Abstract
Objective
This study was done to assess breast density on digital mammography and digital breast tomosynthesis according to the visual Breast Imaging Reporting and Data System (BI–RADS) classification, to compare visual assessment with Quantra software for automated density measurement, and to establish the role of the software in clinical practice.
Materials and methods
We analysed 200 digital mammograms performed in 2D and 3D modality, 100 of which positive for breast cancer and 100 negative. Radiological density was assessed with the BI–RADS classification; a Quantra density cut-off value was sought on the 2D images only to discriminate between BI–RADS categories 1–2 and BI–RADS 3–4. Breast density was correlated with age, use of hormone therapy, and increased risk of disease.
Results
The agreement between the 2D and 3D assessments of BI–RADS density was high (K 0.96). A cut-off value of 21 % is that which allows us to best discriminate between BI–RADS categories 1–2 and 3–4. Breast density was negatively correlated to age (r = −0.44) and positively to use of hormone therapy (p = 0.0004). Quantra density was higher in breasts with cancer than in healthy breasts.
Conclusions
There is no clear difference between the visual assessments of density on 2D and 3D images. Use of the automated system requires the adoption of a cut-off value (set at 21 %) to effectively discriminate BI–RADS 1–2 and 3–4, and could be useful in clinical practice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High breast density is an important risk factor for cancer development and reduces the sensitivity of mammographic evaluation [1–4].
Over the years numerous methods, both qualitative and quantitative, have been developed for the evaluation of mammographic density. One commonly used classification is the “visual” Breast Imaging Reporting and Data System (BI–RADS) proposed by the American College of Radiology [5]. This system is subjective, but nonetheless associated with good intra- and inter-observer reproducibility. The discrepancy in interobserver discrimination between dense (BI–RADS D3–4) and non-dense breasts (BI–RADS D1–2) is not, however, negligible [1, 6, 7], and may represent a problem where different assessments give rise to differing clinical responses (e.g. in the frequency of screening examinations or in the type of monitoring used). For this reason, a method capable of providing greater reproducibility of assessment would be of considerable clinical interest. In recent years, algorithms for the computerised evaluation of breast density have been proposed. These methods have the advantage of a very high reproducibility compared to traditional visual assessment, and a study of correlation between visual and computerised assessments has recently been published [1].
The purpose of our study was to compare the definition of mammographic density between visual assessment with the BI–RADS classification and the automatic Quantra R2 breast density assessment software (1.3 Hologic Corp, Bedford MA, USA). In particular, the objectives of the study were to evaluate the concordance of the visual assessment of breast density based on conventional (2D) digital mammography and tomosynthesis (3D); to determine the correlation between the BI–RADS classification of mammographic density and the automatic Quantra assessment and choose the best Quantra cut-off value for discriminating between BI–RADS density categories 1–2 and 3–4; to estimate correlations between breast density and age, use of hormone therapy and tumour histotypes; to assess whether the Quantra system can be helpful in clinical practice in the case of malignant disease.
Materials and methods
Study population
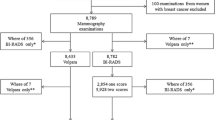
The study sample includes 200 cases of women (mean age 56.04 years; range 35–93) who underwent mammography between February 2010 and December 2011 at the Diagnostic Breast Clinic of the Radiology University of Torino, Department of Diagnostic Imaging and Radiotherapy, Azienda Ospedaliero-Universitaria Città della Salute e della Scienza di Torino. Of the 200 cases, 100 women (mean age 60.40 years; range 37–93), who were symptomatic and referred to our clinic for diagnostic mammography or self-referred for screening mammography in the absence of clinical symptoms, were selected based on the detection of histologically confirmed malignant breast disease; patients undergoing neoadjuvant chemotherapy, those with breast implants or with a previous mastectomy were excluded. The other 100 cases (mean age 51.79 years; range 35–78) were selected from among women who underwent mammography during the same time period and for the same reasons (symptomatic patients referred for diagnostic mammography or asymptomatic patients self-referred for screening mammograms), but negative for radiological signs of malignant breast disease and negative follow-up maintained for at least 1 year; patients with a previous mastectomy were also excluded in this group.
