Abstract
Background
Iatrogenic injuries of the renal artery include pseudoaneurysms (PSA) and pseudoaneurysms with arteriovenous fistula (PSA + AVF). They can cause hematuria, anemization and flank pain. Endovascular treatment is recommended due to its effectiveness.
Objective
To assess the potential difference between the embolization of iatrogenic renal PSA and iatrogenic renal PSA + AVF, in terms of technical and clinical success rate, procedure complexity and impact on the renal function.
Methods
We retrospectively reviewed 30 embolization procedures of iatrogenic renal PSA and renal PSA + AVF in 27 patients in two centers between December 2006 and February 2017, comparing technical and clinical success rate, total procedural time, creatinine before and after the procedure and parenchymal ischemic area after the procedure. All patients underwent CT before embolization procedure and different embolization materials were used.
Results
We identified 15 iatrogenic renal PSA and 15 iatrogenic renal PSA + AVF (causes: 23 nephron-sparing surgery, 2 nephrostomies, 1 lithotripsy, 1 ureteroscopic pyelolithotomy, 1 renal biopsy). Microcoils were used in 21 cases, microcoils and Spongostan in 3 cases, microcoils and controlled-release microcoils in 4 cases and controlled-release microcoils in 1 case. No significant statistical differences were found in the comparison of technical and clinical success rate, total procedural time, creatinine and parenchymal ischemic area after the procedure.
Conclusions
Transarterial embolization can be considered as the first-line treatment for renal artery iatrogenic lesions, considering its effectiveness. No statistical significant differences were found in the comparison of the embolization procedures of iatrogenic renal PSA and PSA + AVF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most common causes of renal hematuria are iatrogenic (i.e., biopsy, percutaneous nephrolithotomy, percutaneous nephrostomy, nephron sparing surgery, guide wire induced arterial perforation) and non-iatrogenic (i.e., angiomyolipomas, cancer, vascular lesions as arteriovenous malformations and fistulas, trauma, spontaneous bleeding, medical renal diseases as end-stage renal disease).
Pseudoaneurysm (PSA) and arteriovenous fistula (AVF) are the most common iatrogenic renal artery lesions that cause hematuria.
A PSA does not comprise all vessel wall layer and it arises from a vessel wall disruption, resulting in an abnormal flow between the intima and media (dissection) or limited only by adventitia or by surrounding tissues (rupture).
Renal artery PSA are rare and their prevalence is unknown, but it ranges 2% [1]. Increasing number and variety of endovascular and surgical procedures and crescent diffusion of cross-sectional imaging studies increase frequency and incidence of iatrogenic renal PSA even as a result of early diagnosis.
Often they are associated with an AVF that is defined as an abnormal communication between an arterial and a venous renal branch.
Endovascular treatment is recommended as the first-line treatment of iatrogenic renal PSA and AVF due to its effectiveness.
The aim of this study was to assess the potential difference of the technical and clinical success rate between the percutaneous embolization of iatrogenic renal PSA without or with AVF in two centers in comparison with the literature [2,3,4,5,6,7,8,9,10,11,12,13], and to compare the renal function and the kidney ischemia after the treatment in the two groups.
Materials and methods
We retrospectively reviewed 30 embolization procedures of endovascular treatment of iatrogenic renal PSA without or with AVF performed from December 2006 to December 2015 in 27 consecutive patients (19 men, 8 women; mean age, 57 years; age range, 16–78 years) in two centers.
Table 1 shows clinic and procedural characteristics and treatment modality for each patient.
All patients underwent diagnostic digital subtraction angiography (DSA) and transarterial embolization after computed tomography (CT) study (Lightspeed VCT, GE Healthcare, Milwaukee, USA and Somatom Sensation, Siemens, Erlangen, Germany) performed before and after administration of 120–150 mL of 350–400 mgI/mL of iodinated contrast medium (Omnipaque 350, GE Healthcare, USA; Ultravist 370, Bayer Healthcare, Brussels, Belgium, and Iomeron 400, Bracco, Milan, Italy) at a flow of 3.5–4 mL/s followed by a bolus chase of 30–40 mL of normal saline solution at the same injection rate with a double head automatic injector (Stellant D, Medrad, Bayer Healthcare, Whippany, NJ, USA).
