Abstract
Purpose
This study was done to evaluate the possibility of reducing the dose of ionising radiation by using dual-source dual-energy computed tomography (CT) in patients undergoing CT angiography of the aorta to search for endoleaks after endovascular aneurysm repair (EVAR).
Materials and methods
One hundred and forty-eight patients (117 M, 31 F; mean age 75 ± 6.5) underwent 171 CT angiography scans for follow-up after EVAR. For each patient we performed a triple-phase acquisition protocol consisting of a nonenhanced phase, an arterial phase and a delayed phase; the latter acquired in dual energy. Two radiologists jointly evaluated the nonenhanced, arterial and delayed phase, and a third radiologist evaluated only the delayed phase and its virtual noncontrast (VNC) reconstruction. Moreover, we compared the cumulative effective doses of the triple-phase acquisition with the dual-energy acquisition.
Results
We detected 34 endoleaks (19.8 %), with 100 % agreement between the triple-phase and dual-energy acquisitions. The effective dose of dual-energy acquisition performed during the delayed phase was 61.7 % lower than that of the triple-phase acquisition.
Conclusions
A dual-energy CT scan acquired during the delayed phase and its VNC reconstruction allow detection of endoleaks with a substantial reduction of effective dose and a complete diagnostic agreement with a triple-phase acquisition protocol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abdominal aortic aneurysm (AAA) is the permanent dilation of the abdominal aorta greater than 3.5 cm in axial diameter or greater than 50 % of its normal diameter [1]. The risk of rupture is directly proportional to the size of the aneurysm and is about 5 % per year for aneurysms 5 cm in diameter, 10 % for aneurysms 6 cm in diameter, 32 % for aneurysms 7 cm in diameter and 25 % every 6 months for aneurysms larger than 8 cm in diameter [2, 3].
Endovascular aneurysm repair (EVAR) is the first choice for the treatment of AAA because it is less invasive and presents less operative mortality than surgery. The indications for EVAR have increased in part thanks to the possibility, with the last generation EVAR, to treat aortic aneurysms with short and angled neck, with smaller peripheral accesses [4, 5]. The most common complication after EVAR is an endoleak, blood flowing outside the stent graft lumen but within the aneurysmal sac, with a potential risk of enlargement of the aneurysmal sac and rupture [6]. Endoleak has an incidence up to 45 % and is the main indication for late surgical conversion; proper management requires following up the patients after EVAR in order to identify and classify the type of endoleak [7].
Computed tomography angiography (CTA) is a fast, safe, and minimally invasive technique which has a high sensitivity and specificity in the search for endoleaks and is the imaging method of choice for the detection of endoleaks after EVAR. The optimal CT acquisition protocol consists of a three-phase acquisition including a noncontrast phase, an arterial phase and a delayed phase [8]: the first phase is useful to differentiate small endoleaks from calcifications inside the aneurysmal sac or in the aortic wall [9].
The high incidence of complications after EVAR requires regular lifelong surveillance [10], which makes dose reduction desirable. Although the triple-phase acquisition protocol provides excellent results, it exposes the patient to ionising radiation with potential risk of carcinogenesis. Some authors proposed a two-phase acquisition protocol, composed of an arterial and a delayed phase after the administration of contrast material [11]. Others showed that a nonenhanced and a delayed phase may be enough for the detection of endoleaks [12]. Recently, single-phase protocols have been introduced, thanks to dual-source dual-energy technology [13] by using post-processing algorithms that allow reconstruction images of a virtual noncontrast (VNC) phase [14].
The aim of this study was to evaluate the possibility of decreasing the radiation dose by performing a single-phase CT scan in dual-source dual-energy mode during the delayed phase in patients undergoing CT angiography of the aorta to search for endoleaks after EVAR.
Materials and methods
Patients
From November 2012 to April 2013, 153 patients who had previously undergone EVAR of AAAs were admitted to the Radiology Department of the San Camillo Forlanini Hospital in Rome, Italy, to perform dual-energy multidetector CTA of the abdominal aorta (Table 1).
