Abstract
Purpose
Retroperitoneal sarcomas (RPS) are rare tumours with an annual reported incidence of 2.7 per million persons. In spite of improvements in both diagnostic imaging and therapeutic strategies, patients afflicted by RPS still have poor prognoses. There are currently many different therapeutic strategies for these rare tumours and combining several different multi-modality strategies have not proved to have superior long-term clinical results. This review analyses the available published data and discusses multi-modality management of this rare entity. In particular, the role of radiation therapy, treatment-related side effects and the use of modern radiation treatment techniques will be discussed.
Materials and methods
A comprehensive literature search was conducted using PubMed in January 2011. Relevant international articles published from January 1980 to January 2011 were assessed. The keywords for search purposes were: retroperitoneum, sarcoma, radiotherapy, and radiation therapy. The search was limited to articles published in English. All articles were read in full by the authors and selected for inclusion based on relevance to this article.
Conclusions
The addition of radiation therapy (RT) to wide surgical excision for RPS has improved local control rates when compared with surgery alone. Preoperative RT is preferred over postoperative RT. New types and delivery techniques in radiation therapy could further improve patient outcomes. Emerging therapies that employ charged particles (such as protons and carbon ions) are expected to be superior in sparing of normal tissues and efficacy over conventional photon therapy radiation, due to their physical and radiobiological properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soft tissue sarcomas (STS) are rare, heterogeneous mesenchymal neoplasms arising from the remnant embryonal mesoderma. Retroperitoneal sarcomas (RPS) are even rarer sarcomas of the extremities and account for 10–15 % of all STS [1]. The annual incidence is equal between both sexes, and has remained stable over time and is reported to be 2.7 per 10 million persons [2]. Patients of all ages can be affected although the median age is 50 years [2]. The common histological variants include liposarcoma, leiomyosarcoma, and malignant fibrous histiocytoma (MFH), which make up 40, 30 and 10 % of all RPS [2].
Biological behaviour and histological subtypes
The distribution of histologic subtypes in RPS differs from that of STS arising at other anatomic sites. Approximately 50 % of RPSs are liposarcoma. The second most frequent histology is leiomyosarcoma; a small proportion will arise from the smooth muscle of the inferior vena cava. Certain RPS subtypes may be responsive to preoperative therapy, such as extraosseous Ewing’s and primitive neuroectodermal tumour. In contrast, the predominant RPS histologies (liposarcoma and leiomyosarcoma) are generally not viewed as highly responsive to chemotherapy and/or radiotherapy. The molecular heterogeneity of fusion gene transcripts has been suggested to predict prognosis in certain sarcoma subtypes. This has been shown in alveolar RMS and synovial sarcoma; both are uncommon in the retroperitoneal space. While molecular genetic testing looks promising, it involves highly complex techniques and the methods are not absolutely sensitive or provide specific results. In addition, technical limitations associated with molecular testing suggest that molecular evaluation should be considered as an ancillary technique. Molecular test results should therefore only be interpreted in the context of the clinical and pathologic features of a sarcoma.
Improvements in diagnosis with the use of magnetic resonance imaging (MRI)
Radiographic imaging is a key component of the evaluation of a patient with a retroperitoneal mass. The preferred diagnostic study is a computed tomography (CT) scan of the abdomen and pelvis to evaluate the primary site, as well as a chest CT to rule out metastatic disease to the lungs. MRI rarely adds important information. In most cases, CT is less sensitive to motion artefacts than MRI, and it better defines the anatomic relationship of the tumour to other abdominal organs. It can also detect metastases to the liver or peritoneum. The radiographic appearance of the primary tumour on CT can also offer clues as to the histologic subtype and grade, which may guide decisions as to the need for pre-treatment biopsy as well as treatment. MRI is the preferred imaging modality for evaluation of soft tissue masses of the extremities, trunk, and head and neck, while CT is the most commonly used imaging technique for RPS.
Positron emission tomography (PET) and PET/CT
A number of studies report that PET and integrated PET/CT using fluorodeoxyglucose (FDG) can distinguish benign soft tissue from sarcomas, with the greatest sensitivity for high-grade sarcomas [3]. However, the ability to differentiate benign soft tissue from low or intermediate grade sarcomas is limited, and PET and PET/CT are not routinely recommended for the initial workup of a soft tissue mass.
Despite the improvement in diagnostic and therapeutic strategies, patients afflicted by RPS have a poor prognosis. The median survival duration is 74 months and the median 5-year overall survival (OS) rate is 36–58 % [4]. This poor prognosis is due to the location of RPS in the retroperitoneal space where they can grow silently until presentation at an advanced stage [5]. Clinical symptoms are nonspecific and include vague abdominal discomfort (from the compression on the gastrointestinal system and neural structures), weight loss, early satiety and swelling of the lower extremities. RPSs are rarely diagnosed in an early stage and also rarely as an incidental finding after performing a CT scan for other reasons [6]. RPS is characterised by local regional relapse, which is the main cause of death. At presentation, these tumours neither spread to loco-regional lymph nodes (3–5 %) nor have distant metastases (15–20 %). In most cases, RPSs remain localised for long periods displacing adjacent organs.
