Abstract
Purpose
Repeatedly negative prostate biopsies in individuals with elevated prostate-specific antigen (PSA) levels can be frustrating for both the patient and the urologist. This study was performed to investigate if magnetic resonance imaging (MRI)-guided transrectal biopsy (MRGB) increases diagnostic performance in individuals with suspected prostate cancer (PCa).
Materials and methods
Twenty-three consecutive men with a total PSA >4 ng/mL, PSA density >0.15, PSA velocity >0.75 ng/mL/year and suspicious MRI findings were included (average age 64 years; age range 53–75 years; total PSA levels ranging from 4.7 to 54 ng/mL; median 9 ng/mL). MRGB was performed with a closed unit at 1.5 Tesla, an MRI compatible biopsy device, a needle guide, and a titanium double-shoot biopsy gun.
Result
At prebiopsy MRI, in the 23 patients, a total of 26 suspicious areas to which the MRGB should be directed were found, 23 of them in the peripheral zone and three in the transitional zone. The needle guide was depicted and could be positioned with MRI guidance in all 23 patients. The duration of the procedure ranged from 35 to 55 min (mean 40 min). MRGB was well tolerated by all patients, and no major complications were observed. The detection rate for the diagnosis of PCa was 80, and 90 % of detected PCa were of intermediate aggressiveness.
Conclusion
MRGB has the potential to improve cancer detection rates in men with suspected PCa to deliver the relevant treatment as soon as possible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the most common malignant tumour in men in the United States and western Europe and is second in frequency among the tumours that lead to death in men [1]. The incidence of PCa has increased with life expectancy, and more cancers are detected as a result of wider screening and determination of prostate-specific antigen (PSA) level in serum. Determination of serum PSA levels is considered useful for the early identification of PCa [2]. The American Urological Association and the American Cancer Society recommend that all men aged 50 years or older undergo an annual digital rectal examination (DRE) and determination of PSA level. Patients with PSA levels greater than 4 ng/mL or with suspicious findings at DRE are candidates for further diagnostic work-up by means of systematic transrectal ultrasonography (TRUS)-guided biopsy. Up to now, TRUS-guided biopsy (TRUSGB), sampling 6–12 cores, one to two for each sextant, has been the diagnostic standard for PCa for many years. With this method, up to 30 % of cancers are missed when performing sextant biopsies, because more than 40 % of PCas are isoechoic and the operator cannot reliably visualise tumour. Therefore, the method has been extended to 45 cores for saturation biopsies [2]. Although taking more cores seems to improve the per-patient detection rate of PCa, the vastly invasive approach of placing up to 45 needles needs to be carefully taken into account. Furthermore, even if random TRUSGB is performed with a saturation or template mapping method, it does not solve the problem due to increased costs, complications, over-detection rates and a small but significant risk of missing high-grade cancer. TRUSGB has a negative predictive value of 70–80 %; thus, up to 20–30 % of patients with a negative biopsy may still have PCa [3], 23 % of whom at high risk of PCa [2]. Patients with a suspected false-negative biopsy are a diagnostic challenge because there is a progressively lower diagnostic yield from subsequent repeat prostate biopsies. Second, third, and fourth repeat biopsies are reported to detect cancer in only 25–27, 5–24, and 4–21 % of cases, respectively [3, 4]. Furthermore, because PCa is multifocal in 85 % of cases, TRUSGB may underestimate the extent and grade of cancer, which can result in Gleason upgrading after radical prostatectomy (RP). It is well documented that approximately 30 % of men who undergo RP for low-grade disease are upgraded on final pathology [5].
At present, multi-parametric magnetic resonance imaging (mp-MRI) which combines anatomical T2-weighted imaging (T2WI) with functional techniques such as diffusion-weighted imaging (DWI) which highlights cell proliferation, dynamic contrast-enhanced imaging (DCEI) which shows neoangiogenesis and MR spectroscopic imaging (MRSI) which displays cell metabolism is considered to be the most reliable imaging biomarker for PCa diagnosis [6–10]. Multiple studies have now shown that mp-MRI can help to identify tumours missed on biopsy, thus increasing biopsy yields with fewer core samples [10]. Many of these tumours are transitional zone tumours which are deep in the prostate and distant from the sites typically reached with a standard TRUSGB approach [10]. Currently, many studies have proved that MR-guided prostate biopsy (MRGB) shows a high detection rate for the diagnosis of PCa compared to standard TRUSGB [11–16]. MRGB can be performed in either dedicated (‘‘interventional’’) low-field systems or clinically widely available MRI scanners (1.5 Tesla field strengths).