CT follow-up timing was chosen based on clinical assessment and procedural difficulty in stent-graft implanting. Exclusion criteria for contrast-enhanced CT were known adverse reactions to iodine-containing contrast material (n = 0) and nephropathy (serum creatinine >1.5 mg/dl) (n = 5).
The local ethics committee approved the study and each patient signed a written consent form. A total of 148 patients (117 M, 31 F) with a mean age of 75 ± 6.5 years (range 62–86) were included in the study for a total of 171 CT angiography examinations.
Dual-energy scanner CT
All CT examinations were performed by using a dual-source dual-energy CT scanner (Somatom Definition, Siemens Medical Solutions, Germany). This CT scanner consists of two CT acquisition systems composed of two X-ray tubes and two corresponding detectors rotating around the patient. The two tubes are arranged inside of the gantry with an angular offset of 90° with respect to each other. The CT scan allows simultaneous generation of different levels of energy during a single scan and can be operated in single- or dual-source mode. When the single-source mode is enabled, data are acquired with tube A only, at a peak voltage of 120 kV and a amperage setting of 190 mAs for the nonenhanced phase, and 120 kV and 220 mAs for the arterial phase; Care Dose 4D (Siemens Medical Systems, Germany) was enabled for tube current modulation. When the dual-source mode is enabled, data are acquired with both X-ray sources operating with independent adjustment of peak voltage and amperage for each tube. Tube A was set at a peak voltage of 140 kV and maximum amperage of 93 mAs and tube B at 80 kV and 395 mAs; Care Dose 4D was enabled for tube current modulation. The field of view (FOV) was 50 cm for tube A, 26 cm for the tube B.
Data acquisition and post-processing
Each patient underwent a CT acquisition protocol composed of a triple phase scan: a nonenhanced phase, an arterial phase and a delayed phase. All acquisitions were performed from the 12th thoracic vertebra to the greater trochanter. The nonenhanced phase and arterial phase were acquired in single-source mode, the delayed phase in dual-source dual-energy mode.
For acquisitions performed after the administration of contrast material, we used iso-osmolar nonionic contrast material (Iopamiro 370 mg/mL Bracco, Milan, Italy) at dose of 80 mL at flow rate of 4 mL/s, followed by 40 mL of 0.9 % NaCl solution at the same rate, administered via an antecubital venous catheter with a power injector (Medrad Stellant, GE Health Care).
For the arterial phase, the bolus tracking technique was used; a region of interest (ROI) of about 0.5 cm2 (0.5 ± 0.1 cm2) was placed over the abdominal aorta at the level of the celiac trunk and an automatic trigger threshold attenuation of 100 HU was chosen. Data acquisition started 7 s after this threshold attenuation was reached; during this time the patient was instructed to maintain an inspiratory breath-hold during the scan and the aorta reached its maximum contrast enhancement.
The delayed phase acquisition started 30 s after the arterial phase and data were acquired in dual-source mode with an overlap of 26 cm between the FOV of the two tubes, enough to allow the dual-energy reconstruction of aorto-iliac axis. All image sets were acquired with a thickness of 1.5 mm (Table 2).
The datasets acquired during the delayed phase (80 and 140 kV) were processed in order to generate a third set of fused images presenting characteristics of a scan acquired with a peak voltage of 120 kV. The attenuation of the fused images was obtained automatically during reconstruction with a composition ratio of 0.3 by the formula: HUfused = 0.3 × HU80kV + (1 − 0.3) × HU140kV [15].
All images were sent to an off-line workstation equipped with post-processing dual-energy software (Syngo Multimodality Workspace, Siemens Medical Solutions, Germany). The images acquired in dual-energy mode were processed by using the LIVER VNC algorithm (Syngo Dual Energy, version VE31A, Siemens Medical Solutions, Germany) which, thanks to the three-material decomposition, enables processing of the 80 and 140 kV images, quantification and subtraction of the iodine content, so as to obtain a VNC phase.