Surgery is the main treatment modality for RPS. However, as often the diagnoses are made in advanced stages, adjuvant therapies have to be integrated. A surgical resection is considered oncologically adequate only when the tumour is macroscopically completely excised and negative microscopic margins (R0) have been achieved. This is possible only when diagnoses are made in the early stages. In advanced disease, a radical surgical approach is necessary, with removal of the surrounding organs infiltrated by tumour [7]. Limited case series published utilising surgery alone, have reported a 5-year local control rate of <50 % [5, 8]. This may be explained by the high risk of residual disease (microscopically and macroscopically) leading to a high risk for local recurrence (37–82 % at 5 years). This high risk for local recurrence supports a multidisciplinary approach and the use of adjuvant radiation treatment and chemotherapy in the management of RPS. The role of frontline aggressive surgery and how this may impact on the need for adjunctive radiotherapy is the subject of clinical trials [7].
Radiotherapy (RT) is important for tumour eradication and yet it is difficult to deliver high doses (>60 Gy) to the tumour and keep within the dose tolerance of the adjacent organs (small bowel, spleen and bone marrow) [9, 10]. The delivery of lower RT doses (<60 Gy) has no advantage over surgery alone. Charged particles therapy is an emerging therapy for RPS and currently only preliminary single-centre experiences have been reported [11]. Although commonly applied for RPS in either the preoperative or postoperative setting, there has been a lack of level-1 evidence for RT specifically in the management of RPS. Therefore, generally data from extremity STS have been extrapolated to RPS. Table 1 shows the results from published studies involving radiotherapy for the treatment of RPS.
The results of systemic as well as intraperitoneal chemotherapy are disappointing [12]. Preoperative regional hyperthermia with systemic chemotherapy is a therapeutic option for high-risk primary or locally recurrent tumours and is the subject of clinical studies.
Biological marker-directed therapy
More recently, a number of targeted therapies have shown promising results in patients with certain histological types of advanced or metastatic STS (outside of the retroperitoneal space). Pazopanib, a multi-targeted tyrosine kinase inhibitor has demonstrated single-agent activity in patients with advanced STS subtypes except liposarcoma. Imatinib and sunitinib have shown efficacy in patients with advanced and/or metastatic STS other than gastrointestinal stromal tumours (GISTs) (alveolar soft part sarcoma, chordoma, pigmented villonodular synovitis/tenosynovial giant cell tumour and solitary fibrous tumour/haemangiopericytoma). Crizotinib, an anaplastic lymphoma kinase inhibitor (ALK) was active in inflammatory myofibroblastic tumour with ALK translocation. Sirolimus has shown promising results in patients with metastatic perivascular epithelioid cell tumours (PEComas) and in patients with recurrent lymphangioleiomyomatosis or angiomyolipomas. Bevacizumab in combination with temozolomide was well tolerated and effective in patients with locally advanced, recurrent, and metastatic hemangiopericytoma and malignant solitary fibrous tumour.
This review analyses the available published literature and discusses the role of radiation therapy, the related side effects and how the modern radiation treatment techniques may improve the care of patients with sarcomas of the retroperitoneum.
Materials and methods
A comprehensive literature search was conducted using PubMed in January 2011. Relevant international articles published from January 1980 to January 2011 were assessed. The keywords for search purposes were: retro-peritoneum, sarcoma, radiotherapy and radiation therapy. The search was limited to articles published in English. Only peer-reviewed publications dealing with patient outcome were selected. RT planning studies; “short communications”; publications without details on RT timing, dose, or technique; publications on paediatric tumours; and publications concerning desmoid tumours, dermatofibrosarcoma protuberans, Kaposi sarcoma, round-cell sarcoma, and angiosarcoma were excluded, because these histopathology types also are excluded from the American Joint Committee on Cancer (AJCC) staging system. Rhabdomyosarcoma, round-cell sarcoma and primitive neuroectodermal tumour were not included because of their well-known radiosensitivity. Series that did not analyse retroperitoneal soft tissue sarcomas (RSTS) separately from other intra-abdominal or extra-abdominal sarcomas were excluded. Publications dealing with recurrent RSTS were included. Review articles were excluded. Finally, articles were selected for this review according to the following criteria:
-
(a)
Series with a minimum of ten patients presenting RPS with at least 24 months of follow-up and addressing various aspects of preoperative, postoperative, intensity-modulated radiotherapy (IMRT), intraoperative radiotherapy (IORT), intraoperative electron radiotherapy (IOERT), brachytherapy (BT), proton therapy and ion therapy.
-
(b)
The outcomes of interest were tumour control and toxicity of radiation therapy.
In total, 425 reports related to radiation for RPS had been retrieved from the initial PubMed search. After applying the inclusion criteria, 43 studies were considered for this review with 26 being the last update of the reported series. All articles were read in full by the authors and selected for inclusion based on relevance to this article.
Results
Preoperative radiation therapy
Although the majority of published data are about postoperative RT, preoperative RT has several potential advantages. Several authors have found preoperative RT as a safe and effective approach for the treatment of RPS. There are several reasons to support the use of preoperative RT:
-
1.
Preoperatively, the gross tumour volume can be more precisely defined on the CT images with smaller safety margins. Potentially the tumour can be downsized with RT (by devitalisation of tumour cells) to facilitate surgical resection.
-
2.
The tumour displaces adjacent normal tissues out of the high-dose region thereby minimising RT-related toxicities of radiosensitive organs [13].
-
3.
Prior to surgery, the tumour is better oxygenated and RT is probably more effective in killing neoplastic cells.
-
4.
