgical shunt, or liver transplant, or at end of the study period (March 2017). The primary endpoint was re-obstruction of the target vein. The secondary endpoints included treatment-related complications and death.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were analyzed by t test and represented as the mean or median. Numerical data were analyzed using the χ2 test or Fisher’s exact test. Patency and survival periods were calculated by Kaplan–Meier curves and the log-rank test. The predictors influencing primary patency duration were determined using univariate and multivariate Cox regression analysis. The covariates incorporated into the multivariate analysis were variables found to be significant at p < 0.1 in the univariate analysis. A p value < 0.05 was considered statistically significant.
Results
Patients
From June 2011 to August 2016, 74 consecutive patients with symptomatic HV-type BCS visited our center. Six patients were not suitable for endovascular treatment because they had diffuse obstruction of three HVs without compensatory AHV. These 6 patients were excluded from this study. Finally, 68 patients who underwent endovascular treatment were enrolled in this study. The baseline data of the 68 patients are demonstrated in Table 1.
Technical success
Endovascular treatment for HV-type BCS was technically successful in all 68 patients (Table 2). Sixty patients underwent balloon dilation, and 8 patients underwent stent insertion. The diameter of the balloons (Cook, Bloomington, IN, USA, Bard, Murray Hill, NJ, USA, or Cordis) ranged from 10 to 16 mm. The stents used were 10–14-mm-diameter Zilver stents (Cook) or Luminexx stents (Bard). Five patients had thrombi in the target vein and underwent catheter-directed thrombolysis before recanalization. The mean HV/AHV pressure decreased from 45.5 ± 5.7 cm H2O before treatment to 17.0 ± 4.3 cm H2O after treatment (p < 0.001). No procedure-related complications occurred in these patients.
Clinical success
Clinical success rate of endovascular treatment for HV-type BCS was 95.6% (65/68). BCS-related symptoms were significantly relieved within 1 week after treatment in all 65 patients who achieved clinical success. Three patients experienced no improvement of BCS-related symptoms after treatment. We performed venography again, which revealed persistent patency of the target vein. Thus, the clinical failure might be caused by the decompensated liver cirrhosis status. Among the 3 patients, 2 patients underwent TIPS and 1 patient abandoned further treatment. The mean aspartate aminotransferase, alanine aminotransaminase, albumin, and total bilirubin concentrations improved from 40.3 ± 59.5 U/L, 45.5 ± 59.9 U/L, 36.3 ± 6.4 g/L, and 41.3 ± 37.0 μmol/L, respectively, before treatment to 32.1 ± 26.3 U/L (p = 0.066), 40.7 ± 44.6 U/L (p = 0.042), 37.2 ± 4.3 g/L (p = 0.065), and 26.0 ± 13.7 μmol/L (p < 0.001) after treatment, respectively.
Follow-up
The 68 patients were followed up for 3 days to 69 months (mean 29.4 ± 13.6 months) after treatment. No patient was lost to follow-up. Re-obstruction was observed in 19 patients. There was no significant difference in re-obstruction rates between patients with and without stent insertion (2/8 vs. 17/60, p = 0.844). These 19 patients underwent revised endovascular recanalization. Two patients underwent TIPS after the second re-obstruction. The cumulative 1-, 2-, and 5-year primary patency rates were 80.0, 72.8, and 67.9%, respectively (Fig. 3a). The cumulative 1-, 2-, and 5-year secondary patency rates were 93.8, 90.3, and 82.9%, respectively (Fig. 3b).
Univariate and multivariate analyses revealed that the independent predictor of prolonged primary patency duration was recanalization of the AHV (hazard ratio: 7.7, 95% confidence interval: 1.0–58.8, p = 0.049, Table 3).
Five patients died 1–28 months (median 15 months) after treatment. The causes of death included hepatic failure (n = 3) and gastrointestinal hemorrhage (n = 2). The cumulative 1-, 2-, and 5-year survival rates were 96.9, 93.4, and 91.2%, respectively (Fig. 4a).
AHV recanalization
Eighteen patients underwent AHV recanalization. The comparison of clinical data between AHV and HV recanalization groups is demonstrated in Table 4. Among these 18 patients, 11 were treated via the trans-jugular route and 7 were treated via the trans-femoral route. During the follow-up, 1 patient experienced re-obstruction of the AHV and 2 patients died. The cumulative 1-, 2-, and 5-year primary patency rates were 100.0, 93.8, and 93.8%, respectively. The cumulative 1-, 2-, and 5-year survival rates were 94.4, 87.7, and 87.7%, respectively. The primary patency duration was significantly longer in the AHV recanalization group than in the HV recanalization group (Table 4, Fig. 3c). There was no significant difference in survival duration between the two groups (Table 4, Fig. 4b).