In view of the improved detection of PCa with mp-MRI, the purpose of this study was to describe our preliminary experience with a biopsy device developed for MRGB with a closed MR unit.
Materials and methods
Patient population
The use of the devices and procedures described here was approved by the Institutional Ethics Committee. All patients were informed in detail before giving their written consent.
Twenty-three consecutive male patients (average age 64 years; age range 53–75 years; total PSA levels ranging from 4.7 to 54 ng/mL; median 9 ng/mL), with highly suspected PCa who referred to our Institution from March 2014 to May 2014, were enrolled in the study. Inclusion criteria were:
(a) Total PSA >4 ng/mL.
(b) PSA density >0.15.
(c) PSA velocity >0.75 ng/mL/year.
(d) Free/total PSA ratio <0.10 when total PSA level was between 4 and 10 ng/mL.
(e) Normal blood clotting parameters.
(f) A suspicious focal zone of PCa at mp-MRI with a prostate imaging-reporting and data system (PI-RADS) score ≥3.
Patients were excluded from the study if they had any of the usual contraindications to 1.5-T MR imaging (e.g. cardiac pacemakers or other metallic implants) or if they did not meet all the inclusion criteria.
MRI acquisition protocol
Pre-biopsy MRI of the pelvis, focused on the prostate gland, was performed using a 1.5 T magnet equipped with a phased-array coil (32 channels) and an endorectal coil. The MRI protocol included the technique and parameters detailed in the ESUR guidelines: T2WI in axial, coronal and sagittal planes: 3 mm thickness; in-plane resolution: 0.5 × 0.5 to 0.7 × 0.7 mm. Diffusion-weighted imaging (DWI) in the axial plane: 4 mm thickness; in-plane resolution: 1.5 × 1.5 to 2.0 × 2.0 mm. We used exponential b values: 0, 500, 1,000 and 2,000 mm2/s. Dynamic contrast-enhanced (DCE)-MRI in the axial plane: 3 mm thickness; in-plane resolution: 1.0 × 1.0 mm; imaging acquisition was continued for 5 min to detect washout. Unenhanced T1WI images from this sequence were used to detect post-biopsy haematomas. MR spectroscopic imaging (MRSI): matrix 8 × 8 × 8 phase-encoding steps with nominal voxel size <0.5 cc; spectral selective suppression of water and lipid signals; automatic or manual shimming up to a line width at half height of the water resonance peak between 15 and 20 Hz.
Analysis of MR images
Two genitourinary radiologists, with 13 and 4 years of experience, respectively, blinded to blood test results, evaluated the images in consensus.
Each MRI technique (T2WI, DWI, DCE and MRSI) was assessed relying on the PI-RADS score [17]. For each patient, the index lesion to which to direct the targeted biopsy, defined as the largest and most aggressive lesion based on MRI patterns, was identified [18]. The measurement and comparison of apparent diffusion coefficient (ADC) values and choline/creatine-to-citrate ratio (CC/C) were helpful for the diagnosis.
In patients with two or more suspected foci of PCa with different PI-RADS scores, the zone assumed to be the index lesion was the one with the highest PI-RADS score. In patients with two or more suspected foci of PCa with the same PI-RADS score, the area reported to be the index lesion was the most aggressive one with the lower ADC values and the highest CC/C [19]. When two or more suspected foci of PCa with a PI-RADS score of 5/5 were detected, the largest in size was biopsied.
MR-guided biopsy
Prostate biopsy was performed with the patient positioned prone in a 1.5 T closed MR. Eight patients underwent MRGB after diagnostic MRI in a single session, and 15 patients underwent MRGB in a second session within 1–2 weeks after the initial MR examination. In the former group, patients were given intravenous antiobiotic coverage before biopsy, whereas patients in the second group received oral antibiotic therapy 3 days before the procedure. Images were acquired with a combination of body and spine phased-array coils.