The tumour is treated “in situ” thereby reducing the risk of peritoneal or locoregional seeding at the time of surgery.
-
5.
Due to the lower incidence of surgical adhesions, higher doses can be delivered to the tumour bed preoperatively. On the contrary, postoperative radiotherapy may be problematic or hazardous in patients with small bowel adhesions.
-
6.
May avoid treatment delay due to postoperative complications.
Combined preoperative RT and chemotherapy
In a Phase I trial, Pisters et al. [14] enrolled 35 consecutive patients with resectable RPS who received preoperative external-beam radiotherapy (EBRT) with concomitant doxorubicin in a dose escalation trial of 18–50.4 Gy, followed by surgery plus IORT (15 Gy). The chemoradiation regimen was completed in an outpatient fashion in 31 of 35 patients (89 %), while four patients (11 %) required hospital admission for dehydration and/or anorexia. Two patients (5 %) who suffered gastrointestinal bleeding secondary to radiation-induced gastritis received 46.8 and 50.4 Gy, respectively. Grade 3–4 gastrointestinal toxicities recorded were: nausea (no vomiting or diarrhoea) in three patients (20 %) at the 46.8 Gy level, and in two patients (18 %) for 50.4 Gy level, while patients at the 41 Gy level did not record any toxicity. The addition of chemotherapy (even if at a fixed dose) to RT is a possible confounder for the impact of dose escalation on treatment morbidity. The authors concluded that 45 Gy of preoperative EBRT was well tolerated and dose escalation to 50 Gy yielded higher GI morbidity rates. However, there is currently insufficient evidence to clarify this issue. Additional well-designed prospective trials are needed to establish the optimal escalation dose above 45 Gy in preoperative EBRT with acceptable toxicity. There have been controversial results about the efficacy and local control with the addition of IORT to preoperative EBRT. The contribution of IORT may be undermined by the selection bias of patients based on the surgeon evaluation [14–19].
Ballo et al. [8] analysed 83 patients treated at MD Anderson Cancer Centre (MDACC) and found no difference in local control regarding RT timing with higher doses (>50 Gy) of EBRT or with the specific use of IORT. In the series from the Massachusetts General Hospital (MGH), Gieschen et al. [18] did not find a significant difference in 5-year local control for 16 patients of preoperative EBRT plus IORT group compared to 13 patients treated by preoperative EBRT alone (83 vs. 61 %, p = 0.19).
Pawlik et al. [19] combined the data from the two prospective trials conducted at the Princess Margaret Hospital (PMH) and MDAAC to analyse long-term recurrence and survival rates. Seventy-two patients with intermediate or high-grade RPS were analysed and were treated with preoperative RT (median dose 45 Gy, range 18.0–50.4 Gy). Eighty-nine percent of patients completed the planned preoperative treatment and only 3 % of patients had interruption due to RT-induced toxicity. Among the 57 patients who underwent surgery with curative intent, macroscopically complete resection (R0 or R1) was achieved in 54 patients (95 %). Among these 54 patients, 22 received boosts with IORT, 12 with postoperative brachytherapy (BT); 20 did not have additional boosts. In both prospective studies, the selection of patients who received IORT or BT was based on the surgeons’ intraoperative assessment. Fifty-four patients completed the planned preoperative RT and underwent R0 or R1 resection with curative intent. The 5-year local recurrence-free, disease-free, and overall survival (OS) rates were 60, 46, and 61 %, respectively. Despite the promising results, preoperative radiation doses ≥45 Gy as well as radiation boost techniques were not shown to have a prognostic impact on recurrence-free, disease-free and overall survival (p = 0.13). Although both BT and IOERT offered similar local control, BT was associated with significantly higher toxicity rates.
In 2003, the American College of Surgeons Oncology Group opened the only multi-institutional phase III trial (ACOSOG Z9031) to determine the effect of preoperative RT on patients with untreated primary RPS, but unfortunately it was closed in 2005 due to poor patient accrual. This randomized controlled trial was needed to obtain definitive data about the role of RT.
Intraoperative radiation therapy (IORT)
IORT uses low energy X-rays directed to the tumour bed at the time of surgery, commonly by a mobile linear accelerator, which produces electrons (IOERT). IORT allows irradiation of areas at risk for recurrences as defined by the surgeon at time of surgery. IORT may allow delivery of high RT doses to high-risk areas while limiting small bowel toxicity by direct shielding during IORT delivery.
To date, a trial performed between 1980 and 1985 at the National Cancer Institute represents the single published randomized control trial involving RT for patients with RPS [20]. In this trial, 20 patients were treated with postoperative EBRT alone (35–40 Gy to an extended field, and 15 Gy to a boost field). These patients were randomly compared with 15 patients who received intravenous misonidazole (a radio-sensitizer) plus IOERT (20 Gy), administered using abutting or overlapping fields with high-energy electrons of 11–15 megaelectron volts (MeV), followed by postoperative EBRT (35–40 Gy). All patients underwent macroscopically complete resection [20]. After 5 years of follow-up, 60 % of patients who received IOERT were free from local recurrences compared with 20 % of patients treated with EBRT alone (p < 0.001). RT morbidity was more significant in the IORT arm: 60 % of the patients in the IOERT arm versus 5 % in the EBRT arm developed peripheral neuropathy; 13 % of patients in IORT-EBRT arm versus 50 % in EBRT without IORT developed enteritis. In another trial, Krempien et al. [21] reported on 67 patients who underwent surgery and IOERT (median dose, 15 Gy); 45 patients underwent additional postoperative EBRT (median 45 Gy) and the 5- and 10-year actuarial loco-regional control rates were 40 and 33 %, respectively.