The bolus tracking technique was utilized for achieving an optimal arterial phase placing the region of interest (ROI) at the suprarenal abdominal aorta with an enhancement threshold of 100 UH.
A venous phase was performed 70 s after the contrast medium administration and an urographic phase was performed 8–10 min after contrast medium administration.
CT criterion of arterial renal PSA was defined as a cavity communicating with a ruptured renal branch.
CT criterion of renal AVF was defined as a direct communication between an arterial and a venous renal branch, or an early opacification of the renal vein in the arterial phase of CT scan.
Endovascular procedure
All embolization procedures were performed in the angiographic room and a written informed consent and an anesthesiological evaluation were obtained, if possible.
After local anesthesia (lidocaine 2%), the common femoral artery (CFA) was percutaneously punctured and a standard 5F sheath (Radifocus, Terumo, Tokyo, Japan) was inserted.
If the origin of the renal artery was down angulated, an alternative brachial access was used.
Selective catheterism of the target renal artery was performed by a 5F diagnostic catheter (RDC or Cobra or Simmonds, Tempo Aqua, Cordis, Miami, FL, USA or Glidecath, Terumo, Tokyo, Japan) and superselective catheterism of the target arterial vessel was performed by coaxial technique with a 2.7F microcatheter (Progreat, Terumo, Tokyo, Japan).
Whenever stable catheterization of the target renal artery was not possible by standard diagnostic catheter, a coaxial technique with a guiding catheter (RDC or HS Vista brite, 7F, Cordis, Miami, FL, USA; Contra, 8F, Boston Scientific, Natick, MA, USA) was used.
Aim of the embolization was distal as possible to minimize the extent of infarction of the renal parenchyma. Embolization materials were microcoils (Vortx, Boston Scientific, Natick, MA, USA; Tornado, Cook, Bloomington, IN, USA) delivered by saline bolus injection or coil pusher device (Coil pusher, Boston Scientific, Natick, MA, USA), spongostan (Spongostan, Johnson & Johnson Ethicon Inc, New Brunswick, NJ, USA) and detachable microcoils (Concerto, ev3, Covidien, Plymouth, MA, USA).
After the treatment, a completion DSA was performed to confirme no blood extravasion and disappearance of the PSA sac and/or AVF.
Finally, hemostasis of the puncture site was obtained by normal compression or a closure device (Angioseal 6 or 8F, Saint Jude, Minnetonka, MN, USA).
Clinic and laboratory follow-up was performed in all patients by symptoms and laboratory parameters control, and a 3-phase CT scan was performed after the procedure to confirm residual bleeding absence and to evaluate the renal parenchyma loss.
“Technical success” was defined as complete embolization without endoleak or immediate reperfusion signs on completion angiography.
“Clinical success” was defined as freedom from death, rebleeding in post-embolization period, continuous anemization end/or gross haematuria, and stabilization of blood pressure and haematocrit level.
All complications were recorded and classified according to the Society of Interventional Radiology classification.
“Technical failure” was defined as incomplete embolization or endoleak or reperfusion on completion angiography.
“Clinical failure” was defined as event of death, rebleeding in post-embolization period, continuous anemization end/or gross haematuria, and unstabilization of blood pressure and haematocrit level.
Creatinine serum level was compared before, 1 and 3 days after transarterial embolization to exclude the transitory mild worsening of renal function very common after every renal treatment.
Kidney ischemia was evaluated on final bidimensional arteriogram as percentage of devascularizated parenchymal wedge-shaped areas in comparison with the total renal area.
The parenchymal loss was categorized into < 20, 20–40, 40–60, 60–80 and > 80% using DSA.
Methods
For statistical analysis, statistical significance was assumed at p < 0.05.
Statistical analyses were performed with exact Fisher test for categorial variables and Wilcoxon test for independent samples for quantitative variables.