Discussion
This study evaluated the clinical effectiveness and long-term outcomes of endovascular treatment for HV-type BCS. The technical and clinical success rates were 100 and 95.6%, respectively. The cumulative 5-year primary patency and survival rates were 75.7 and 91.2%, respectively. These satisfactory results may indicate that endovascular treatment is effective for HV-type BCS.
A step-wise procedure, according to the sequence of “medical treatment-endovascular treatment-TIPS-liver transplantation,” is recommended for treating BCS [16]. However, medical treatment had been considered ineffective and is associated with poor long-term results [8]. Therefore, endovascular treatment is usually used as the first-line treatment option for BCS [1,2,3,4,5,6,7,8]. TIPS is usually used when endovascular treatment fails in either technical or clinical effectiveness [9,10,11,12,13,14,15]. If the preoperative radiological examination reveals a diffuse obstruction of the HV, TIPS can be performed directly [14].
The strategy of endovascular treatment for HV-type BCS is recanalization of one HV with the shortest obstruction length [5,6,7,8]. Single HV is sufficient to drain the entire liver because of the well-established intrahepatic collateral vessels in the liver of BCS patients [5,6,7,8]. At present, the clinical application of the AHV in BCS is currently being highlighted in the literature [17,18,19]. AHV recanalization is also an effective method for HV-type BCS, and its use has expanded the scope of endovascular treatment for BCS [18].
In this present study, the cumulative 1-, 2-, and 5-year primary patency rates were 80.0, 72.8, and 67.9%, respectively. These rates are comparable to those reported in previous studies involving endovascular treatment for HV-type BCS patients [5,6,7]. The cumulative 1-, 2-, and 5-year secondary patency rates were 93.8, 90.3, and 82.9%, respectively. These rates suggest that endovascular treatment is repeatable. However, the cumulative 5-year primary patency rate in Ding’s study was 90% [8]. Ding et al. [8] used large balloons (12–20 mm in diameter) to dilate the HVs. In this study, the largest balloon was 16 mm in diameter. Dilation of the obstructed HV with a large balloon catheter can provide sufficient radial mechanical force to disrupt the fibrotic and organized structure of the vascular wall [8]. Therefore, it is reasonable that the cumulative 5-year primary patency rate in this study was lower than that in Ding’s study.
The type of BCS is an important risk factor for recurrence of BCS after endovascular treatment [20]. In previous studies, both Zhang et al. [21] and Gao et al. [20] considered that recurrence was more likely to occur in patients with BCS caused by HV obstruction, than in patients with BCS caused by IVC obstruction. Among the patients with HV-type BCS, segmental obstruction (> 1 cm) of the target vein was considered an independent risk factor of recurrence after endovascular treatment [6].
In this study, we found that the primary patency duration of the AHV was significantly longer than that of the HV. The extent of AHV obstruction was ostial (≤ 1 cm) in all 18 patients in the AHV recanalization group. However, in the HV recanalization group, eight patients had segmental obstruction. A dilated AHV is a compensatory mechanism in patients with BCS [17,18,19]. AHV obstruction occurs because the ostium of the AHV is restricted by the IVC wall and does not dilate along with AHV stem dilation [17]. Therefore, AHV obstruction usually presents with an ostial obstruction. These findings may explain why AHV had a longer primary patency duration than did the HV after endovascular treatment.
On the other hand, the mechanism of obstruction between HV and AHV may be different. HV obstruction is usually caused by primary venous diseases in patients with BCS [20]. However, AHV obstruction occurs because the ostium of the AHV is restricted by the IVC wall and does not dilate along with the AHV stem dilation [17]. That is, the AHV itself has no venous disease. This also may be the reason that AHV had a longer primary patency duration than did the HV after endovascular treatment. In this study, the cumulative 1-, 2-, and 5-year primary patency rates were 100.0, 93.8, and 93.8%, respectively. These rates are comparable to those reported in the previous study [8].
The cumulative 1-, 2-, and 5-year survival rates in this study were 96.9, 93.4, and 91.2%, respectively. These rates are comparable to those in previous studies about endovascular treatment for HV-type BCS [5,6,7]. Han et al. [1] found that re-obstruction was a risk factor for decreased survival following endovascular treatment of BCS. Repeat recanalization or TIPS should be performed in a timely manner if patients experience the re-obstruction.
This study has some limitations. First, this is a retrospective review, therefore, there is a selection bias inherently associated with such studies. Second, there was no control group. Therefore, there was no means of comparing this approach to other treatment options for HV-type BCS. Further prospective, controlled trials should be performed. Third, although we found that AHV recanalization may achieve a longer patency duration, not all patients had the compensatory AHV; thus, use of the AHV is only suitable for selected patients.