Each patient’s history was collected taking special care to investigate family history of breast disease, previous breast surgery and past and present hormone treatment.
Image acquisition
After giving their informed consent, patients underwent two-dimensional (2D) digital mammography and 3D tomosynthesis with a single compression (Combo mode) using the same imaging session. The mammograms were performed with a Lorad Selenia Dimensions digital mammography system (Hologic Corp, Bedford MA, USA) set for tomosynthesis in the two standard craniocaudal (CC) and mediolateral oblique (MLO) projections. For tomosynthesis, the scan was performed along an angle of 15° (±7.5°) and 15 exposures were acquired (one exposure for each degree).
Image analysis
A radiologist with 23 years of experience in breast imaging and 4 years in tomosynthesis reviewed in a single session the images acquired in 2D and 3D modes and defined visual density according to the American College of Radiology (ACR) BI–RADS classification [5]. All medical records were available to the reader. Only the 2D images were subsequently processed by Quantra R2 automatic software (Quantra® 1.3, Hologic Corp, Bedford MA, USA) [8]. This consists of a new automatic mathematical algorithm designed for the volumetric assessment of digital breast mammograms. This evaluation algorithm is based on a physical model that correlates the attenuation of the X-ray due to the breast tissue and the digital mammography images provided to the radiologist. The assessments are based on physical parameters specific to the breast tissue and imaging system, as well as on information relating to individual X-ray exposures including attenuation coefficients for breast tissue [9], radiographic spectra of the target material [10], kVp, mAs and thickness of the tissue reproduced in the images. Quantra estimates the amount of fibroglandular and fatty tissue that an X-ray must have crossed to deposit a specific amount of energy on the detector, and it provides a result (height) in centimetres of fibroglandular tissue penetrated corresponding to each pixel of the image.
After completing the analysis of the pixels (excluding the pectoral muscle), the system combines the values in the volume of the fibroglandular tissue, expressed in cubic centimetres. The system then calculates the ratio between the volume of the fibroglandular tissue and the total estimated breast volume in order to determine the percentage of fibroglandular tissue volume in the breast, for both the left and right side (combined CC and MLO projections) [11]. The result is shown by a single command on the Hologic workstation (Fig. 1) [1, 12, 13].
Many methods have been developed to assess breast density. They may be divided into descriptive systems and quantitative systems. The former are based on the individual perception of density and include the original Wolfe classification, modified and improved by the two most commonly used classifications, Boyd [14] and BI–RADS [5]. The latter requires frequent operator interaction to outline the tissue and often calculate the area of the density rather than the volume of the dense tissue in the breast. These systems “quantify” breast density in terms of absolute or percentage ratio of dense tissue. Among the quantitative systems, interactive thresholding proposed by Byng et al. [15] requires the involvement of an operator and makes the assessment of the relationship between total and glandular breast tissue possible. This technique was first applied to analogue images and then, thanks to semiautomatic procedures, to digital mammograms [16]. More recently, a system developed by Shepherd et al. [17] makes use of a phantom as a calibration tool for evaluating breast density. The image is then analysed to determine breast thickness, percentage of fibroglandular tissue in each pixel and dense tissue in the mammogram. Among fully automated volumetric systems, the standard mammographic form (SMF) is a technique that divides breast tissue into “interesting” (healthy normal tissue and neoplastic tissue) and “uninteresting” (adipose tissue) and is based on different X-ray attenuation coefficients: the volume resulting from the volume of “interesting” tissue is regarded as the volume of dense tissue [18]. A first experience with a fully automatic system for the evaluation of the breast density applicable to mammographic images in tomosynthesis was also recently proposed by Tagliafico et al. [19].