All statistical analysis was performed with SAS 9.4 (SAS Institute Inc., Cary, NC, USA) for Windows.
Results
Thirty transarterial embolization procedures in 27 consecutive patients (19 men, 8 women; mean age, 57 years; age range, 16–78 years) with iatrogenic renal PSA (group A, n = 15) or PSA with AVF (group B, n = 15) were performed from December 2006 to December 2015 in two Institutions. In two patients the embolization was repeated 5 and 22 days after the first procedure, respectively, because rebleeding occurred. In one patient a renal artery vasospasm not allowed to complete the procedure and the successful embolization was performed in the other center.
All kidneys were native and clinical and procedural characteristics of the study population are given in Table 1.
Causes of iatrogenic renal PSA and PSA with AVF were laparoscopic nephron-sparing surgery (n = 23), percutaneous nephrostomy (n = 2), lithotripsy (n = 1), percutaneous nephrolithotomy (n = 1), and percutaneous biopsy (n = 1) (Table 2).
Presentation symptoms and signs were haematuria, flank pain and anemization in all cases.
All patients were hemodinamically stable at presentation and underwent multidetector CT study before endovascular treatment.
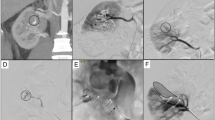
Pre-procedural imaging revealed an intraparenchymal PSA in 15/30 (50.0%) cases (Fig. 1a, b) and a PSA with AVF in 15/30 (50.0%) cases (Fig. 2a, b).
a Axial CT-angiography (arterial phase) in a 24-year-old female patient after partial nephrectomy for reninoma. The image shows a renal artery pseudoaneurysm (long white arrow) in the third medium of the right kidney with simultaneous opacification of the renal vein (short white arrows) with arteriovenous fistula. b DSA before the embolization confirming a round-shape pseudoaneurysm (long black arrow) at the third medium renal with arteriovenous fistula and simultaneous opacification of the renal vein (white arrows) and of the inferior vena cava (short black arrows)
a Coronal multi planar reformatting CT-angiography (arterial phase) in a 56-year-old female patient after partial nephrectomy for renal cell carcinoma. The image shows a small pseudoaneurysm at the superior renal third (white arrow) without arteriovenous fistula signs. b DSA before the embolization confirming an oval-shape pseudoaneurysm (white arrow) at the superior renal third without opacification of the renal vein nor of the inferior vena cava
The mean and median size of the pseudoaneurysmal sac were, respectively, 12.6 and 10.0 mm in the group A (range diameter, 4–20 mm) and 17.6 and 15.0 mm in the group B (range diameter, 7–40 mm) (p = 0.12) (Fig. 3).
Embolization materials were microcoils in 21/30 (70.0%) cases, microcoils and Spongostan in 3/30 (10.0%) cases, microcoils and detachable microcoils in 4/30 (13.3%) cases and detachable microcoils in 1/30 (3.3%) cases (Table 3).
Embolization results of PSA and PSA with AVF are given in Table 4.
Overall technical success rate was 27/30 (90.0%), because in two cases (2/30, 6.7%) only partial pseudoaneurysm exclusion was obtained after endovascular treatment, but haematuria spontaneously stopped and no PSA and/or AVF were observed at 1 week CT study of follow-up; in one case (1/30, 3,3%) there was a primary technical failure due to vasospasm that not allowed to complete the embolization. After the patient was transferred to the other center, the embolization was successfully performed. Technical success rate was 86.7% (13/15) in the group A and 93.3% (14/15) in the group B, respectively, because in two cases of the group A (2/15, 13.3%) and in one case of the group B (1/15, 6.7%) only partial PSA exclusion was obtained after endovascular treatment.
Overall clinic success rate was 88.9% (24/27) because two (7.4%) patients had rebleeding 5 days and 22 days after the first successful embolization, respectively, but a second treatment of the reperfused pseudoaneurysmal sac was successful and uneventful (secondary overall clinical success rate, 26/27, 96.3%). Clinical success rate was 84.6% (11/13 patient) in the group A and 92.9% (13/14 patient) in the group B, respectively, because in two cases of the group A (2/13, 15.4%) and in one case of the group B (1/14, 7.1%) only partial PSA exclusion was obtained after endovascular treatment (Fig. 4).