Data interpretation
Images were assessed for endoleaks: two radiologists jointly evaluated the nonenhanced, arterial and delayed phase, and a third radiologist evaluated only the delayed phase and VNC. All radiologists had the opportunity to evaluate images on an external workstation with multiplanar reconstructions.
Endoleaks occur when there is blood flow inside the aneurysmal sac outside the EVAR. At CT evaluation it appears as high-attenuating material in the aneurysmal sac that is not appreciable during the nonenhanced phase [16]. Endoleaks are classified into five types: type I is related to the incomplete attachment of the proximal (Ia) or distal (Ib) end of the stent-graft to the native artery; type II endoleak is due to the retrograde blood flow through aortic branches into the aneurysmal sac (generally lumbar arteries and inferior mesenteric artery); type III endoleak is related to a defect of the stent-graft with disconnection between prosthetic components or to holes in the prosthesis; type IV endoleak is related to porosity of the prosthesis; type V endoleak or endotension is the expansion of the aneurysmal sac without the presence of an endoleak [9, 17].
The triple-phase acquisition protocol was considered the reference standard to detect endoleaks.
Radiation dose estimates
When the single-source mode is enabled, the radiation dose is the same as that of a single-energy CT scan; when the dual-source mode is enabled, the total radiation dose is the sum of the radiation doses of each of the two tubes.
Effective dose (ED) is the product of the dose–length product (DLP) of each phase (included in the CT scanner patient protocol) and the conversion coefficient (k), where k (0.017 mSv/mGy × cm) is the mean of both region-specific conversion coefficients, abdomen (k = 0.015 mSv/mGy × cm) and pelvis (k = 0.019 mSv/mGy × cm). Estimation of the cumulative ED of a triple-phase acquisition was compared to that of a dual-energy acquisition. The triple-phase acquisition cumulative ED was considered the sum of the ED of nonenhanced phase and the double ED of arterial phase acquired in single-energy mode, whereas the dual-energy cumulative ED was the ED of the delayed phase. Data were compared using the unpaired t test. A probability value of less than 0.05 was considered significant.
Results
Detection of endoleaks
All CT scans were completed and all phases could be reconstructed for each patient. We did not have any complication during the acquisition phase.
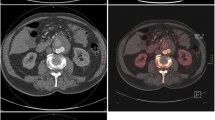
Evaluation of the VNC and delayed phase revealed 34 endoleaks (19.8 %) with an agreement of 100 % with the triple-phase evaluation (Fig. 1). We detected two type I endoleaks and 32 type II endoleaks (Table 3). We did not detect any type III, IV or V endoleaks. Type I endoleaks were both type Ia, whereas among type II endoleaks there were 5 cases of retrograde flow from the inferior mesenteric artery and 27 cases from the lumbar arteries.
Type II endoleak in a patient after endovascular aneurysm repair. a Arterial phase; b delayed phase dual-energy; c nonenhanced phase; d virtual noncontrast reconstruction of dual-energy delayed phase. Arterial (a) and delayed phase (b) show contrast-enhancement in the aneurysmal sac (arrowhead), which the nonenhanced phase (c) and virtual noncontrast reconstruction (d) do not show
Radiation dose estimates
The mean scanning length was 407 ± 33 mm and it was the same for all CT scans (nonenhanced, arterial phase, and delayed phase) in each patient.
The mean calculated ED was 7.8 ± 1.2 mSv for the nonenhanced phase, 9.8 ± 1.7 mSv for the arterial phase and 10.5 ± 1.8 mSv for the dual-energy delayed phase (Table 4).
The cumulative estimated ED was 27.4 ± 2.6 mSv for the triple-phase acquisition protocol and 10.5 ± 1.8 mSv for the dual-energy acquisition. Therefore, the estimated ED of a dual-energy acquisition during the delayed phase was 61.7 % lower than that delivered in a triple-phase protocol. The cumulative effective doses were significantly different between the protocols (p < 0.01).
Discussion
During the follow-up of patients who have undergone EVAR of AAA, ultrasound and magnetic resonance imaging are good tools, although CT is the reference standard and is routinely used for the follow-up of aortic stent-graft procedures [18–25].