Statistical analysis
Intraobserver concordance of visual assessment of breast density (BI–RADS) between 2D digital mammograms and tomosynthesis (3D) was calculated by using Cohen’s kappa statistic. Conventionally, k values equal to 0–0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80 and 0.81–1.00 correspond to a poor, modest, moderate, substantial and almost perfect correlation, respectively. To provide a practical criterion and relate the Quantra measurements to the BI–RADS visual scale, the percentage of Quantra breast density which best discriminated dense (BI–RADS 3–4) from non-dense breast (BI–RADS 1–2) was sought. The software’s ability to discriminate between the two BI–RADS categories was assessed buy using ROC curve (receiver operating characteristics) analysis. The first step was to calculate the two density distributions for the two classes to be discriminated: any technique able to identify the two distributions should only allow for a small overlap. Therefore, the best Quantra density value (cut-off value) discriminating between the two BI–RADS categories was chosen, evaluating sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and diagnostic accuracy (DA). This cut-off was used for the subsequent analysis of the results. The correlation between breast density and age, both in positive and negative patients, and the correlation between breast density and history of hormone therapy were quantified by Pearson’s linear correlation coefficient. A paired Student’s test was used to analyse, in positive patients and in particular in the subgroup of patients who had or were taking hormone therapy, the difference in Quantra density between the pathological (case) and healthy side (control), and also subdividing the patients into two age groups (≤50 and >50 years). In all other cases Anova analysis of variance was used to compare continuous variables. Categorical variables were studied by contingency tables and the Chi-square test. In the case of 2 × 2 tables the Yates correction was applied.
Results
Intraobserver concordance in assigning BI–RADS density with 2D and 3D modes
The 2D visual evaluation of breast density (range 5–100 %) was classified as D1, 2, 3 and 4 in 45, 59, 62 and 34 cases, respectively; the density of the corresponding distribution for the same cases using 3D visual assessment was D1 in 44 cases, D2 in 58, D3 in 65 and D4 in 33, with a intraobserver concordance estimated with Cohen’s kappa coefficient of 0.96 [95 % confidence interval (CI), 0.93–0.99; SE, 0.016]. Limiting the classification to two groups (D1–D2 vs. D3–D4), of greater practical clinical use, good intraobserver agreement is demonstrated by k = 0.98 (95 % CI, 0.95–1; SE, 0.014) (Table 1).
Analysis of Quantra data and comparison with BI–RADS classification
The density values provided by the Quantra software for the 2D mammograms are distributed from 8 to 51 %, while those of the visual method range from 5 to 100 % (D1–D4). Considering both breasts in all patients, a BI–RADS D1 density corresponds to Quantra density values between 8 and 27 % (mean 13.4 ± 3.6 %; 95 % CI, 12.3–14.5), D2 corresponds to values between 10 and 32 % (mean 16.8 ± 4.2 %; 95 % CI, 15.7–17.9), D3 to values between 11 and 45 % (mean 24.5 ± 7.2 %; 95 % CI, 22.7–26.3), and D4 to values ranging from 9 to 51 %, with mean = 32.8 ± 8.3 % (95 % CI, 29.3–35.1). The ANOVA test on the Quantra density values corresponding to the four groups agree with what was indicated by the 95 % CIs that do not overlap, showing a strong statistical significance (p < 10−7). The plot of these data identifies a direct linear relationship between Quantra density and BIRADS density with the expression D Quantra = 6.4 DBI–RADS + 5.7, with a Pearson’s linear correlation coefficient of r = 0.99.
The ability of the Quantra software to discriminate between the BI–RADS density categories 1–2 and 3–4, evaluated with the ROC curve, is fully demonstrated by the value of the area under the curve (AUC) which is close to the maximum value of 1 (AUC = 0.903; 95 % CI, 0.872–0.934). The ROC curve parameters indicate 21 % as a cut-off value corresponding to 78.0 % sensitivity, 88.9 % specificity, 86.6 % positive predictive value (PPV), 82.8 % negative predictive value (NPV), and 84.1 % diagnostic accuracy (Fig. 2a–d).