These authors emphasised that dose escalation with the combination of IOERT and postoperative EBRT decreased the risk of recurrence, after macroscopically complete resection. Furthermore, the 5-year local-control rate within the IOERT field was 72 % regardless of the resection status, but increased to 84 % after gross resection and 95 % after complete resection. The abdominal (loco-regional) control was significantly affected by resection status (R0 vs. R1/2, p < 0.001) and for the 12 patients, who completed IOERT and EBRT after R0 resection, 5- and 10-year loco-regional control (LRCR) was 100 % compared with 0 % for 12 patients having R2 resection.
The main limitation of IORT is the volume of tissue safely treated in a single field. Neurotoxicity is hardly increased if the intraoperative dose is limited to less than 15 Gy [22]. Other techniques to deliver dose to the primary tumour and margin of normal tissue may be better. Intensity-modulated radiation therapy (IMRT) utilising a simultaneous integrated boost has been used for further dose escalation to high risk margins [6].
Postoperative radiation therapy
The natural history of RPS and the high risk for local recurrences support the use of postoperative local treatments. Postoperative RT is widely used for its advantages that it does not delay the surgery and it can provide selective treatment in the high-risk group depending on biopsy results or when the complete resection is not possible. Therefore, it is performed mainly in the cases of high grade, incomplete resection or inadequate resection margins.
Therefore, RT in addition to surgery is widely accepted in the management of RPS. Radiotherapy for RPS is complex because of the large treatment volumes required and proximity of critical normal tissues, especially the bowel and liver. The ureters, kidneys, spinal cord and peripheral nerves are also at risk for late radiation-related injury. Due to the rarity of RPS, most of the published data are from retrospective studies and therefore results are difficult to compare across different studies.
Catton et al. [23] reviewed the treatment outcomes of 104 patients from the Princess Margaret Hospital, Toronto, Canada. Thirty-six of 45 patients who had undergone macroscopic total resection and postoperative RT had a delayed time to local recurrence compared to patients treated with surgery alone (p = 0.06) for radiation doses higher than 35 Gy. When only patients with in-field recurrences are considered, the difference reached statistical significance (p = 0.02), suggesting a theoretical role of further dose escalation and boosting the margins at higher risk of recurrence, to improve local control and survival.
Stoeckle et al. [5] published the largest retrospective study showing a benefit on local control with postoperative RT after complete macroscopic excision. Sixty patients, who received adjuvant RT (median dose 50 Gy), were compared with 34 patients treated with surgery alone. Superior actuarial 5-year local recurrence-free survival was found in the former group (55 vs. 23 %; p = 0.0021). In multivariate analyses, a positive prognostic impact of RT on local control was shown (p = 0.0002).
In RPS, higher doses correlate with better local control rates. In a series of 17 patients treated with postoperative RT, Tepper et al. [24] observed a local control rate of 83 % for doses >60 Gy compared with only 33 % for those receiving <50 Gy. Fein et al. [25] reported on 21 patients (19 of 21 irradiated postoperatively) and found radiation doses >55.2 Gy yielded 75 % of local control, whereas 62 % was obtained for doses <55 Gy. The outcomes of postoperative RT for RPS and the experiences extrapolated from extremity sarcomas [26–28] support a theoretical optimal tumour killing dose 60 Gy or higher, to achieve a significant improvement of local control. The ability to deliver such high doses in the retro-peritoneum is limited by the close proximity of radiosensitive organs such as the small bowel, which cannot tolerate more than 45 Gy [29]. In a postoperative setting, dose escalation is possible by delivering a boost in a focal area at high risk for local recurrence. Different delivery techniques have been suggested to overcome the limit related to bowel toxicity, targeting the dose selectively to areas at risk for tumour recurrence.
Discussion
Levels of evidence of current protocols
As opposed to limb sarcoma, adjuvant RT in RPS is not considered a standard, since no randomised trial has addressed the question of surgery combined with adjuvant RT, compared with surgery alone. Thus, decisions for every individual patient are based on results of retrospective studies. In a multi-institutional study where surgery for RPS was “classical” (limited to tumour only), adjuvant postoperative RT reduced the risk of local relapse by a factor of 3 [5].
Level 2A: based on lower level evidence, there is uniform consensus that the intervention is appropriate
For resectable disease
Biopsy should be performed (to exclude GIST or desmoid tumours), surgery ± IORT, surgery + postoperative radiotherapy ± chemotherapy. If a biopsy was not done, further management will be guided by intraoperative frozen section (i.e., in the case of IORT) or final pathology evaluation.
For unresectable or stage IV disease
Preoperative chemotherapy, radiotherapy or combination may be considered to downsize the tumour and surgery may be considered when the tumour becomes resectable. Otherwise, management will be palliative in nature and be guided by patients’ symptoms and performance status.
Level 2B: based on lower level evidence, there is consensus that the intervention is appropriate
After biopsy, preoperative RT may be considered. After surgery, consider postoperative RT in patients with R1 resection if no preoperative RT is given. If the patient had preoperative RT, postoperative boost RT is recommended. Highly selected patients (high risk factors) with R0 resection may be offered postoperative RT.