For guidance of the 18-gauge, fully automatic, core-needle, titanium double-shot biopsy gun, a needle guide filled with a gadolinium-chelate dotted gel for better visualisation on T2WI was used (Fig. 1). For fixation and adjustment of this needle guide, a portable biopsy device was used (DynaTRIM, Fig. 2). The biopsy gun needle has a length of 150–170 mm and a total probe feed of 25 mm. DynaCAD software for prostate advanced visualisation and interventional planning was used (Fig. 3).
Photograph shows biopsy device without base plate and cushion for patient positioning. The stand is variable in height and rests on a base plate containing a track that enables movement of the device along the longitudinal axis of the MR unit. Except for small parts such as screws, the device is made of synthetic materials that are fully compatible with MR imaging
After the patient was positioned, the needle guide was inserted into the rectum and connected to the arm of the biopsy device. The arm enables the needle guide to be rotated, moved forwards and backwards, and adjusted in height. In addition, the insertion angle can be changed by rotating the needle guide about a point inside the rectum. The needle guide can be rotated and moved forwards and backwards from outside the MR unit by means of a telescopic rod. It is thus possible to direct the needle guide to the desired prostate region with MRI guidance.
To reproduce the prebiopsy diagnostic MRI findings, after repositioning of the patient or on a different day, coronal, sagittal and axial T2W images (single-shot) were performed; moreover, axial T1W images for detection of haemorrhage with a section thickness of 3 mm were performed before biopsy in patients who underwent a previous TRUSGB. Adjustments of the needle guide system and planning for biopsy were based on oblique transversal T2W images. The needle guide was positioned according to image findings, and the position was reported after each repositioning by T2W images. T2WI was adopted in a double-oblique orientation, parallel to the long axis of the needle guide.
As a rule, two biopsy cores were obtained in each patient from the suspicious zone. In three patients, a suspicious lesion to which to direct the targeted biopsy was detected both in the peripheral and transitional zone, so that four biopsy cores were obtained, two for each lesion.
No local anaesthesia was offered prior to the procedure, nor were medications for bowel movement reduction or local anaesthetics or sedatives used, since the procedure was very well tolerated.
The overall 52 samples were separately placed in formalin solution and labelled for histopathological evaluation. All specimens were reviewed by a single pathologist with 18 years of experience in genitourinary pathology. The length of the tissue core was measured and documented after fixation. All patients received prophylactic antibiotic therapy before and after biopsy. The duration of the procedure, from the time the patient was positioned until the patient left the room, was determined. Complications were recorded during questioning of the patient after the procedure. Patients were instructed to present to the Department of Urology if they developed complications such as prolonged haematuria, fever, or pain.
Results
At prebiopsy mp-MRI, in the 23 patients, a total of 26 suspicious areas to which to direct the MRI-guided targeted biopsy were found, 23 of which in the peripheral zone and three in the transitional zone. Seven suspicious areas had a PI-RADS score 3/5, seven areas a PI-RADS scores 4/5 and 12 suspicious areas a PI-RADS scores 5/5. The needle guide was depicted and could be positioned with MRI guidance in all 23 patients (Fig. 4). The length of the biopsy cores after fixation ranged from 4 to 20 mm (median 13 mm). Histological examination demonstrated PCa in 3/7 zones with a PI-RADS score of 3/5 (average Gleason score 6), two of which were located in the transitional zone; in 6/7 areas with a PI-RADS score 4/5 (average Gleason score 7); in all the 12 areas with a PI-RADS score of 5/5 (average Gleason score 8). Histological examination in the four areas with a PI-RADS score of 3/5 negative for PCa revealed high-grade prostatic intraepithelial neoplasia in two zones and prostitis in the other two areas; whereas in the area with a PI-RADS score of 4/5 negative for PCa, it detected a capsular prostatic adenoma.