a Correlation between BI–RADS and Quantra in patients with breast cancer (positive) and without breast cancer (negative). b Linear correlation between Quantra density and BI–RADS classification. c Distribution of the density value measured by the Quantra software, considering cases classified as BI–RADS 1–2 and BI–RADS 3–4. d ROC curve in which the value of the area under the curve (c > 0.9) corresponds to a very good ability of the Quantra software to discriminate between BI–RADS 1–2 and BI–RADS 3–4
Density in relation to age
In the present study, 82 patients were aged below 50 (mean 44.28 years) and 118 had an age greater than or equal to 50 (mean 64.21 years). The average Quantra density was 24.89 % (95 % CI, 22.82–26.96) for patients aged <50 years and 17.28 % (95 % CI, 16.01–18.55) for those aged ≥50 years. Figure 3 and Table 2 show a negative correlation between density and age, with increasing age corresponding to a reduction in density. The Pearson correlation coefficient was r = −0.45 (95 % CI, −0.55, −0.33) and, in particular, r = −0.37 (95 % CI, −0.53, −0.19) for negative patients and r = −0.42 (95 % CI, −0.57, −0.24) for positive patients. Comparison of these last two correlation coefficients does not show a different relationship between density and age between the groups of patients with positive and negative (p = 0.69).
Correlation between breast density and hormone therapies
Considering the whole group of patients, 95 out of 200 women reported having taken hormone therapy in a time interval between a few months and 30 years (oestrogen–progestin therapy in 77 cases, hormone replacement therapy in 8 and tamoxifen in 10). Comparing the breast density values provided by the Quantra software in relation to exposure to hormone therapies we observed that this affects breast density significantly. Indeed patients reporting no exposure to hormone therapies had a mean density of 18.61 ± 7.77 % (range 10–46 %), while with exposure had a mean density of 22.90 % (range 8–49 %) (p = 0.0004).
Density analysis of both breasts in relation to breast disease, hormone therapy and age
Because hormone therapy affects density, we analysed only the positive subgroup of patients with unilateral disease (n = 94) to identify correlations between the presence of malignant disease and density, and evaluated differences in density between healthy breast and diseased breast. Overall, the affected side had a mean density of 19.45 ± 8.50 and the healthy side of 18.12 ± 8.30. Comparison with paired Student’s t test (considering the diseased side as a case and the healthy one as a control) clearly indicated (p = 0.001) that the density of the affected side was statistically significantly greater than that of the healthy side. Analysis by age groups showed that in the group ≤50 years (n = 30) the density of the affected side was equal to 25.07 ± 10.52 and that of the healthy side was 23.47 ± 9.60 (p = 0.06, a limit value related to the small number of patients in this subgroup which reduces the power of the test π to well below 80 % of the conventionally accepted value); in the group >50 years (n = 64) the density was 16.81 ± 5.80 and 15.61 ± 6.26, respectively (p = 0.009). Comparing the two age subgroups, the difference in density between the healthy and the diseased sides is 1.60 ± 4.48 in the group ≤50 years, and 1.20 ± 3.59 in the group >50 (p = 0.65). The comparison by age groups of the above density data, studied with ANOVA, shows that density is higher in group ≤50 years (p = 5 × 10−6) (Fig. 4).
44-year-old patient. In the left breast mammogram it is difficult to assess a clinically palpable lump reported by the patient in the lower quadrants; at ultrasound examination we found an inhomogeneous hypoechoic area. The BI–RADS visual classification was D4. Definitive histological examination was invasive ductal carcinoma associated with ductal carcinoma in situ measuring 2 cm. The density values assessed by Quantra software were 34 % for the right breast, and 50 % for the left breast (difference between the right and the left breast was 16 %)
Albeit with low test power (π = 7 %), a greater tendency to disease could be seen in patients in the group aged ≤50 years with density greater than 21 % compared to those with density lower than 21 % (threshold value obtained for Quantra), respectively, with a probability of 26.9 % (17.7–38.6 %) and 22.6 % (13.3–35.7 %).