Several factors have contributed to a lack of quality evidence regarding optimal management. Due to the rarity of this disease and the controversial role of postoperative RT, it would be difficult to conduct a randomised study directly comparing three-dimensional conformal radiotherapy (3DCRT), IMRT and IORT or pre- and postoperative RT. Furthermore, it is also unknown how the benefit might vary among treatment modalities regarding different disease stages, characterised by tumour size, grade, and lymph node involvement. Although there have been reports on several cohort studies or case series based on single-institution experiences with surgical treatment and RT, it is unclear to what extent these findings from small numbers of patients can be generalised to most patients with RPS. There may be substantial variation among surgical (margin status) and radiotherapy techniques (treatment volumes, RT dose) in the various series.
Radiation therapy-related toxicity (comparison among different approaches)
Preoperative RT has been associated with lower morbidity rates compared to postoperative RT. Zlotecki [30] compared 15 patients treated with preoperative RT and 25 patients with postoperative RT, and found that 80 % of the postoperative RT patients experienced acute enteritis compared to 36 % in the preoperative group (p = 0.0098). Furthermore, all six patients with severe complications that required hospitalisation (infection, haemorrhage, and bowel obstruction) were treated with postoperative RT.
The majority of published studies that concern preoperative EBRT for RPS have been retrospective series and/or EBRT has been combined with IORT for dose escalation to regions with higher risk of recurrence. Therefore, a separate evaluation of morbidity attributable only to preoperative EBRT is not possible and it is difficult to interpret the available clinical data.
Gieshcen et al. [18] reported on 37 patients treated at the Massachusetts General Hospital with preoperative RT. Of the 25 patients who received a boost with IORT, four patients had severe side effects. Out of these patients, three (12 %) received IORT to 10–20 Gy and experienced gastrointestinal/genitourinary toxicity versus 8 % of whole series treated with preoperative EBRT (median dose 45 Gy). Patients who received EBRT doses up to 50 Gy did not have an increase in the rates of complications.
The trials from the Princess Margaret Hospital and the MD Anderson Cancer Centre are informative because acute toxicities of preoperative RT were prospectively and separately recorded from other toxicities [14, 31]. The latter study combined chemotherapy with preoperative EBRT, which could be a confounding factor [14]. The prospective series of the Princess Margaret Hospital reported on 46 patients who underwent complete gross surgical resection [31]. Forty-one patients were treated with preoperative EBRT and 23 of them had postoperative BT. Preoperative EBRT was delivered at a median dose of 45 Gy and associated with acute toxicity scores of the Radiation Therapy Oncology Group (RTOG) grade ≤2 in all patients. Wound complications were observed in 5 % of patients, whereas acute toxicity of RTOG grade ≥3 was recorded in 39 % of patients after receiving surgery and/or BT. Four of six patients developed grade 4 acute toxicity postoperatively and all six patients who experienced late toxicity had received BT. Moreover, 58 % of patients who underwent BT needed hospitalisation with two deaths and one life-threatening illness owing to upper abdominal BT. Consequently, the upper abdominal BT was subsequently excluded from the treatment protocol.
Regarding radiation tolerance of healthy tissues, Krempien et al. [21] recorded grade 2 or higher late toxicity in 21 % of the patients: 13 % of acute gastrointestinal morbidity, 4.5 % gastrointestinal fistulae, 6 % small bowel stenosis and 7 % neuropathies. In general, severe gastrointestinal morbidity results in patients treated with IORT plus postoperative EBRT using a dose of 45–55 Gy [20, 21, 32, 33], and drastically decreased for doses <45 Gy. Such low doses (<45 Gy), on the other hand, even if well tolerated by the small bowel, are not sufficient to have a tumoricidal effect [20]. The dose-related radiation toxicity in STS can be influenced by the particular situation of postoperative RT for RPS. The small and large bowels tend to occupy the space in the surgical bed where tumour was located. In addition, surgical adhesions increase the toxic effect of EBRT, by restricting the same section of bowel loops receiving high RT doses [34]. Peripheral neuropathy represents a well known and expected dose-related complication specifically associated with IOERT. In the randomised study from the National Cancer Institute, all patients received the same dose with IOERT boosts (20 Gy) and the rate of peripheral neuropathy was 60 %. In the published reports where a median dose 15 Gy of IOERT was administered, lower rates (6–37 %) were observed [16–18, 21, 32, 33].
Radiation treatment volumes for preoperative RT
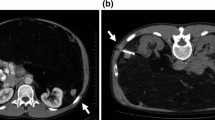
Preoperative radiotherapy for RSTS is classically delivered to the entire gross tumour volume (GTV) (Fig. 1). The clinical tumour volume (CTV) includes the GTV with 3–5 cm margins and includes the area in contact with the adjacent organs. Such regions are at a higher risk for local relapse. Conventionally, the radiation beam arrangements that have been employed are antero-posterior and oblique fields using computed tomography (CT) treatment planning to conform to the target volume and shield the organs at risk. With such techniques, the risk of small bowel toxicity is high and limits the feasibility of dose escalation. Safe doses are considered to be 45–50.4 Gy in 1.8–2 Gy/fraction as determined by the dose limit of the small bowel.