There were no statistical significant differences of technical success and clinical success between the two groups (p > 0.05).
No death was observed and no surgical intervention was necessary for rebleeding in the follow-up period, and resolution of hematuria and symptoms were observed in all patients, except the only two patients who underwent two embolization procedures.
No renal complications were observed, nor puncture site complications (i.e. hematoma, dissection, arteriovenous fistula, infection) occurred.
Complexity of the procedure was evaluated by the total procedural time. There were no statistical significant differences of the total procedural time (mean time 68 min, time range 14–180 min in group A; mean time 60 min, time range 22–129 min in group B) between the two groups (p > 0.05) (Fig. 5).
Median serum creatinine before the procedure was 118 µmol/L (58–207 µmol/L) in the group A and 92 µmol/L (51–166 µmol/L) in the group B; one day after the procedure, it was 105 µmol/L (60–194 µmol/L) in the group A and 100 µmol/L (49–194 µmol/L) in the group B. Three days after the procedure, it was 101 µmol/L (64–190 µmol/L) in the group A and 96 µmol/L (54–212 µmol/L) in the group B (p > 0.05) (Fig. 6).
The post-procedural renal ischemic area was < 20% in 65.4% of cases (17/26 patients), between 20 and 40% in 19.2% of cases (5/26 patients) and between 40 and 60% in 15.4% of cases (4 patients). In group A, the post-procedural renal ischemic area was < 20% in 83.3% of cases (10/12 patients) and between 20 and 40% in 16.7% of cases (2/12 patients). In group B, the post-procedural renal ischemic area was < 20% in 50.0% of cases (7/14 patients), between 20 and 40% in 21.4% of cases (3/14 patients) and between 40 and 60% in 28.6% of cases (4/14 patients). There was no statistical significant difference of the mean post-procedural renal ischemic area between the two groups (p > 0.05), but it was basically larger in group B (Fig. 7).
Discussion
Iatrogenic renal PSA are uncommon, but their incidence is increasing because percutaneous urological procedures (biopsy, nephrostomy, nephrolithotomy) and nephron sparing surgery are increasing in number in last years. PSA can be associated with AVF, but a comparison of the results of theirs endovascular treatment is missing in the literature.
To the best of our knowledge, this is the first large study of comparison of the endovascular treatment of iatrogenic renal PSA (n = 15) and PSA with AVF (n = 15), in terms of the potential difference of technical and clinical success rate, renal function and kidney ischemia after the treatment.
Most PSA are small and asymptomatic, and spontaneously disappeared; in most cases, a conservative management is usually preferred. A strategy of treatment is considered mandatory for all PSA regardless of their size or symptomatology (anemization, flank pain, renal disfunction), due to the risk of rupture and bleeding [14]. Endovascular treatment by microcoils or stent graft has become the first choice of management of the renal PSA because superselective catheterization enables successfully embolization with a minimum loss of normal renal parenchyma and has reduced invasiveness, high success rate and lower risk of complications.
Iatrogenic renal PSA can be associated with AVF and this, theoretically, increase technical difficulty of their endovascular embolization due to the risk of distal dislodgement of the embolization material as coils and particles.
Complications related to endovascular procedures are rare and the most frequent are nonselective embolization, non-target occlusion, and additional arterial trauma. These can lead to a parenchymal loss, hypertension or renal failure.
To the best of our knowledge, this is one of the largest series reported in the literature today and it was consecutively performed in two different centers, but a team member and the strategy of treatment were the same (between 2006 and 2012, center A, n = 6 cases; between 2012 and 2015, center B, n = 21 cases).
In comparison with Ierardi et al. [4], they had a higher number of patients, but only 6 were iatrogenic renal PSA. Sildiroglu et al. [3] had only 8 cases and Spiliopoulus et al. [2] had 16 renal PSA, but only 12 were iatrogenic.