Density and pathological analysis
All patients testing positive for malignant disease on percutaneous biopsy were subjected to surgical treatment, except for three women, one aged 82 years whose clinical condition precluded surgery and two who on the waiting list for surgery when data collection was completed. Definitive histological examination revealed the presence of 66 infiltrating ductal carcinomas (IDC), four IDC associated with lobular component (IDC + ILC), one IDC with ductal carcinoma in situ component (DCIS + IDC), 14 DCIS, seven infiltrating lobular carcinomas (ILC), one lobular carcinoma in situ (LCIS), one infiltrating tubular carcinoma (ITC), one IDC associated with infiltrating tubular component (IDC + ITC), one papillary carcinoma, and one mucinous carcinoma. Evaluating the most representative definitive histological results, DCIS had a mean density of 16.4 ± 6.5 %, IDC of 18.4 ± 9.1 % and ILC of 25.7 % ± 14.4R. Applying the ANOVA analysis of variance we observed that DCIS and IDC have equivalent density (p = 0.44), while a borderline difference (p = 0.08) was noted between them and ILC due to the small number of ILC.
Discussion
All we know today about the meaning and impact of mammographic density on the risk of disease and failure to diagnose is based primarily on visual classification. While waiting for controlled studies on the value of the automatic density as a risk factor to be repeated, the adoption of a simple system to convert the Quantra value into a visual value is currently the only solution if we are to continue using automatic evaluation in current practice. The advantages would be undeniable, in particular a very high reproducibility and speed of calculation (the density category may be part of image processing and be displayed on the monitor at the time of reporting).
In the present study, the first evaluation regards the comparison between visual BI–RADS breast density on 2D and 3D mammograms. Total intraobserver concordance was found to be almost perfect considering both the four categories (D1–D4) and grouping the less dense (D1–D2) and denser (D3–D4) categories, with Cohen kappa correlation coefficient values of 0.96 and 0.98, respectively. Similar results were reported by Rafferty [20] and Bakic [21], although the latter author indicates a better interobserver correlation of the judgement expressed on tomosynthesis (ρ = 0.85 ± 0.05) than on 2D digital mammography (ρ = 0.75 ± 0.05). In our study it was not possible to assess this correlation, because the mammograms were read by a single observer. The density values calculated with the Quantra software in our study are between 8 and 51 %, a much narrower range than the visual BI–RADS classification. The Quantra values for the different BI–RADS categories overlap considerably. In fact, we found a positive correlation between BI–RADS density and performance of Quantra. The adoption of a Quantra cut-off value of 21 % allowed us to discriminate visual values very consistently, in particular with 78.8 % sensitivity, 88.9 % specificity and 84.1 % diagnostic accuracy. In a recent study by Ciatto et al., the equivalent cut-off was 22 %, with correct classification of 89 % of cases compared to the BI–RADS classification (D1–D2 vs. D3–D4). The similarity of the results between the two studies also applies to the range of Quantra values for the individual BI–RADS categories, except for category D4, in which we observe a discrepancy in the value of the lower range. In particular, in the study by Ciatto et al. [1], the following correspondence in density is reported: D1 (7–19 %), D2 (10–32 %), D3 (16–47 %), D4 (24.5–50.5 %). The difference in the range of D4 values (9–51 % in our study vs. 24.5–50.5 % in Ciatto et al.’s study) could be accounted for by the fact that in some cases the observer overestimated the BI–RADS category compared to the actual density. In particular, the discrepancy of values occurred in three cases in which the total breast volume was very high. In these cases, the visual assessment overestimated the actual glandular volume which was only correctly quantified by Quantra. In fact, leaving aside those three cases, category D4 corresponds to a Quantra density range between 22 and 51 %, with a mean of 32.61 %.