Radiation treatment volumes for postoperative RT
The CTV is defined based on preoperative imaging, the surgeon’s findings and the histological results. Co-registration between pre-surgery and post-surgery CT should be done when possible to facilitate the delineation of the posterior involvement of the tumour in the retroperitoneal space. The retroperitoneal surface at higher risk of recurrence is defined as the contact area between the resected preoperative GTV and remaining adjacent organ structures (ipsilateral psoas muscle, ipsilateral muscles of the posterior abdominal wall, pre-vertebral surface around the great vessels). A margin is then added to the CTV to obtain the planning target volume (PTV). In postoperative RT, the treatment area is typically larger than that in preoperative RT and includes more of the intestinal cavity. Typical doses prescribed range between 45 and 50.4 Gy.
Intensity-modulated radiotherapy (IMRT) and image-guided radiotherapy (IGRT)
Over the past decade, technical advances have improved tumour targeting and conformal RT delivery. Dosimetric studies suggest enhanced target coverage and improved sparing of non-target normal tissue with IMRT compared to that of conventional three-dimensional conformal photon therapy. Recently, IMRT has been introduced as a strategy to deliver preoperative RT in RPS. This highly conformal technique allows a superior coverage of tumour volume and a sparing of dose to critical normal structures such as the kidneys, small bowel and liver. IMRT allows delivering of differential doses to different areas at risk, selectively escalating the dose, and hopefully local control. Preoperative IMRT has the potential to further improve the therapeutic index by permitting dose escalation to the area of the tumour while minimising the dose to normal tissues at risk for radiation toxicity [13]. It is possible to reduce overall treatment time using an integrated boost concept with simultaneously increased dose per fraction in parts of the target volume which are at increased risk for incomplete resection during planned surgery. Compared to 3DCRT, IMRT using 6–9 coplanar beams greatly reduced the high dose irradiated volume and decreased the radiation exposure above 25 Gy to the intestinal cavity. The conformity index was compellingly better (doubled) with IMRT, while conserving the integral dose delivered to the whole body [35]. The recent development of IGRT with the use of daily (kilovoltage or megavoltage) CT-based target verification allows reduction of the CTV to PTV expansion margins, thereby potentially reducing the incidence of toxicity and/or allowing for further dose escalation.
In a prospective, non-randomized study, 45 Gy (1.8 Gy/fraction) was prescribed to the whole tumour plus margin, which is the small bowel tolerance dose, and then a further boost dose of 57.5 Gy (2.3 Gy/fraction) to the posterior abdominal wall at high risk for positive surgical margins was delivered [36]. Other than grade 3 nausea/vomiting, no other severe acute toxicity was noted in 16 patients. However, the small patient numbers and short follow-up period prevented assessment of long-term toxicity to peripheral nerves. Bossi et al. [13] described a novel approach for the definition of the CTV for liposarcoma of the retroperitoneum. The suggested CTV included only the region between the tumour and the posterior abdominal wall. This method may reduce the acute toxicities of preoperative RT for RPS without compromising the rate of resectability. Eighteen consecutive patients were irradiated preoperatively with the IMRT technique: the complication rates were found to be comparable with historical data on non-irradiated patients.
New radiation therapies
Charged particle therapy
Proton beam radiotherapy (PBRT): Protons are charged particles that deposit most of their energy at a depth proportional to their energy, resulting in the characteristic dose distribution known as the Bragg peak. There are several theoretical advantages of PBRT over IMRT and 3DCRT. Protons have a reduced entrance dose compared to photons and almost no exit dose. Thus, PBRT reduces the radiation of adjacent normal organs and tissues by approximately 60 %. This allows delivery of the prescription dose to the tumour with much greater sparing of adjacent organs and structures than can be possible with photons. A comparative planning study evaluated 3D-conformal proton radiotherapy (3DCPT), IMRT, and 3DCRT to determine the optimal RT technique for RPS. Results indicate that IMRT and 3DCPT result in more conformal and homogenous plans than 3DCRT. Based on Qualitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) benchmarks, the dosimetric advantage of proton therapy may be less gastrointestinal and genitourinary toxicity [37].
MGH reported a study of PBRT and/or IMRT (median dose of 50 Gy) with IOERT (single fraction 10–20 Gy) in 28 patients with RPS [38]. Surgical complications occurred in eight patients (28.6 %), and radiation-related complications occurred in four patients (14 %). After a median follow-up of 33 months, only two patients (10 %) with primary disease experienced local recurrence (LR), while three patients (37.5 %) with recurrent disease experienced local recurrence. This strategy may minimise radiation-related morbidity and reduce local recurrence, especially in patients with primary disease.
Current trials in proton therapy
At the moment, there are two active trials in North America to address the issue of proton therapy in RPS. The University of Pennsylvania is conducting a Phase I trial to study the side effects and best dose of PBRT in treating patients with retroperitoneal sarcoma. The University of Florida is assessing treatment feasibility of preoperative proton therapy for resectable intermediate or high-grade retroperitoneal sarcoma.
High linear energy transfer (LET) radiotherapy
Sarcoma has been traditionally considered to be resistant to conventional LET radiation. The reason may be from the mechanism of cell damage: low LET radiations generate injuries indirectly through the formation of free radicals and/or directly with “ionizations” in the patient. The formation of radicals depends heavily on oxygenation level. Consequently, the sensitivity of tissue to low LET radiation depends on the degree of blood supply to the tissues and also on the types of tissues, presence of free radical scavengers and cell cycle phase.