The triple-phase CT acquisition protocol consists of a nonenhanced phase, an arterial phase and a delayed phase and is considered the gold standard for endoleak detection [8]. Complications after EVAR are frequent, so these patients need a lifelong imaging surveillance which is performed 1–3 months after the interventional procedure and then every 6–12 months if the aneurysmal sac is stable or decreases in diameter [26, 27].
Endoleaks are the most frequent complications after EVAR, they occur in 2–45 % of patients and their management is different according to endoleak type [7]. Type I and type III endoleaks should be repaired immediately after diagnosis by an interventional angioplasty procedure (type I) or by placing endograft covered stents (type III); other endoleak types require conservative management. When type II endoleaks are detected, the afferent branches with retrograde flow can occlude spontaneously, so closer surveillance of the size of the aneurysmal sac may be advised and interventional embolisation is reserved for enlarging aneurysmal sacs [28, 29]. Type IV endoleaks are becoming less frequent and occur in the postoperative period, when the patient is severely uncoagulated; usually it resolves spontaneously when coagulation is normalised and requires only surveillance [30]. Type V endoleaks are also uncommon and have a low risk of early rupture, so they only require surveillance; progressive enlargement of the aneurysm sac requires surgical conversion [17].
Ionising radiation is the main limitation of CTA because of the risk of inducing carcinogenesis, particularly in patients who require lifelong surveillance; thus, there is a duty to deliver as low as reasonably achievable radiation doses [31–33]. The cumulative dose increases with the number of phases, so reducing the acquired phases may be a strategy for reducing radiation dose to these patients. For patients after EVAR the lack of a nonenhanced phase can make it impossible to differentiate a high-attenuating calcifying thrombus within the aneurysmal sac from an endoleak, and according to recent studies, the lack of the arterial phase does not reduce diagnostic accuracy [12, 34]. Dual-energy technology allows a single-phase acquisition protocol to be performed during the delayed phase and a virtual noncontrast phase to be reconstructed by means of a post-processing reconstruction algorithm [35].
In this study, we tested the feasibility of a single-phase CTA acquisition protocol for endoleak detection by performing a dual-energy scan during the delayed phase, without reducing diagnostic accuracy. Diagnostic concordance in endoleak detection was 100 % between the triple-phase and the dual-energy acquisition protocol; VNC reconstruction was enough to determine whether high-attenuation material within the aneurysmal sac was a calcifying thrombus or an endoleak (Fig. 2). All endoleaks were detected during the delayed phase, but in five cases a type II endoleak was not detected during the arterial phase (Fig. 3).
Type II endoleak in a patient after endovascular aneurysm repair. a Nonenhanced phase; b virtual noncontrast reconstruction of dual-energy delayed phase; c arterial phase; d dual-energy delayed phase. Both the nonenhanced phase (a) and the virtual noncontrast reconstruction (b) show high-attenuating areas caused by the graft (white arrow) and calcifying thrombus in the aneurysm sac (black arrow) and on the vessel wall (black arrowhead). Only the delayed phase shows an endoleak (white arrowhead) (d)
In our study, the estimation of ED of the dual-energy scan was 6.6 % higher than the ED of single-energy arterial phase, but the dual energy scan allowed reconstruction of also the VNC phase. Estimation of the cumulative ED of the dual-energy mode acquisition (delayed phase) was 40.3 % lower than the sum of the two acquisitions in single-energy mode (nonenhanced and arterial phase) and 61.7 % lower than the estimate of cumulative ED of a triple-phase acquisition (noncontrast, 2× arterial phase).
Limitations
In our study only type I and type II endoleaks were detected, so it was not possible to assess the diagnostic performance of dual-energy acquisitions in detecting type III, IV or V endoleaks; however, the latter are rarely observed [7, 9, 30, 35, 36]. Furthermore, the overlap in the scanning field of view of both tubes was only 26 cm in diameter; this enabled reconstruction of the dual-energy and VNC images of the aorto-iliac axis, but did not allow assessment of all abdominal organs. Finally, the images processed by using the LIVER VNC algorithm were noisier and qualitatively a little lower than the nonenhanced images and in some cases we noticed a trend to oversubtract calcifications, even though we did not perform any qualitative evaluation using objective parameters.