In Rafferty’s study, the Quantra cut-off result was 13 %. Indeed, the intention of these authors was not to achieve the best overall prediction of the visual classification but, on the contrary, to ensure maximum sensitivity for visual grades D3–4. If one applies the same intent to Ciatto et al’s study, the cut-off value that correctly identifies all D3–4 visual cases is <15 %, whereas in our study this corresponds to a cut-off value <9 %. The literature contains numerous studies that show a negative correlation between age and breast density, whereby increased age corresponds to reduced density. In a recent work by Skippage et al. [11] who used Quantra as evaluation software for mammographic density, the Pearson correlation coefficient between the age and density parameters was −0.20 (p < 0.0001); specifically, menopausal status showed a significant correlation with breast density with peri- or post-menopausal women having a lower density compared to premenopausal women. Our study as well found a negative correlation between density and age: Pearson’s linear correlation coefficient r was equal to −0.45 (p < 0.0001). From the comparison of positive and negative patients, with coefficient r equal to −0.42 and −0.37, respectively, there was, however, no statistically significant difference between the two correlation coefficients (p = 0.69) .
Analysing the relationship between breast density and the use of previous or current hormone therapies, such as oral contraceptives or hormone replacement therapy, in both positive and negative groups, it was observed that more patients taking hormonal therapies were present in the groups with high density. Even the above study by Skippage et al. [11], which unlike our study makes reference to the use of hormone replacement therapy (HRT), demonstrates this correlation: non-use versus use of HRT for a period of less than 5 years shows a significant negative correlation (16.5 vs. 24; p = 0.0014) so that women who took HRT had a higher breast density than the others. In our study, according to the Quantra density data, patients not exposed to hormone therapy had a mean density of 18.61 ± 7.77 % (range 10–46 %), while those exposed had a mean density of 22.90 % (range 8–49 %) (p = 0.0004).
Although the sample size is limited, the results observed regarding the correlation between the presence of malignant disease and density appear to support the utility of the automatic volumetric system to detect differences in density and, especially in extremely dense breasts, highlight differences between the two breasts in particular in the presence of pathology. In the literature the relationship between breast density and risk of developing breast cancer is well known. It was Wolfe who in 1976 first proposed a positive association between breast density and risk of carcinoma. In particular, he analysed the cases of breast cancer developed between 1967 and 1973 comparing the density patterns as expressed with his classification. In that study, he noted a gradual increase in incidence in relation to different density patterns: 0.14, 0.52, 1.96 and 5.22 %, respectively, for N1 (mainly fatty breast structure), P1 (fatty parenchyma and ductal structures prevailing in the anterior portion or extending to a quadrant as a thin band), P2 (severe prevalence of ductal structures occupying more than a quarter of the volume of the breast) and DY (severe dysplasia, which often masks the underlying ductal structures) [2, 22]. Numerous other authors, over the years, have confirmed the relationship between breast density and increased risk of development of carcinoma. McCormack et al. [23] in a large meta-analysis including 42 studies used 21 % as a cut-off density value stating that the risk of developing breast cancer in women with dense breasts (>21 %) was up to 4.6 times higher compared to those with lower density.
The literature reports many experiences related to a reduction in the sensitivity of mammography in the detection of breast cancer in dense breasts. Among them is the study by Mandelson et al. conducted on women participating in mammography screening from 1988 to 1993 in whom primary invasive breast cancer was diagnosed within 24 months of a screening examination and therefore before the next inspection. The sensitivity of mammography was found to be 80 % among women with fatty breasts, but only 30 % in women with very dense breasts. The odds ratio (OR) for interval cancers among women with very dense breasts was 6.14 (95 % CI, 1.85–19.4) compared with women with extremely fatty breasts [24]. With the evolution of technology, studies have been conducted to compare qualitative and quantitative methods of density assessment. The meta-analysis of Byng et al. compared Wolfe’s qualitative classification with a quantitative classification, finding that the latter was characterised by a better risk prediction. Specifically, a summary OR of 3.6 (95 % CI, 2.7–4.8) was calculated for the quantitative method, compared to an OR of 1.8 (95 % CI, 1.5–2.1) for the Wolfe method. Many studies using quantitative methods for the evaluation of breast density reported a higher OR, in terms of developing breast cancer, in women with dense breasts compared to studies in which subjective evaluation methods were used. Boyd et al. [25, 26] confirmed the importance of using precise methods for evaluating mammographic density since they found a 2 % increase in the relative risk of breast cancer for each percentage point increase in breast density. Pinker et al., in a study conducted with the aim of validating the new Quantra volumetric density measurement system as a marker for the risk of developing breast carcinoma, analysed 200 cases positive for tumour and 200 controls and found that the percentage of breast density ranged between 6 and 63 % and decreased significantly with age (p < 0.001).