Larger studies as that of Li et al. [10] and Sam et al. [13] reported 86/144 cases (69 PSA and 17 PSA + AVF) and 30/50 cases (21 PSA and 9 PSA + AVF), respectively, but these studies were not targeted for evaluation of the results of the transarterial embolization.
In our experience, high technical (90.0% overall) and clinical (88.9% overall) success were possible because of the fast clinical evaluation and multidisciplinary management of the symptoms and signs at debut. Gross haematuria, acute flank pain, anemization and recent history of invasive and/or mini-invasive renal procedures are crucial for suspicion of iatrogenic renal vascular injuries.
Doppler ultrasound (US), colour-Doppler US, contrast-enhanced US (CEUS) and contrast-enhanced CT were evaluated and compared in previous studies to evaluate sensitivity and specificity. CT has the advantage to image the entire urinary tract and to exam even the urinary phase of the contrast-enhancement enabling to detect eventually an arterial-urinary fistula (AUF). All of our patients were preliminarily studied by CT scan and DSA was targeted to treatment.
In the literature, the reported technical success was until 100.0% [2,3,4,5], but in our series the only 2 cases of partial embolization had a spontaneous complete clinical resolution.
The clinical success reported in literature was until 95.0% [4, 10] and similar to our study in which the only 2 cases of rebleeding were successfully treated by a second embolization procedure.
Technical (PSA group 86.7%, PSA + AVF group 93.3%) and clinical (PSA group 84.6%, PSA + AVF group 92.9%) success rates were not statistically different (p > 0.05), despite the hypothesis of major complexity of the iatrogenic renal PSA embolization when associated with an AVF.
An explication can be the same technical procedure of embolization of a PSA with or without AVF; the only additional risk factor for the embolization of an AVF is the distal migration of the embolization material, but the detachable microcoils were used in few cases of PSA (2/15 cases) and PSA + AVF (3/15 cases) with no statistical difference (p > 0.05), and no cases of distal embolization.
In case of iatrogenic vascular renal injuries as PSA or AVF, there are two options of treatment: surgical or endovascular management. In some cases, PSA spontaneously heal and no treatment is necessary. If the bleeding is massive or the renal function progresses to impairment, a medical treatment is mandatory. In recent years, transarterial embolization (TAE) is became the first and effective method to control the hemorrhagic urological emergency; previous studies have shown that the embolization of iatrogenic renal arterial injuries is a safe, tissue preserving treatment method, associated with high clinical and technical success rate, using different embolic agents [15,16,17].
In the endovascular management, the choice of the embolic material is important for a superselective and complete embolization of the target lesion and the material should be chosen according to the its characteristics (size, flow, material availability, experience of the interventionalist and, last but not least, costs). Coils, detachable coils, vascular plugs, stent grafts or liquid agents are the possible choices of treatment.
Our treatment preferred material was microcoils that allow a superselective embolization as distally as possible, to have a minimal parenchymal loss and are more controllable in particular in small vessels [18].
The embolization using coils is not disadvantage-safe; often more than a coil is required to have a complete embolization, and it is time-consuming.
In two cases, we used Spongostan to complete the sac embolization, because the coils were not enough to have a complete embolization; as seen in most recent cases, it was not needful. In fact in recent years, we used the detachable coils, which allow a higher precision to embolize the target. Using detachable coils, we did not need to use the Spongostan.
We obtained high technical and clinical success rate (90.0 and 88.9%, respectively) with a low complication rate and minimal sacrifice of renal parenchyma area (< 20%); only in 4/15 cases, we had a loss of 40–60% of the kidney, all in patients who had extended PSA AVF- associated (p > 0.05). An explication can be the proximal site of the AVF when associated with PSA, resulting in a proximal embolization of a larger renal artery branch and then in a greater renal parenchyma loss.
In two patients only partial PSA exclusion was obtained at the first endovascular treatment, but in one of these hematuria spontaneously stopped and no PSA was observed at 1 week CT study of follow up; the other patient had rebleeding 22 days after the first successful embolization, but a second TAE of the reperfused PSA sac was successful and uneventful.