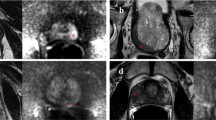
a, b Coronal T2-weighted image, c T2-weighted sagittal image and d axial diffusion-weighted image of a 69-year-old patient (PSA, 7.98 ng/mL) with only slight hypertrophy of the central gland and the index lesion located in the left third middle periapical aspect of the gland showing a PI-RADS score of 5/5. Note the needle guide pointing at the suspicious lesion
The duration of the procedure ranged from 35 to 55 min (mean 40 min). MRGB was well tolerated by all patients, and no major complications were either observed during the procedure or reported afterwards. In three patients, MRI revealed a small haemorrhage between the prostate and the rectal wall without affecting the procedure. Urinary, bowel and sexual function after the intervention were reported to be normal in all cases, and no haematuria was observed. According to our preliminary data, the detection rate for the diagnosis of PCa with a MR-guided targeted biopsy was 80 %. Ninety percent of PCa cases had intermediate grade aggressiveness: 34 % of them were Gleason score 7 (of which 22 % 3 + 4 and 12 % 4 + 3) and 66 % Gleason score 8 (4 + 4). The remaining 10 % of PCa detected had low-grade aggressiveness (Gleason score 6, 3 + 3), 66 % of which were located in the transitional zone.
Discussion
The prognosis and likelihood of distant metastases in PCa correlate with the tumour volume, TNM stage, and degree of differentiation [20]. Early detection of PCa enables identification of the tumour at an early stage at which it can be treated with a curative therapy. The determination of serum PSA level is an important tool for identifying early PCa [2]. Nevertheless, in some patients with PSA levels greater than 4 ng/mL, the first TRUSGB biopsy may yield no histological evidence of tumour. Some improvement in the targeting of suspicious areas at biopsy may be expected with a preliminary mp-MR. Mp-MRI examination shows promising results in identifying suspected PCa foci suitable for a re-biopsy in patients with persistently elevated PSA level and negative TRUSGB [21, 22].
Moreover, because mp-MRI is the most accurate imaging modality for the localisation of PCa, MRGB offers the possibility of more precise targeting with the possibility to perform fewer core biopsies [23]. MRGB techniques are becoming increasingly available, but there is no current consensus on the optimal technique [13, 24, 25]. In-bore approaches are exclusively MRI-based, using prebiopsy MRI to define the targets and real-time MRI to guide and control, with image confirmation, all the steps of the procedure. Out-of-bore approaches use US to guide and control the procedure; in a fusion US-MRI prostate biopsy, previously obtained prostate MR images are fused with the US images at the time of biopsy to guide the operator to the target.
Open and closed in-bore MRI settings could be used. Several different types of biopsy robots [26], some with complex software, are used to guide the needle. Target regions are determined using combinations of different MRI techniques. Some physicians use a transrectal approach, whereas others prefer a transperineal methodology. Movement of the prostate during the biopsy procedure is one of the biggest challenges in taking biopsies of the prostate [27]. Several solutions to this problem have been suggested, from fixation using needles to rendering real-time images [28].
Some authors experimented a transgluteal approach. Zangos et al. [29] used a device for transgluteal biopsies in a closed-bore system. Transgluteal biopsies minimise the risk of injury to the bladder, bowel, and iliac vessels, and no intestinal germs are introduced into the prostate. Disadvantages of this method are the need for local or general anaesthesia and the longer biopsy pathway. This technique was used first in a cadaver study, and currently it is not widely available.
The advantage of open MR scanners over closed MR scanners is that a physician has direct patient access. Unfortunately, open MR scanners are known for a low signal-to-noise ratio related to low-field strength (typically 0.5 T). The result is image quality that is too low to adequately localise tumours. To reliably identify the target regions, at least 1.5 T images must be obtained prior to the biopsy procedure by means of a closed-bore scanner.