Analysis of the results by density, revealed a striking increase in the cancer risk for density volumes exceeding 35 % (OR, 1.8) [12]. In our study, albeit with low test power (π = 7 %), we found that the group of patients aged ≤50 years and density greater than 21 % had a greater tendency to disease (probability of 26.9 %) compared to the group with density <21 %.
Moreover, comparison between definitive histology and breast density showed that IDC and DCIS have equivalent density (p = 0.44), while there was a borderline difference (p = 0.08) between these and ILC due to the small number of ILC which reduced the statistical power (π = 23 %) (probability of false negative, 77 %). To reach the conventionally accepted power of 80 %, the number of patients in the ILC group should be increased by at least 50 %.
In the literature, there are conflicting data on the subject. For example, within a retrospective study, screening mammograms performed on 4,866 showed a statistically significant correlation between tumour histotype and breast density (p = 0.02). Specifically, the average density in negative cases, IDC, DCIS and ILC was 32, 33, 35 and 44 %, respectively [27]. Conversely in two other recent studies, the data failed to demonstrate a statistically significant correlation between density and tumour histology. In particular, in the study by Eriksson et al., which analysed 1,747 patients diagnosed with tumour between 1993 and 1995, the breast density estimated by computerised algorithm was found to be associated with tumour size (regression coefficient 0.031, p = 0.017), but not with histopathological classification or receptor status. The only borderline association (OR, 1.56; p = 0.069) was found with grade 3 tumour type [28]. Similarly, Pollan et al. [29], who analysed 1,172 patients with breast cancer seen in a screening context and selected cases by separating DCIS from invasive cancers, found that the risk for the high-density categories (>75 %) compared to those with lower density (<10 %) was similar for DCIS (OR, 3.47; 95 % CI, 1.46–8.27) and invasive tumours (OR, 2.95; 95 % CI, 2.01–4.35). In these cases, the risk was particularly high for interval cancers (OR, 7.72; 95 % CI, 4.02–14.81) compared to screen-detected cases (OR, 2.17; 95 % CI, 1.40–3.36).
Conclusions
Quantra seems to provide a more accurate assessment of breast density in clinical practice, and may allow better correlation between density and breast disease. However, to provide a practical criterion for correlating the BI–RADS classification and the Quantra results, an appropriate cut-off value is required that separates dense from non-dense breasts. This was quantified as 21 %, in line with the data proposed in the literature.
References
Ciatto S, Bernardi D, Calabrese M et al (2012) A first evaluation of breast radiological density assessment by QUANTRA software as compared to visual classification. Breast 21:503–506
Wolfe JN (1976) Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer 37:2486–2492
Ciatto S, Zappa M (1993) A prospective study of the value of mammographic patterns as indicators of breast cancer risk in a screening experience. Eur J Radiol 17:122–125
Ciatto S, Visioli C, Paci E et al (2004) Breast density as a determinant of interval cancer at mammographic screening. Br J Cancer 90:393–396
American College of Radiology (2003) Breast Imaging Reporting and Data System Atlas (BI–RADS® Atlas). Reston, VA. http://www.acr.org. Last accessed June 2012
Ciatto S, Houssami N, Apruzzese A et al (2005) Categorizing breast mammographic density: intra-and interobserver reproducibility of BI–RADS density categories. Breast 14:269–275
Bernardi D, Pellegrini M, Di Michele S et al (2012) Interobserver agreement in breast radiological density attribution according to BI–RADS quantitative classification. Radiol Med 117:519–528
Tuncbilek N, Sezer A, Uğur U et al (2009) Qualitative and quantitative analysis of fibroglandular tissue in the digital environment. In: Proffered paper at 10th National Congress of Breast Diseases, Izmir, Turkey