In one case, there was vasospasm during the procedure, unfortunately followed by a technical problem; because of that the patient underwent the procedure in another hospital.
A significantly impairment of the renal function was not observed; in almost all cases, we observed a transient increase in the serum creatinine (1 day after the procedure) [19] followed by a non-significant decrease 3 days after the procedure, as reported in the literature [20]. Transient elevation of the serum creatinine level is probably due to the amount of contrast material used during the procedure and before that in CT diagnosis [21].
Our technical success rate (90.0%) was quite similar and comparable to that reported by other authors and our complication rate was much better than that reported by Guneyli et al. [22], who has a higher number of cases, but not as large as in our study after nephron sparing surgery.
Other complications reported in the literature such as perirenal hematoma and surgical conversion [23, 24] have not been noted in our study; this indicates a limited parenchymal or renal function loss.
No local complications in the puncture site occurred, even using mechanical hemostasis devices.
Complexity of the procedure was evaluated by the total procedural time. This is a surrogate index of the procedural complexity, because a better index could be the fluoroscopy time. The fluoroscopy time was not available for the majority of patients and then we used the total procedural time that may be affected by other factors (such as vasospasm and difficult anatomy) that may dilate the procedural time but not necessarily the fluoroscopy time.
There were no statistical significant differences of the total procedural time between the two groups but this does not allow to state no statistical significant differences of the procedural complexity. Assumption of a greater complexity of the embolization of a PSA with AVF versus a PSA cannot be confirmed from our study; no study in literature evaluated this aspect nor the comparison of the procedural and fluoroscopy time in the two groups [24,25,26].
Serum creatinine was assumed as an indicator of the renal function. We compared the serum creatinine before the procedure, one day and 3 days after the procedure to evaluate the renal function injury. The serum creatinine after 3 days was considered a good index to evaluate the recovery of the renal function after the procedure because it allows the intravascular volume recovery and the contrast agent elimination. We noted a transitory increase of the serum creatinine 1 day after the procedure, due to impairment of the renal function caused by the contrast agent used during the preliminary CT study and during embolization procedure [19, 21]. The serum creatinine 3 days after the procedure was similar to the base creatinine and there were no statistical significant differences between the two groups.
The post-procedural renal ischemic area was considered a good index of the superselectivity of the embolization because the procedural goal was a peripheral embolization to minimize the renal parenchymal loss. It is well-known that the kidney had a terminal vascularization and the occlusion of an arterial branch determine the complete ischemia of the renal tissue corresponding. Our post-procedural renal ischemic area was similar to other study in the literature [3], but we at first tried a stratification of the patients by the type of iatrogenic lesion. We noted that the mean size and the mean post-procedural renal ischemic area were basically larger in PSA + AVF group versus PSA group. Despite there was no statistical significant difference between the two groups (p > 0.05), the renal tissue sacrifice seems to be greater in PSA + AVF group. A possible explication was the proximal site of the communication between the arterial and venous renal branches that required a proximal embolization procedure. Another study [25] examined the change in renal parenchymal volume before and after procedure by quantitative evaluation of the renal volume, but the mean loss of volume of 25.2% was determined in a small subgroup (10/25, 40%) of the patients.
The main limitations of our study are the small sample size, the retrospective study nature instead of a randomized prospective trial, and the absence of a surgical control group; these are due to the rarity of this type of lesions and because the nephron sparing surgery is a relative new surgical approach. Because of that we decided to make a retrospective multicenter study to collect a number as large as possible of cases.
Recently, a larger study in a single center including 27 patients with iatrogenic renal arterial PSA (n = 16), AVF (n = 7) or PSA + AVF (n = 4) was reported [26], but no comparison between the three subgroups of lesions was performed.
In conclusion, the percutaneous embolization of the iatrogenic renal artery PSA without or with an AVF is safe and effective; these iatrogenic renal artery injuries can be embolized with coils ± spongostan with a minimal renal tissue loss. PSA with AVF could be most challenging to embolize in comparison with only PSA and require advanced technical skills and detachable embolization materials because they can determine a major renal tissue loss.