The degree of ionisation of low LET radiation is sparse in tissue; the mean distance between two ionisation events is far greater than the diameter of deoxyribonucleic acid (DNA) double helix. This dose deposition pattern at the microscopic level may produce DNA breaks that can be recognised and repaired quickly and easily. This compares with the theoretical, physical and radiobiological advantages of high LET radiation. The physical advantages are low dose deposition in the entry channel, followed by a steep dose deposition in the target region (the Bragg Peak), and then a sharp dose fall-off with no exit dose. From the biological point of view, heavy ions can produce dense ionisations within the tumour, resulting in multiple clustered DNA breaks, which are more difficult to be repaired by the intrinsic cellular repair pathways. In addition, all the factors necessary to reduce tumour response (mentioned in the preceding paragraph) to low LET RT are less relevant with heavy ions.
Carbon ion beams have the same favourable physical properties of proton beams. Minor differences between the two are a sharper lateral penumbra and a dose tail beyond the Bragg peak for the carbon beam. Like all charged particles, carbon ions have a variable LET that increases with the depth of penetration into tissues. Carbon ions therefore produce sparse ionisations in the entrance channel and dense ionisations in the Bragg peak. In the last part of their track, carbon ions release a higher dose and results in a higher effectiveness in the target volume (compared to protons). The effectiveness of a radiation is measured by its radiobiological effectiveness (RBE). Carbon has an RBE ratio in the Bragg peak to RBE in the plateau region higher than other ions. Therefore, carbon ion radiotherapy (CIRT) has the potential to combine the optimal dose conformality of proton therapy with the increased local control of neutron therapy without the increase in unwanted side effects of the latter [39–41]. Carbon ion beams have improved dose localisation properties, and this can potentially produce a large effect on tumours while minimising normal tissue damage. Moreover, CIRT possesses various biologic advantages such as high LET radiation, decreased oxygen enhancement ratio (OER), diminished capacity for repair of sublethal and potentially lethal damage, and diminished cell cycle-dependent radiosensitivity when compared with those observed with low LET radiation.
From 1993, CIRT has been performed at the National Institute of Radiological Sciences (NIRS) in Chiba, Japan. The optimal dose for inoperable, residual or recurrent bone and STS has been determined within dose escalation trials to be at 70.4 GyE (Gray equivalent) in 16 fractions over 4 weeks [42–44]. The definition of Gray equivalent has previously been reported [45]. Results of a small series of 24 patients with inoperable RPS treated with CIRT at NIRS have been published [11]. Patients were heterogeneous in terms of tumour histology (malignant fibrous histiocytoma, liposarcoma, malignant peripheral nerve sheath tumour, Ewing/primitive neuroectodermal tumours, etc.). Median volume of CTV was 525 ml (range 57–1,194 ml) and patients received doses of 52.8–73.6 GyE. Median follow-up was 36 months (range 6–143 months). The 5-year local control rate was 69 % and the 5-year overall survival rate was 50 %. No acute or late grade 3 toxicity was detected and there was no gastrointestinal toxicity. These results appear promising in such unfavourable patients. Therefore, CIRT could be considered for patients with RPS with residual macroscopic disease after surgery due to infiltration of surrounding organs or unresectable tumours. Existing and planned carbon ion facilities in Asia and Europe are activating clinical protocols in RPS and more time is needed to verify these preliminary experimental data.
Conclusions
A multidisciplinary team with expertise in STS should manage all patients with RPS. Complete resection is the only potentially curative treatment. The addition of RT to wide surgical excision for RPS has improved local control rates when compared with surgery alone, but does not improve overall survival. Preoperative RT is preferred to postoperative RT especially in patients with high-grade or intermediate-grade RPS and for selected cases of low-grade RPS (e.g., extremely large or appearing unresectable or borderline resectable). In this setting, RT alone is recommended rather than chemo-radiation, unless the patient is being treated within a clinical trial. For patients with either intermediate- or high-grade histology or positive margins who have undergone surgical resection without preoperative RT, postoperative RT is a reasonable option. IORT with/without pre- or postoperative EBRT is also a reasonable option, especially for patients with tumour close to critical organs or gross residual disease after surgery. New types and delivery techniques in RT could further improve patient outcomes. Due to their physical and radiobiological properties, charged particle RT (such as protons and carbon ions) are expected to be better in sparing normal tissues and with higher efficacy than conventional photon therapy RT. However, in the absence of a randomized controlled trial, it is impossible to determine whether any putative improvement in tumour outcome is a consequence of selection bias or other confounding factors. The rarity of RPS makes organising appropriately powered prospective studies difficult. Larger collaborative prospective controlled studies among sarcoma centres need to be conducted to establish more powerful conclusions regarding the management of these complex neoplasms.