Johns PC, Yaffe MJ (1987) X-ray characterization of normal and neoplastic breast tissue. Phys Med Biol 32:675–695
Boone JM, Fewell TR, Jennings RJ (1997) Molybdenum, rhodium, and tungsten anode spectral models using interpolating polynomials with application to mammography. Med Phys 24:1863–1874
Skippage P, Wilkinson L, Allen S et al (2013) Correlation of age and HRT use with breast density as assessed by Quantra™. Breast J 19:79–86
Pinker K, Perry N, Milner S et al (2010) Validation of a new automated volumetric breast density measurement system as a marker of breast cancer risk. Breast Cancer Res 12(Suppl 3):O1. doi:10.1186/bcr2648 (Published online 2010 October 25)
Hartman K, Hoghnam R, Warren R et al (2008) Volumetric assessment of breast tissue composition from FFDM images. In: Krupinksi EA (ed) Proceedings of the international workshop on digital mammography, Tucson, AZ, USA, IWDM 2008, LNCS 5116, pp 33–39
Boyd NF, Byng JW, Jong RA et al (1995) Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Ins 87:670–675
Byng JW, Boyd NF, Fishell E et al (1994) The quantitative analysis of mammographic densities. Phys Med Biol 39:1629–1638
Byng JW, Yaffe MJ, Jong RA et al (1998) Analysis of mammographic density and breast cancer risk from digitised mammograms. Radiographics 18:1587–1598
Shepherd JA, Kerlikowske KM, Smith-Bindman R et al (2002) Measurement of breast density with dual X-ray absorptiometry: feasibility. Radiology 223:554–557
Highnam R, Brady M, Shepstone B (1996) A representation for mammographic image processing. Med Image Anal 1:1–18
Tagliafico A, Tagliafico G, Astengo D et al (2012) Mammographic density estimation: one-to-one comparison of digital mammography and digital breast tomosynthesis using fully automated software. Eur Radiol 22:1265–1270
Rafferty E, Smith A, Niklason L (2009) Comparison of three methods of estimating breast density: BI–RADS density scores using full field digital mammography, BI–RADS density scores using breast tomosynthesis, and volumetric breast density. In: Presented RSNA, SSM01-04, Wednesday, December 2 2009, 15:30–15:40, Chicago, IL, USA
Bakic PR, Carton AK, Kontos D et al (2009) Breast percent density: estimation on digital mammograms and central tomosynthesis projections. Radiology 252:40–49
Wolfe JN (1976) Breast patterns as an index of risk for developing breast cancer. Am J Roentgenol 126:1130–1137
McCormack VA, dos Santos SI (2006) Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 15:1159–1169
Mandelson MT, Oestreicher N, Porter PL et al (2000) Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst 92:1081–1087
Boyd NF, Martin LJ, Bronskill M (2010) Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst 102:1224–1237
Boyd NF, Lockwood GA, Byng JW et al (1998) Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev 7:1133–1144
Lip G, Zakharova N, Duffy SW et al (2010) Breast density as a predictor of breast cancer risk. Breast Cancer Res 12(Suppl 3):P1. doi:10.1186/bcr2654 (Published online 2010 October 25)
Eriksson L, Czene K, Rosenberg L et al (2012) The influence of mammographic density on breast tumor characteristics. Breast Cancer Res Treat 134:859–866. doi:10.1007/s10549-012-2127-0
Pollán M, Ascunce N, Ederra M et al (2013) Mammographic density and risk of breast cancer according to tumor characteristics and mode of detection: a Spanish population-based case-control study. Breast Cancer Res 15:R9
Conflict of interest
E Regini, G. Mariscotti, M. Durando, G. Ghione, A. Luparia, P.P. Campanino, C.C. Bianchi, L. Bergamasco, P. Fonio, G. Gandini declare that they have no conflict of interest related with the publication of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Regini, E., Mariscotti, G., Durando, M. et al. Radiological assessment of breast density by visual classification (BI–RADS) compared to automated volumetric digital software (Quantra): implications for clinical practice. Radiol med 119, 741–749 (2014). https://doi.org/10.1007/s11547-014-0390-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-014-0390-3