Abstract
Purpose
The main objective of this study was to describe the characteristic imaging features of cerebral and spinal hydatid disease (HD) in magnetic resonance imaging (MRI) and computed tomography (CT) in order to provide more effective differential diagnoses in endemic regions. We also aimed to use MRI and CT to evaluate whether the World Health Organization’s (WHO) new classification of hepatic cystic echinococcosis (CE) could be used in the classification of cerebral HD.
Materials and methods
We retrospectively reviewed the CT and MR images of 30 patients who were diagnosed with cerebral and spinal HD between 1990 and 2014. The imaging findings were noted. All hydatid cysts were classified according to the WHO classification of hepatic CE, consisting of six types.
Results
The study group consisted of 49 CEs in 27 patients with cerebral HD and 12 CEs in three patients with spinal HD. Of the cysts, 14 were type CL (cystic lesion), 29 were type CE1, 11 were type CE2 and seven were type CE3. In other words, 54 cysts were in the active group and seven were in the transition group. Most of the cysts were type CL and CE1.
Conclusions
Even though characteristic imaging features could be used in the differential diagnosis of HD, sometimes the differentiation of HD from other cystic lesions may be difficult. The use of WHO classification will provide standardisation of uniform treatment modality, as the treatment of HD, according to the stage of the disease, may be surgical or medical.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydatid disease (HD) is a systemic zoonosis that is endemic in sheep and cattle farming areas of Asia, North and East Africa, South America and the Middle East [1–3]. In Europe, HD is seen only in immigrants or persons with a history of travel to an endemic area [1, 2, 4, 5]. Recently, due to increased immigration, the prevalence of the disease has increased in Europe and North America [6]. Although HD may affect any part of the body, it most often affects the liver (59–75 %), followed by the lung (27 %) [1, 7, 8]. HD of the central nervous system is extremely rare. Cerebral and spinal HD accounts for only 1–2 % of all cases of HD [1, 8, 9].
Preoperative diagnosis of HD is essential, because rupture and dissemination of the cysts may result in recurrence and life-threatening complications. Although the diagnosis of hydatid cyst (HC) relies on serological tests and imaging techniques [10], in primary cerebral HD, serologic tests are not diagnostic because of the blood brain barrier, and imaging studies such as magnetic resonance imaging (MRI) and computed tomography (CT) are necessary for the preoperative diagnosis. Classical imaging findings in HD are well known but differential imaging findings of HD in unusual locations such as spine and cerebrum are less described in the literature.
The first objective of our study was to describe the characteristic MRI and CT imaging features of cerebral and spinal HD in an attempt to provide an improved modality for the differential diagnosis of central nervous system HD in endemic regions. The second objective was to use MRI and CT to evaluate whether the new World Health Organization (WHO) classification of hepatic cystic echinococcosis (CE) could be used in the classification of cerebral HD.
Materials and methods
The study was approved by the institutional review board and protocol review committee. We retrospectively reviewed the CT and MR images of 32 patients with cerebral and spinal HD diagnosed between 1990 and 2014. Two patients were excluded from the study because of incomplete characterisation of lesions on imaging or histopathological examination. As a consequence, 30 patients (27 cerebral HD and three spinal HD) were included in this study.
The diagnosis of HD was confirmed by histopathology in 25 patients after surgery. The remaining lesions with similar or characteristic radiological appearance were accepted as HD.
CT examinations were performed on two CT scanners: 16 detectors, (Toshiba Activion; Toshiba Medical Systems, Tochiqi-ken, Japan) and 64 detectors, (Brilliance CT scanner; Philips Medical Systems, Cleveland, Ohio). MR examinations were performed on MRI devices supplied with 1.5T and 3T magnetic power (Achieva, Philips Medical Systems, The Netherlands) with a standard head coil.
Five patients were examined with MRI only and nine with CT only. Sixteen patients were examined with both MRI and CT. All MR scans were performed with and without contrast in 21 patients. The CT scans were performed with contrast in 11 patients, without contrast in three patients, and with and without contrast in 11 patients.