Conclusions
Dual-energy technology with single-phase acquisition protocol during the delayed phase and its VNC reconstruction allows identification of endoleaks in patients after EVAR. This acquisition protocol substantially reduces the effective dose to the patient while having complete diagnostic agreement with a triple-phase acquisition protocol.
References
Fleming C, Whitlock EP, Beil TL et al (2005) Screening for abdominal aortic aneurysm: a best-evidence systematic review for the preventive services task force. Ann Intern Med 142:203–211
Sterpetti AV, Cavallaro A, Cavallari N et al (1991) Factors influencing the rupture of abdominal aortic aneurysms. Surg Gynecol Obstet 173(3):175–178
Hatakeyama T, Shigematsu H, Muto T (2001) Risk factors for rupture of abdominal aortic aneurysm based on three-dimensional study. J Vasc Surg 33:453–461
Eefting D, Ultee KH, Von Meijenfeldt GC et al (2013) Ruptured AAA: state of the art management. J Cardiovasc Surg 54(Suppl 1):47–53
Karkos CD, Sutton AJ, Bown MJ et al (2011) A meta-analysis and metaregression analysis of factors influencing mortality after endovascular repair of ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 42:775–786
Görich J, Rilinger N, Sokiranski R et al (1999) Leakages after endovascular repair of aortic aneurysms: classification based on findings at CT, angiography and radiography. Radiology 213:767–772
Iezzi R, Cotroneo AR, Filippone A et al (2006) Multidetector-row CT in abdominal aortic aneurysm treated with endovascular repair: are unenhanced and delayed phase enhanced images really effective for endoleak detection? Radiology 241:915–921
Iezzi R, Cotroneo AR, Marano R et al (2008) Endovascular treatment of thoracic aortic diseases: follow-up and complications with multidetector computed tomography angiography. Eur J Radiol 65:365–376
Stavropoulos SW, Charagundla SR (2007) Imaging techniques for detection and management of endoleaks after endovascular aortic aneurysm repair. Radiology 243:641–655
Mancuso M, Pasquali E, Leonardi S et al (2008) Oncogenic bystander radiation effects in patched heterozygous mouse cerebellum. Proc Natl Acad Sci USA 105:12445–12450
Clevert DA, Minaifar N, Kopp R et al (2009) Imaging of endoleaks after endovascular aneurysm repair (EVAR) with contrast-enhanced ultrasound (CEUS). A pictorial comparison with CTA. Clin Hemorheol Microcirc 41:151–168
Macari M, Chandarana H, Schmidt B et al (2006) Abdominal aortic aneurysm: can the arterial phase at CT evaluation after endovascular repair be eliminated to reduce radiation dose? Radiology 241:908–914
Chandarana H, Godoy MC, Vlahos I et al (2008) Abdominal aorta: evaluation with dual-source dual-energy multidetector CT after endovascular repair of aneurysms–initial observations. Radiology 249:692–700
Johnson TR, Krauss B, Sedlmair M et al (2007) Material differentiation by dual energy CT: initial experience. Eur Radiol 17:1510–1517
Fayngersh V, Passero M (2009) Estimating radiation risk from computed tomography scanning. Lung 187:143–148
Motta R, Rubaltelli L, Vezzaro R et al (2012) Role of multidetector CT angiography and contrast-enhanced ultrasound in redefining follow-up protocols after endovascular abdominal aortic aneurysm repair. Radiol Med 117:1079–1092
Carrafiello G, Recaldini C, Laganà D et al (2008) Endoleak detection and classification after endovascular aneurysm treatment of abdominal aortic aneurysm: value of CEUS over CTA. Abdom Imaging 33:357–362
Nyheim T, Staxrud LE, Rosen L et al (2013) Review of postoperative CT and ultrasound for endovascular aneurysm repair using Talent stent graft: can we simplify the surveillance protocol and reduce the number of CT scans? Acta Radiol 54:54–58