References
Jain S, Nyrenda T, Yates J, Munver R (2013) Incidence of renal artery pseudoaneurysm following open and minimally invasive partial nephrectomy: a systematic review and comparative analysis. J Urol 189:1643–1648. https://doi.org/10.1016/j.juro.2012.11.170
Spiliopoulos S, Sabharwal T, Karnabatidis D, Brontzos E, Katsanos K, Krokidis M, Gkoutzios P, Siablis D, Adam A (2012) Endovascular treatment of visceral aneurysms and pseudoaneurysms: long-term outcomes from a multicenter European study. Cardiovasc Interv Radiol 35:1315–1325. https://doi.org/10.1007/s00270-011-0312-x
Sildiroglu O, Saad WE, Hagspiel KD, Matsumoto AH, Turba UC (2012) Endovascular management of iatrogenic native renal arterial pseudoaneurysms. Cardiovasc Interv Radiol 35:1340–1345. https://doi.org/10.1007/s00270-011-0325-5
Ierardi AM, Floridi C, Fontana F, Duka E, Pinto A, Petrillo M, Kehagias E, Tsetis D, Brunese L, Carrafiello G (2014) Transcatheter embolisation of iatrogenic renal vascular injuries. Radiol Med 119:261–268. https://doi.org/10.1007/s11547-013-0343-2
Balderi A, Antonietti A, Ferro L, Peano E, Pedrazzini F, Fonio P, Grosso M (2012) Endovascular treatment of visceral artery aneurysms and pseudoaneurysms: our experience. Radiol Med 117:815–830. https://doi.org/10.1007/s11547-011-0776-4
Loffroy R, Favelier S, Pottecher P, Genson PY, Estivalet L, Gehin S, Cercueil JP, Krausé D (2015) Endovascular management of visceral artery aneurysms: when to watch, when to intervene? World J Radiol 28:143–148. https://doi.org/10.4329/wjr.v7.i7.143
Hemp JH, Sabri SS (2015) Endovascular management of visceral arterial aneurysms. Tech Vasc Interv Radiol 18:14–23. https://doi.org/10.1053/j.tvir.2014.12.003
Tulsyan N, Kashyap VS, Greenberg RK, Sarac TP, Clair DG, Pierce G, Ouriel K (2007) The endovascular management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg 45:276–283
Vignali C, Lonzi S, Bargellini I, Cioni R, Petruzzi P, Caramella D, Bartolozzi C (2004) Vascular injuries after percutaneous renal procedures: treatment by transcatheter embolization. Eur Radiol 14:723–729
Li L, Zhang Y, Chen Y, Zhu KS, Chen DJ, Zeng XQ, Wang XB (2015) A multicentre retrospective study of transcatheter angiographic embolization in the treatment of delayed haemorrhage after percutaneous nephrolithotomy. Eur Radiol 25:1140–1147. https://doi.org/10.1007/s00330-014-3491-4
Ngo TC, Lee JJ, Gonzalgo ML (2010) Renal pseudoaneurysm: an overview. Nat Rev Urol 7:619–625. https://doi.org/10.1038/nrurol.2010.163
Pan H, Xia D, Wang S, Wang Z, Zhong B, Zhou X, Peng Z (2014) Embolization of renal artery pseudoaneurysm following laparoscopic partial nephrectomy for central renal tumor: a report of two cases. Oncol Lett 7:2118–2120
Sam K, Gahide G, Soulez G, Giroux MF, Oliva VL, Perrault P, Bouchard L, Gilbert P, Therasse E (2011) Percutaneous embolization of iatrogenic arterial kidney injuries: safety, efficacy, and impact on blood pressure and renal function. J Vasc Interv Radiol 22:1563–1568. https://doi.org/10.1016/j.jvir.2011.06.020
Barbiero G, Battistel M, Susac A, Miotto D (2014) Percutaneous thrombin embolization of a pancreatico-duodenal artery pseudoaneurysm after failing of the endovascular treatment. World J Radiol 6:629–635. https://doi.org/10.4329/WJR.V6.I8.629