The CT and MR findings were retrospectively assessed by the same experienced radiologist. The location, number, stage of the cysts, contour, calcification, internal structure, contrast enhancement and peripheral oedema were noted. Also the simultaneous organ involvement and distribution of cysts were noted.
All HCs were classified according to the WHO classification of CE [11, 12] (Table 1).
Results
The patient sample comprised 20 males (66.6 %) and 10 females (33.3 %) aged 7–50 years. Most of the cerebral HD patients were children, with 19/27 patients (70.3 %) between 7 and 15 years old. All of the spinal HD patients were over 25 years old. Eighteen (66.6 %) patients with cerebral HD and 2/3 patients (66.6 %) with spinal HD were male.
All of the cerebral cysts were supratentorial and the parietal lobe was most frequently involved. The size of the HCs ranged from 1 to 12 cm and most of the cysts were solitary. Distribution of the age, sex, location, type and CT/MRI findings for the patients with cerebral HD are shown in Table 2.
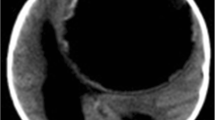
CL type cysts identified in the study were unilocular, spherical, homogenous, isodense/isointense to cerebrospinal fluid (CSF) and lacking a wall on CT and MRI (Fig. 1). CE1 cysts were unilocular, spherical or oval, isodense/isointense to CSF with a well-defined wall (isodense to hyperdense on CT, hypointense on T2-weighted MR images) (Fig. 2). CE2 cysts were multivesicular and multiseptated. Cyst septations produce “rosette-like”, “honeycomb-like” or “wheel-like” appearances (Fig. 3). In CE3 cysts, detachment of laminated membrane from the cyst wall produced a floating membrane appearance (water-lily sign).The cysts were oval shaped with a hypointense wall on T2-weighted MR images (Fig. 4). There were no cases of type CE4 or CE5 cysts in our study.
CE1 cyst. Axial T2-weighted image a shows a unilocular, well-defined, cyst located in the left frontal lobe. The cyst has a hypointense rim (visible cyst wall) with mild surrounding oedema along its anterior aspect. Axial T1-weighted image b shows homogeneous low intensity of the cyst. Note the mass effect and the displacement of the interhemispheric fissure to the right
Noncomplicated or noninfected lesions demonstrated smooth, well-defined, thin-walled, spherical, homogeneous appearance, without calcification, peripheral oedema or contrast enhancement which had inner density/intensity similar to CSF on CT and MRI. Infected cysts demonstrated spherical well-defined wall with surrounding oedema and complete or incomplete rim enhancements (Fig. 5). Two of the patients had multiple HC (one of them had three cysts and the other one had 21), and all of them were supratentorial, noncomplicated cysts. In five patients, the mass effect of the HC induced a midline shift.
We included only three cases of spinal HD in this study. All of them were primary and had multilevel, extradural-intraspinal components. Table 3 shows the level, location and type of the spinal CEs.
All of our spinal HD cases had characteristic imaging features; multiple hypointense masses in the vertebral bone, extradural-intraspinal and paravertebral areas on T1-weighted MRI. On T2-weighted MRI multiple hyperintense masses with daughter cysts and low-signal-intensity rims were observed (Fig. 6).
Extradural-intraspinal and paravertebral hydatid disease. Axial T1-weighted image a shows multiple hypointense masses in the paravertebral and extradural-intraspinal areas. The corresponding axial T2-weighted image b shows multiple areas of increased signal intensity. Note that the extradural-intraspinal masses have low-signal-intensity rims. c Macroscopic appearance of the daughter cysts after surgical excision
The most common symptoms and clinical findings were vomiting, headache, seizures, visual disturbances in cerebral HD patients, and weakness of the limbs, and back pain in spinal HD patients.