Collins JT, Boros MJ, Combs K (2007) Ultrasound surveillance of endovascular aneurysm repair: a safe modality versus computed tomography. Ann Vasc Surg 21:671–675
Clevert DA, Minaifar N, Weckbach S et al (2008) Color duplex ultrasound and contrast-enhanced ultrasound in comparison to MS-TC in the detection of endoleak following endovascular aneurysm repair. Clin Hemoreol Microcirc 39:121–132
Giannoni MF, Fanelli F, Citrone M et al (2007) Contrast ultrasound imaging: the best method to detect type II endoleak during endovascular aneurysm repair follow-up. Interact Cardiovasc Thoracic Surg 6:259–262
Napoli V, Bargellini I, Sardella SG et al (2004) Abdominal aortic aneurysm: contrast-enhanced US for missed endoleaks after endoluminal repair. Radiology 233:217–225
van der Laan MJ, Bartels LW, Viergever MA et al (2006) Computed tomography versus magnetic resonance imaging of endoleaks after EVAR. Eur J Vasc Endovasc Surg 32:361–365
Pitton MB, Schweitzer H, HHerber S et al (2005) MRI versus helical CT for endoleak detection after endovascular aneurysm repair. AJR Am J Roentgenol 185:1275–1281
Scaglione M, Pinto A, Pinto F et al (2001) Role of contrast-enhanced helical CT in the evaluation of acute thoracic aortic injuries after blunt chest trauma. Eur Radiol 11:2444–2448
Görich J, Rilinger N, Söldner J et al (1999) Endovascular repair of aortic aneurysm: treatment of complications. J Endovasc Surg 6:136–146
Wolf YG, Tillich M, Lee WA et al (2002) Changes in aneurysm volume after endovascular repair of abdominal aortic aneurysm. J Vasc Surg 36:305–309
El Batti S, Cochennec F, Roudot-Thoraval F (2013) Type II endoleaks after endovascular repair of abdominal aortic aneurysm are not always a benign condition. J Vasc Surg 25:S0741–S5214
Patatas K, Ling L, Dunning J et al (2012) Static sac size with a type II endoleak post-endovascular abdominal aortic aneurysm repair: surveillance or embolization? Interact Cardiovasc Thorac Surg 15:462–466
Saba L, Montisci R, Sanfilippo R et al (2009) Imaging of the endoleak after endovascular aneurysm repair procedure by using multidetector computer tomography angiography. J Cardiovasc Surg 50:515–526
Ron E (2003) Cancer risks from medical radiation. Health Phys 85:47–59
Berrington de Gonzalez A, Darby S (2004) Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet 363:345–351
Brenner DJ, Hall EJ (2007) Computed tomography: an increasing source of radiation exposure. N Engl J Med 357:2277–2284
Sommer WH, Graser A, Becker CR et al (2010) Image quality of virtual non-contrast images derived from dual energy CT angiography after endovascular aneurysm repair. J Vasc Interv Radiol 21:315–321
Stolzmann P, Frauenfelder T, Pfammatter T et al (2008) Endoleaks after endovascular abdominal aortic aneurysm repair: detection with dual-energy dual-source CT. Radiology 249:682–691
Brägelmann A, Bunck A, Donas K et al (2013) Dual-energy CT in the follow-up after endovascular abdominal aortic aneurysm repair. Rofo 185:351–357
Conflict of interest
Vitaliano Buffa, Antonio Solazzo, Valeria D’Auria, Alessandra Del Prete, Andrea Vallone, Monica Luzietti, Manuela Madau, Roberto Grassi, Vittorio Miele declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buffa, V., Solazzo, A., D’Auria, V. et al. Dual-source dual-energy CT: dose reduction after endovascular abdominal aortic aneurysm repair. Radiol med 119, 934–941 (2014). https://doi.org/10.1007/s11547-014-0420-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-014-0420-1