Engelhard et al. [11] used a closed in-bore approach in a study with 37 patients who had previous negative prostate biopsies. The researchers concluded that suspicious lesions with a diameter greater than 10 mm could be successfully punctured using this device. PCa was detected in 14 of 37 patients (38 %). In a study of 27 patients with previous negative TRUSGB, Anastasiadis et al. [12] also used this device and found PCa in 15 (55.5 %) patients. The detection rates after one negative biopsy round ranged between 38 and 55.5 % [11–13]; these data are promising and demonstrate the potential clinical value of MRGB. To investigate the positive biopsy rate of closed in-bore MRGB in a routine clinical setting, Roethke et al. [14] included 100 patients with at least one negative TRUSGB, persistently elevated or rising PSA and at least one lesion suspicious for PCa on diagnostic 1.5 Tesla endorectal coil MRI. In 52/100 (52.0 %) patients, PCa was detected, showing a high tumour detection rate of over 50 %.
The results reported by Penzkofer et al. [15] have shown that to date in-bore MRGB is very reliable and relatively easy, and the targeted approach has high yield with more positive lesions from ADC- and DCE-positive sites.
One study reported on 71 consecutive men with at least two negative TRUSGB who then underwent mp-MRI: 70 had an MRI-suspicious region and 68 underwent in-gantry MRGB. The cancer detection rate was 59 %, of which 93 % were clinically significant cancers; MRGB was compared to a matched reference group who underwent repeat TRUSGB, and the authors found that MRGB detected significantly more tumours than standard repeat TRUSGB (22 % for second and 15 % for third TRUSGB) [16]. In a separate study by the same research group, 34 men underwent mp-MRI, then MRGB of DWI-derived targets, followed by RP; the biopsy-to-prostatectomy Gleason upgrading rate was compared with that of a matched cohort of 64 men who underwent standard TRUS 10 core biopsy followed by prostatectomy. The authors reported that Gleason grade on DWI-guided biopsy accurately predicted the Gleason grade at RP in 88 % of cases, whereas Gleason grade on standard 10-core biopsy predicted the Gleason grade at RP in only 55 % of cases [30]. This supports the hypothesis that MRGB more accurately risk stratifies PCa than standard biopsy. A large series reported a detection rate of 41 % in 96 men, but this study has been criticised for only using single parameter T2WI at 1–1.5 T to identify suspicious regions for biopsy [31].
As in-bore biopsies require MR scanners, and thus valuable device time, they are associated with a higher organisational overhead as a result of the magnetic field hazards. Thus, in-bore MRGB can be both time-consuming and expensive. However, it does offer the only method which can image the target and the biopsy needle within it prior to sampling, and thus the only true image-targeted biopsy. However, the limited space inside the MRI prevents physicians from making real-time intervention under direct MRI guidance. Procedures combining the virtues of US with the ability of MRI to delineate PCa may represent a promising way of overcoming this limit [32, 33]. A proposed solution for this dilemma is the ‘out-of-bore’ approach with fusion or registration of pre-procedural prostate MRI data to TRUSGB which combines the detection capabilities of MRI with the comparably easy set-up of TRUS [15]. Therefore, real-time TRUS and MRI fusion-guided biopsy are proposed as methods for using the high-contrast-sensitive MRI data to detect the tumour and the real-time character of TRUS to follow the motions of the prostate during biopsy [34, 35].
The most straightforward approach for TRUS/MRI-guided biopsies is cognitive fusion, in which TRUSGB is performed knowing the localisation of MRI-suspicious lesions derived from peri-procedural MRI [15]. No specialised equipment is required other than an MRI scanner and a conventional TRUS biopsy device [36]. Despite the fact that cognitive fusion seems to improve biopsy protocols, more sophisticated devices have been developed for MRI/TRUS fusion that use different ways of registering the intraprocedural US coordinates to the MRI coordinates [15]. Pinto et al. [37] studied a group of 101 patients from three different risk categories derived from imaging aspects (low, moderate, high) on a TRUS/MRI system. All patients received 12-core standard systematic and MRI/TRUS-fused prostate biopsies in the same setting. Cancer was detected in 27.9, 66.7 and 89.5 % of the cases for the low, intermediate and high-risk groups, respectively. In this setting, MRI/TRUS fusion-guided prostate biopsies detected more cancers per core than the standard 12-core approach (20.6 versus 11.7 %). Sonn et al. [38] in a recent study concluded that TRUS/MRI biopsy is three times as likely to yield cancer diagnoses (21 % of performed targeted biopsies versus 7 % of systematic biopsies); on a per-patient basis, many cancers were detected by systematic biopsy alone (84 total positive diagnoses, 38 by both methods, 15 by MRI/TRUS alone, 31 by systematic TRUSGB alone). Thus, the combination of systematic and TRUS/MRI-guided biopsy seems to be the key in the detection of more cancers.