There was extracerebral organ involvement in eight cases of cerebral HD. Except for one patient, who had cardiac and muscular involvement, all patients (n = 7) had concomitant liver involvement and one patient had a disseminated infection.
Discussion
Since diagnosis and treatment decisions are driven by imaging, it is important to be familiar with the imaging features of HD. The diagnostic accuracy of MRI and CT is high for cerebral and spinal HD with specific imaging characteristics like daughter cysts, floating membrane and hypointense walls on T2-weighted images. However, the imaging features of HD may overlap with those of other cystic lesions.
The differential diagnosis of cerebral HCs includes numerous cystic lesions such as simple cysts, arachnoid cysts, porencephalic cysts, cystic tumour of the brain, pyogenic abscess and neurocysticercosis [9].
-
1.
Simple cysts and complete liquid-type HCs (CL and CE1) display isodensity/isointensity on CT and MR images, with well-defined margins. Type CE1 usually has a low-signal-intensity well-defined wall on T2-weighted MR image (rim sign) which makes them easy to distinguish from simple cysts. On CT scan the cyst wall appears iso/hyperdense. However, in CL type cysts there is no visible cyst wall so the differential diagnosis requires clinical information and laboratory findings. While secondary cerebral HD could cause high antibody titres, in primary cerebral HD, these are absent or lower. Therefore, if there is another involved organ, the antibody titres will be high, which is helpful for the diagnosis.
-
2.
Arachnoid cysts are extra-axial masses. They are neither spherical nor surrounded by brain tissue [13]. Arachnoid cysts may deform the adjacent brain tissue and scallop the calvaria.
-
3.
Porencephalic cysts are not generally spherical and they usually have a direct communication with the ventricular system. Porencephalic cysts are typically lined by gliosis that can easily be demonstrated with MR imaging.
-
4.
Cystic tumours could be differentiated by surrounding oedema, peripheral and mural nodular enhancement. HCs, especially complicated cysts, may demonstrate complete or incomplete peripheral enhancements, but in cystic tumours peripheral enhancement is more prominent. Three of our cases were infected cysts displaying fine peripheral enhancement and oedema.
-
5.
In pyogenic abscess, peripheral oedema is mostly observed and the rim enhancement is prominent. Satellite lesions are commonly present. Diffusion-weighted MRI could be used for differentiation between abscess and HCs. While pyogenic abscess shows diffusion restriction [14], HCs do not [15]. As the stage of the HCs progress, the ADC values decrease due to the protein, lipid and polysaccharide content [16].
-
6.
Neurocysticercosis shows a smooth thin-walled cyst that is CSF-like on CT and MR images in the early vesicular stage. A mural nodule that represents the viable larval scolex, “cyst with a dot” appearance, and oedema and contrast enhancement can be used in differentiation.
Our demographic findings were similar to those reported in the literature. Czermak et al. [17] reported that cerebral HD is most commonly seen in children, and El-Shamam et al. [18] reported that cerebral HCs are usually seen in males. In our study, 19 patients out of 27 (70.3 %) were between 7 and 15 years old, and 18 (66.6 %) patients with cerebral HD were male. All of the cerebral cysts were supratentorial and the parietal lobe was most frequently involved.
Daughter cysts are pathognomonic for HD but this finding has rarely been reported [19]. In accordance with the literature, three of the cerebral HCs and all of the spinal HCs were multilocular in our series. Primary multiple HCs of the brain are rare [20]. Multiple larval intake and embolisations from a ruptured cyst into the circulation are causes of multiple cerebral cysts. We had only two cases with multiple cerebral HCs, consistent with the literature (Fig. 1).
The differential diagnosis of spinal HCs includes cystic masses, tuberculous spondylitis, chronic osteomyelitis, abscess and haematoma [21, 22]. The imaging characteristics of spinal HD can aid in differentiation. Mentioned characteristics include features such as spinal HCs usually having a multilocular structure, low-signal-intensity rims on T2-weighted imaging, no enhancement (except in complicated cysts), and paraspinal extension, usually without calcification, and osteoporosis and sclerosis in bone.