In conclusion, endovascular treatment is an effective method that resulted in excellent long-term patency and survival in patients with HV-type BCS. If patients have the compensatory AHV, AHV recanalization can be considered in order to achieve a longer patency duration.
References
Han G, Qi X, Zhang W et al (2013) Percutaneous recanalization for Budd–Chiari syndrome: an 11-year retrospective study on patency and survival in 177 Chinese patients from a single center. Radiology 266:657–667
Fu YF, Xu H, Wu Q et al (2015) Combined thrombus aspiration and recanalization in treating Budd–Chiari syndrome with inferior vena cava thrombosis. Radiol Med 120:1094–1099
Qi XS, Ren WR, Fan DM et al (2014) Selection of treatment modalities for Budd–Chiari Syndrome in China: a preliminary survey of published literature. World J Gastroenterol 20:10628–10636
Fu YF, Li Y, Cui YF et al (2015) Percutaneous recanalization for combined-type Budd–Chiari syndrome: strategy and long-term outcome. Abdom Imaging 40:3240–3247
Sang HF, Li XQ (2014) Endovascular treatment of Budd–Chiari syndrome with hepatic vein obstruction in China. J Laparoendosc Adv Surg Tech A 24:846–851
Cui YF, Fu YF, Li DC et al (2016) Percutaneous recanalization for hepatic vein-type Budd–Chiari syndrome: long-term patency and survival. Hepatol Int 10:363–369
Tripathi D, Sunderraj L, Vemala V et al (2017) Long-term outcomes following percutaneous hepatic vein recanalization for Budd–Chiari syndrome. Liver Int 37:111–120
Ding PX, Zhang SJ, Li Z et al (2016) Long-term safety and outcome of percutaneous transhepatic venous balloon angioplasty for Budd–Chiari syndrome. J Gastroenterol Hepatol 31:222–228
Tripathi D, Macnicholas R, Kothari C et al (2014) Good clinical outcomes following transjugular intrahepatic portosystemic stent-shunts in Budd–Chiari syndrome. Aliment Pharmacol Ther 39:864–872
Zahn A, Gotthardt D, Weiss KH et al (2010) Budd–Chiari Syndrome: long term success via hepatic decompression using transjugular intrahepatic porto-systemic shunt. BMC Gastroenterol 10:25
Qi X, Guo W, He C et al (2014) Transjugular intrahepatic portosystemic shunt for Budd–Chiari syndrome: techniques, indications and results on 51 Chinese patients from a single centre. Liver Int 34:1164–1175
Hayek G, Ronot M, Plessier A et al (2017) Long-term outcome and analysis of dysfunction of transjugular intrahepatic portosystemic shunt placement in chronic primary Budd–chiari syndrome. Radiology 283:280–292
Fitsiori K, Tsitskari M, Kelekis A et al (2014) Transjugular intrahepatic portosystemic shunt for the treatment of Budd–Chiari syndrome patients: results from a single center. Cardiovasc Interv Radiol 37:691–697
He F, Zhao H, Dai S et al (2016) Transjugular intrahepatic portosystemic shunt for Budd–Chiari syndrome with diffuse occlusion of hepatic veins. Sci Rep 6:36380
Rosenqvist K, Sheikhi R, Eriksson LG et al (2016) Endovascular treatment of symptomatic Budd–Chiari syndrome–in favour of early transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol 28:656–660
Seijo S, Plessier A, Hoekstra J et al (2013) Good long-term outcome of Budd–Chiari syndrome with a step-wise management. Hepatology 57:1962–1968
Fu YF, Wei N, Wu Q et al (2015) Use of accessory hepatic vein intervention in the treatment of Budd–Chiari syndrome. Cardiovasc Interv Radiol 38:1508–1514
Fu YF, Xu H, Zhang K et al (2015) Accessory hepatic vein recanalization for treatment of Budd–Chiari syndrome due to long-segment obstruction of the hepatic vein: initial clinical experience. Diagn Interv Radiol 21:148–153
Mammen T, Keshava S, Eapen CE et al (2010) Intrahepatic collateral recanalization in symptomatic Budd–Chiari syndrome: a single-center experience. J Vasc Interv Radiol 21:1119–1124
Gao X, Gui E, Lu Z et al (2015) Risk factors of recurrence among 471 Chinese patients with Budd–Chiari syndrome. Clin Res Hepatol Gastroenterol 39:620–626
Zhang CQ, Fu LN, Xu L et al (2003) Long-term effect of stent placement in 115 patients with Budd–Chiari syndrome. World J Gastroenterol 9:2587–2591
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Chen, ZK., Fan, J., Cao, C. et al. Endovascular treatment for hepatic vein-type Budd–Chiari syndrome: effectiveness and long-term outcome. Radiol med 123, 799–807 (2018). https://doi.org/10.1007/s11547-018-0907-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-018-0907-2