In a recent paper, de Rooij et al. [39] determined the cost-effectiveness of mp-MRI and MRGB compared with TRUSGB and found that the MRI strategy is cost-effective in diagnosing PCa compared with the TRUSGB strategy, assuming a sensitivity of MRGB ≥20 %. They concluded that although the MRI strategy is initially more expensive, these extra costs are compensated for by reducing treatment costs as a result of fewer false positives and a better estimation of tumour aggressiveness.
Our preliminary experience confirms the promising results of closed-bore MRGB in the early diagnosis of PCa and we hope that this new approach will become widespread so that the relevant treatment can be delivered as soon as possible.
References
Cooperberg MR, Broering JM, Carroll PR (2010) Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol 28:1117–1123
Scattoni V, Raber M, Abdollah F et al (2010) Biopsy schemes with the fewest cores for detecting 95% of the prostate cancers detected by a 24-core biopsy. Eur Urol 57:1–8
Djavan B, Milani S, Remzi M (2005) Prostate biopsy: who, how and when. An update. Can J Urol 12:44–48
Lujan M, Paez A, Santonja C et al (2004) Prostate cancer detection and tumor characteristics in men with multiple biopsy sessions. Prostate Cancer Prostatic Dis 7:238–242
Freedland SJ, Kane CJ, Amling CL et al (2007) Upgrading and downgrading of prostate needle biopsy specimens: risk factors and clinical implications. Urology 69:495–499
Ahmed HU, Kirkham A, Arya M et al (2009) Is it time to consider a role for MRI before prostate biopsy. Nat Rev Clin Oncol 6:197–206
Valerio M, Panebianco V, Sciarra A et al (2009) Classification of prostatic diseases by means of multivariate analysis on in vivo proton MRSI and DCE-MRI data. NMR Biomed 22:1036–1046
Dickinson L, Ahmed HU, Allen C et al (2013) Clinical applications of multiparametric MRI within the prostate cancer diagnostic pathway. Urol Oncol 31:281–284
Hoeks CM, Schouten MG, Bomers JG et al (2012) Three-Tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. Eur Urol 62:902–909
Hoeks CM, Hambrock T, Yakar D et al (2013) Transition zone prostate cancer: detection and localization with 3-T multiparametric MR imaging. Radiology 266:207–217
Engelhard K, Hollenbach HP, Kiefer B et al (2006) Prostate biopsy in the supine position in a standard 1.5-T scanner under real time MR-imaging control using a MR-compatible endorectal biopsy device. Eur Radiol 16:1237–1243
Anastasiadis AG, Lichy MP, Nagele U et al (2006) MRI-guided biopsy of the prostate increases diagnostic performance in men with elevated or increasing PSA levels after previous negative TRUS biopsies. Eur Urol 50:738–748 (discussion 748–749)
Beyersdorff D, Winkel A, Hamm B et al (2005) MR imaging-guided prostate biopsy with a closed MR unit at 1.5 T: initial results. Radiology 234:576–581
Roethke M, Anastasiadis AG, Lichy M et al (2012) MRI-guided prostate biopsy detects clinically significant cancer: analysis of a cohort of 100 patients after previous negative TRUS biopsy. World J Urol 30:213–218
Penzkofer T, Tempany-Afdhal CM (2013) Prostate cancer detection and diagnosis: the role of MR and its comparison with other diagnostic modalities—a radiologist’s perspective. NMR Biomed 27:3–15
Hambrock T, Somford DM, Hoeks C et al (2010) Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol 183:520–527
Barentsz JO, Richenberg J, Clements R et al (2012) ESUR prostate MR guidelines 2012. Eur Radiol 22:745–746
Riaz A, Miller FH, Kulik LM et al (2010) Imaging response in the primary index lesion and clinical outcomes following transarterial locoregional therapy for hepatocellular carcinoma. JAMA 303:1062–1069
Kobus T, Vos PC, Hambrock T et al (2012) Prostate cancer aggressiveness: in vivo assessment of MR spectroscopy and diffusion-weighted imaging at 3 T. Radiology 265:457–467