Additionally, it is important to know the different stages of CE in order to determine the treatment protocol. Today, imaging-based, stage-specific treatment is accepted and four options exist for the treatment of CE. These are surgery, percutaneous treatment of the hydatid cysts with the PAIR (puncture, aspiration, injection, re-aspiration) technique, anti-infective drug treatment and ‘watch and wait’ [23]. In cerebral and spinal HD, three (surgery, medical treatment and “watch and wait”) of these options could be used. The most common treatment modality is surgical removal of the cysts. Medical treatment [24] could be used for inoperable cysts and for cases with a high surgical risk. Furthermore, leaving type CE4 and CE5 cysts (inactive forms) untreated and just monitoring them is a treatment method used in liver hydatid cysts. Therefore, a “watch and wait” approach may be used for cysts which are calcified, inactive and do not lead to increased intracranial pressure in asymptomatic patients [25]. The choice of treatment protocol should be made according to the risks, benefits, indications and contraindications for each case.
We used the WHO classification of CE for 49 CEs in 27 patients with cerebral HD and 12 CEs in three patients with spinal HD. Of the cysts, 14 were type CL, 29 were type CE1, 11 were type CE2 and seven were type CE3. In other words 54 cysts were in the active class and seven were in the transitional class. Utilisation of this classification can differentiate fertile active cysts from the transitional and inactive forms of CE.
Cerebral and spinal HD is well demonstrated by CT and MRI. Calcification can easily be detected with CT but MRI is better in detecting multiplicity and demonstrating cyst wall and anatomical relationships. Consequently, MRI is more helpful in surgical planning.
This study has several technical limitations. First of all, the images of 30 patients were obtained from four different devices (two CT and two MRI). Another limitation is that our study included no patients with type CE4 and type CE5.
In conclusion, the differential diagnosis of HD is usually possible with the combined use of characteristic imaging features, laboratory and clinical information. However, sometimes the differential diagnosis of HD from other cystic lesions may be challenging. The radiologist must be diligent, particularly in endemic areas, as HD has a broad differential diagnosis including simple cyst or cystic tumour. Since the treatment of HD may be surgical or medical, determined by the stage of the disease, the use of such classification will provide standardisation of a uniform treatment modality.
References
Polat P, Kantarci M, Alper F et al (2003) Hydatid disease from head to toe. Radiographics 23:475–494. doi:10.1148/rg.232025704
McManus DP, Zhang W, Li J, Bartley PB (2003) Echinococcosis. Lancet 362:1295–1304. doi:10.1016/S0140-6736(03)14573-4
Dahniya MH, Hanna RM, Ashebu S et al (2001) The imaging appearances of hydatid disease at some unusual sites. Br J Radiol 74:283–289
Pedrosa I, Saíz A, Arrazola J et al (2000) Hydatid disease: radiologic and pathologic features and complications 1 (CME available in print version and on RSNA link). Radiographics 20:795–817
Kilani T, El Hammami S, Horchani H et al (2001) Hydatid disease of the liver with thoracic involvement. World J Surg 25:40–45
Turgut AT, Altınok T, Topçu S, Koşar U (2009) Local complications of hydatid disease involving thoracic cavity: imaging findings. Eur J Radiol 70:49–56. doi:10.1016/j.ejrad.2008.01.002