Cimsit NC, Baltacioglu F, Cengic I, Akpinar IN, Ilker Y, Turkeri L (2008) Transarterial glue embolization in iatrogenic renovascular injuries. Int Urol Nephrol 40:875–879. https://doi.org/10.1007/s11255-008-9380-5
Negoro H, Kawakita M, Koda Y (2005) Renal artery pseudoaneurysm after laparoscopic partial nephrectomy for renal cell carcinoma in a solitary kidney. Int J Urol 12:683–685
Mavili E, Dönmez H, Ozcan N, Sipahioglu M, Demirtas A (2009) Transarterial embolization for renal arterial bleeding. Diagn Interv Radiol 15:143–147
Ikeda O, Nakasone Y, Tamura Y, Yamashita Y (2010) Endovascular management of visceral artery pseudoaneurysms: transcatheter coil embolization using the isolation technique. Cardiovasc Interv Radiol 33:1128–1134. https://doi.org/10.1007/s00270-010-9973-0
Tinto HR, Di Primio M, Tselikas L, Rico AP, Pellerin O, Pagny JY, Sapoval M (2014) Selective arterial embolization of life-threatening renal hemorrhage in four patients after partial nephrectomy. Diagn Interv Imaging 95:601–609. https://doi.org/10.1016/j.diii.2014.02.005
Irwine C, Kay D, Kirsch D, Milburn JM (2013) Renal artery embolization for the treatment of renal artery pseudoaneurysm following partial nephrectomy. Ochsner J 13:259–263
Heye S, Maleux G, Van Poppel H, Oyen R, Wilms G (2005) Hemorrhagic complications after nephron-sparing surgery: angiographic diagnosis and management by transcatheter embolization. AJR Am J Roentgenol 184:1661–1664
Güneyli S, Gök M, Bozkaya H, Cinar C, Tizro A, Korkmaz M, Akin Y, Parildar M, Oran I (2015) Endovascular management of iatrogenic renal arterial lesions and clinical outcomes. Diagn Interv Radiol 21:229–234. https://doi.org/10.5152/dir.2014.14286
El-Nahas AR, Shokeir AA, Mohsen T, Gad H, El-Assmy AM, El-Diasty T, el-Kappany HA (2008) Functional and morphological effects of postpercutaneous nephrolithotomy superselective renal angiographic embolization. Urology 71:408–412. https://doi.org/10.1016/j.urology.2007.10.033
Loffroy R, Guiu B, Lambert A, Mousson C, Tanter Y, Martin L, Cercueil JP, Krausé D (2008) Management of post-biopsy renal allograft arteriovenous fistulas with selective arterial embolization: immediate and long-term outcomes. Clin Radiol 63:657–665. https://doi.org/10.1016/J.CRAD.2007.11.014
Strobl FF, D’Anastasi M, Hinzpeter R, Franke PS, Trumm CG, Waggershauser T, Staehler M, Clevert DA, Reiser M, Graser A, Paprottka PM (2016) Renal pseudoaneurysms and arteriovenous fistulas as a complication of nephron-sparing partial nephrectomy: technical and functional outcomes of patients treated with selective microcoil embolization during a ten-year period. Rofo 188:188–194. https://doi.org/10.1055/s-0041-110136
Hongjie G, Chengen W, Min Y, Xiaoqiang T, Jian W, Haitao G, Li S, Yinghua Z (2017) Management of iatrogenic renal arteriovenous fistula and renal arterial pseudoaneurysm by transarterial embolization—a single center analysis and outcomes. Medicine 96:40(e8187). https://doi.org/10.1097/md.0000000000008187
Acknowledgements
The authors wish to acknowledge Elisabetta Balestro for English assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Barbiero, G., Groff, S., Battistel, M. et al. Are iatrogenic renal artery pseudoaneurysms more challenging to embolize when associated with an arteriovenous fistula?. Radiol med 123, 742–752 (2018). https://doi.org/10.1007/s11547-018-0906-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-018-0906-3