Miller GJ, Cygan JM (1994) Morphology of prostate cancer: the effects of multifocalityon histological grade, tumor volume and penetration. J Urol 152:1709–1713
Yuen JS, Thng CH, Tan PH et al (2004) Endorectal magnetic resonance imaging and spectroscopy for the detection of tumor foci in men with prior negative transrectal ultrasound prostate biopsy. J Urol 171:1482–1486
Panebianco V, Sciarra A, Ciccariello M et al (2010) Role of magnetic resonance spectroscopic imaging ([1H]MRSI) and dynamic contrast-enhanced MRI (DCE-MRI) in identifying prostate cancer foci in patients with negative biopsy and high levels of prostate-specific antigen (PSA). Radiol Med 115:1314–1329
Bonekamp D, Jacobs MA, El-Khouli R et al (2011) Advancements in MR imaging of the prostate: from diagnosis to interventions. Radiographics 31:677–703
D’Amico AV, Tempany CM, Cormack R et al (2000) Transperineal magnetic resonance image guided prostate biopsy. J Urol 164:385–387
Fischer GS, DiMaio SP, Iordachita II, Fichtinger G (2007) Robotic assistant or transperineal prostate interventions in 3 T closed MRI. Med Image Comput Comput Assist Interv 10:425–433
Cleary K, Melzer A, Watson V et al (2006) Interventional robotic systems: applications and technology state-of-the-art. Minim Invasive Ther Allied Technol 15:101–113
Stone NN, Roy J, Hong S et al (2002) Prostate gland motion and deformation caused by needle placement during brachytherapy. Brachytherapy 1:154–160
Dattoli M, Waller K (1997) A simple method to stabilize the prostate during transperineal prostate brachytherapy. Int J Radiat Oncol Biol Phys 38:341–342
Zangos S, Herzog C, Eichler K et al (2007) MR-compatible assistance system for punction in a high-field system: device and feasibility of transgluteal biopsies of the prostate gland. Eur Radiol 17:1118–1124
Hambrock T, Hoeks C, Hulsbergen-Van De Kaa C et al (2012) Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol 61:177–184
Engehausen DG, Engelhard K, Schwab SA et al (2012) Magnetic resonance image-guided biopsies with a high detection rate of prostate cancer. Sci World J 2012:975971
Turkbey B, Xu S, Kruecker J et al (2011) Documenting the location of prostate biopsies with image fusion. BJU Int 107:53–57
Hadaschik BA, Kuru TH, Tulea C et al (2011) A novel stereotactic prostate biopsy system integrating preinterventional magnetic resonance imaging and live ultrasound fusion. J Urol 186:2214–2220
Singh AK, Kruecker J, Xu S et al (2008) Initial clinical experience with real-time transrectal ultrasonography-magnetic resonance imaging fusion-guided prostate biopsy. BJU Int 101:841–845
Kaplan I, Oldenburg NE, Meskell P et al (2002) Real-time MRI-ultrasound image guided stereotactic prostate biopsy. Magn Reson Imaging 20:295–299
Moore CM, Robertson NL, Arsanious N et al (2013) Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol 63:125–140
Pinto PA, Chung PH, Rastinehad AR et al (2011) Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol 186:1281–1285
Sonn GA, Natarajan S, Margolis DJA et al (2012) Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol 189:86–91
de Rooij M, Crienen S, Witjes JA et al (2014) Cost-effectiveness of magnetic resonance (MR) imaging and MR-guided targeted biopsy versus systematic transrectal ultrasound-guided biopsy in diagnosing prostate cancer: a modelling study from a health care perspective. Eur Urol 66:430–436
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panebianco, V., Barchetti, F., Manenti, G. et al. MR imaging-guided prostate biopsy: technical features and preliminary results. Radiol med 120, 571–578 (2015). https://doi.org/10.1007/s11547-014-0490-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-014-0490-0