Çeçe H, Gündoğan M, Karakaş Ö et al (2013) The role of diffusion-weighted magnetic resonance imaging in the classification of hepatic hydatid cysts. Eur J Radiol 82:90–94. doi:10.1016/j.ejrad.2012.08.015
Tüzün M, Hekimoğlu B (1998) Hydatid disease of the CNS: imaging features. AJR Am J Roentgenol 171:1497–1500. doi:10.2214/ajr.171.6.9843277
Osborn AG, Preece MT (2006) Intracranial cysts: radiologic–pathologic correlation and imaging approach. Radiology 239:650–664. doi:10.1148/radiol.2393050823
Torgerson PR, Deplazes P (2009) Echinococcosis: diagnosis and diagnostic interpretation in population studies. Trends Parasitol 25:164–170. doi:10.1016/j.pt.2008.12.008
Working Group WI (2003) International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop 85:253–261. doi:10.1016/S0001-706X(02)00223-1
Razek AAKA, El-Shamam O, Wahab NA (2009) Magnetic resonance appearance of cerebral cystic echinococcosis: World Health Organization (WHO) classification. Acta Radiol Stockh Swed 1987 50:549–554. doi:10.1080/02841850902878161
Borja MJ, Plaza MJ, Altman N, Saigal G (2013) Conventional and advanced MRI features of pediatric intracranial tumors: supratentorial tumors. AJR Am J Roentgenol 200:W483–W503. doi:10.2214/AJR.12.9724
Chang S-C, Lai P-H, Chen W-L et al (2002) Diffusion-weighted MRI features of brain abscess and cystic or necrotic brain tumors: comparison with conventional MRI. Clin Imaging 26:227–236. doi:10.1016/S0899-7071(02)00436-9
Kitis O, Calli C, Yunten N (2004) Report of diffusion-weighted MRI in two cases with different cerebral hydatid disease. Acta Radiol Stockh Swed 1987 45:85–87
Inan N, Arslan A, Akansel G et al (2008) Diffusion-weighted imaging in the differential diagnosis of cystic lesions of the pancreas. Am J Roentgenol 191:1115–1121. doi:10.2214/AJR.07.3754
Czermak BV, Unsinn KM, Gotwald T et al (2001) Echinococcus granulosus revisited: radiologic patterns seen in pediatric and adult patients. AJR Am J Roentgenol 177:1051–1056. doi:10.2214/ajr.177.5.1771051
El-Shamam O, Amer T, El-Atta MA (2001) Magnetic resonance imaging of simple and infected hydatid cysts of the brain. Magn Reson Imaging 19:965–974
Haliloglu M, Saatci I, Akhan O et al (1997) Spectrum of imaging findings in pediatric hydatid disease. AJR Am J Roentgenol 169:1627–1631. doi:10.2214/ajr.169.6.9393178
Yurt A, Avcı M, Selçuki M et al (2007) Multiple cerebral hydatid cysts: report of a case with 24 pieces. Clin Neurol Neurosurg 109:821–826. doi:10.1016/j.clineuro.2007.07.011
Oğüt AG, Kanberoğlu K, Altuğ A, Cokyüksel O (1992) CT and MRI in hydatid disease of cervical vertebrae. Neuroradiology 34:430–432
Tsitouridis I, Dimitriadis AS (1997) CT and MRI in vertebral hydatid disease. Eur Radiol 7:1207–1210
WHO Informal Working Group on Echinococcosis (1996) Guidelines for treatment of cystic and alveolar echinococcosis in humans. Bull World Health Organ 74:231–242
Seckin H, Yagmurlu B, Yigitkanli K, Kars HZ (2008) Metabolic changes during successful medical therapy for brain hydatid cyst: case report. Surg Neurol 70:186–189. doi:10.1016/j.surneu.2007.05.047
Köktekir E, Erdem Y, Gökçek C et al (2011) Calcified intracranial hydatid cyst: case report. Türkiye Parazitolojii Derg Türkiye Parazitoloji Derneği Acta Parasitol Turc Turk Soc Parasitol 35:220–223. doi:10.5152/tpd.2011.56
Acknowledgments
We are grateful to Dicle University DUBAP for their sponsorship of the English editing of this manuscript.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teke, M., Göçmez, C., Hamidi, C. et al. Imaging features of cerebral and spinal cystic echinococcosis. Radiol med 120, 458–465 (2015). https://doi.org/10.1007/s11547-014-0475-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-014-0475-z