References
Strauss DC, Hayes AJ, Thomas JM (2011) Retroperitoneal tumours: review of management. Ann R Coll Surg Engl 93:275–280
Porter GA, Baxter NN, Pisters PW (2006) Retroperitoneal sarcoma: a population-based analysis of epidemiology, surgery, and radiotherapy. Cancer 106:1610–1616
Roberge D, Vakilian S, Alabed YZ et al (2012) FDG PET/CT in initial staging of adult soft-tissue sarcoma. Sarcoma 2012:960194
Perez EA, Gutierrez JC, Moffat FL Jr et al (2007) Retroperitoneal and truncal sarcomas: prognosis depends upon type not location. Ann Surg Oncol 14:1114–1122
Stoeckle E, Coindre JM, Bonvalot S et al (2001) Prognostic factors in retroperitoneal sarcoma: a multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer 92:359–368
Van De Voorde L, Delrue L, van Eijkeren M, De Meerleer G (2011) Radiotherapy and surgery—an indispensable duo in the treatment of retroperitoneal sarcoma. Cancer 117:4355–4364
Le Pechoux C, Musat E, Baey C et al (2013) Should adjuvant radiotherapy be administered in addition to front-line aggressive surgery (FAS) in patients with primary retroperitoneal sarcoma? Ann Oncol 24:832–837
Ballo MT, Zagars GK, Pollock RE et al (2007) Retroperitoneal soft tissue sarcoma: an analysis of radiation and surgical treatment. Int J Radiat Oncol Biol Phys 67:158–163
Alford S, Choong P, Chander S et al (2013) Outcomes of preoperative radiotherapy and resection of retroperitoneal sarcoma. ANZ J Surg 83:336–341
Lee HJ, Song SY, Kwon TW et al (2011) Treatment outcome of postoperative radiotherapy for retroperitoneal sarcoma. Radiat Oncol J 29:260–268
Serizawa I, Kagei K, Kamada T et al (2009) Carbon ion radiotherapy for unresectable retroperitoneal sarcomas. Int J Radiat Oncol Biol Phys 75:1105–1110
Bonvalot S, Cavalcanti A, Le Pechoux C et al (2005) Randomized trial of cytoreduction followed by intraperitoneal chemotherapy versus cytoreduction alone in patients with peritoneal sarcomatosis. Eur J Surg Oncol 31:917–923
Bossi A, De Wever I, Van Limbergen E, Vanstraelen B (2007) Intensity-modulated radiation therapy for preoperative posterior abdominal wall irradiation of retroperitoneal liposarcomas. Int J Radiat Oncol Biol Phys 67:164–170
Pisters PW, Ballo MT, Fenstermacher MJ et al (2003) Phase I trial of preoperative concurrent doxorubicin and radiation therapy, surgical resection, and intraoperative electron-beam radiation therapy for patients with localized retroperitoneal sarcoma. J Clin Oncol 21:3092–3097
Willett CG, Suit HD, Tepper JE et al (1991) Intraoperative electron beam radiation therapy for retroperitoneal soft tissue sarcoma. Cancer 68:278–283
Petersen IA, Haddock MG, Donohue JH et al (2002) Use of intraoperative electron beam radiotherapy in the management of retroperitoneal soft tissue sarcomas. Int J Radiat Oncol Biol Phys 52:469–475
Bobin JY, Al-Lawati T, Granero LE et al (2003) Surgical management of retroperitoneal sarcomas associated with external and intraoperative electron beam radiotherapy. Eur J Surg Oncol 29:676–681
Gieschen HL, Spiro IJ, Suit HD et al (2001) Long-term results of intraoperative electron beam radiotherapy for primary and recurrent retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys 50:127–131
Pawlik TM, Pisters PW, Mikula L et al (2006) Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol 13:508–517
Sindelar WF, Kinsella TJ, Chen PW et al (1993) Intraoperative radiotherapy in retroperitoneal sarcomas. Final results of a prospective, randomized, clinical trial. Arch Surg 128:402–410
Krempien R, Roeder F, Oertel S et al (2006) Intraoperative electron-beam therapy for primary and recurrent retroperitoneal soft-tissue sarcoma. Int J Radiat Oncol Biol Phys 65:773–779
Shaw EG, Gunderson LL, Martin JK et al (1990) Peripheral nerve and ureteral tolerance to intraoperative radiation therapy: clinical and dose–response analysis. Radiother Oncol 18:247–255
Catton CN, O’Sullivan B, Kotwall C et al (1994) Outcome and prognosis in retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys 29:1005–1010
Tepper JE, Suit HD, Wood WC et al (1984) Radiation therapy of retroperitoneal soft tissue sarcomas. Int J Radiat Oncol Biol Phys 10:825–830
Fein DA, Corn BW, Lanciano RM et al (1995) Management of retroperitoneal sarcomas: does dose escalation impact on locoregional control? Int J Radiat Oncol Biol Phys 31:129–134
Pisters PW, O’Sullivan B, Maki RG (2007) Evidence-based recommendations for local therapy for soft tissue sarcomas. J Clin Oncol 25:1003–1008
Yang JC, Chang AE, Baker AR et al (1998) Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 16:197–203
O’Sullivan B, Davis AM, Turcotte R et al (2002) Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359:2235–2241
Emami B, Lyman J, Brown A et al (1991) Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 21:109–122
Zlotecki RA, Katz TS, Morris CG et al (2005) Adjuvant radiation therapy for resectable retroperitoneal soft tissue sarcoma: the University of Florida experience. Am J Clin Oncol 28:310–316
Jones JJ, Catton CN, O’Sullivan B et al (2002) Initial results of a trial of preoperative external-beam radiation therapy and postoperative brachytherapy for retroperitoneal sarcoma. Ann Surg Oncol 9:346–354
Gilbeau L, Kantor G, Stoeckle E et al (2002) Surgical resection and radiotherapy for primary retroperitoneal soft tissue sarcoma. Radiother Oncol 65:137–143
Alektiar KM, Hu K, Anderson L et al (2000) High-dose-rate intraoperative radiation therapy (HDR-IORT) for retroperitoneal sarcomas. Int J Radiat Oncol Biol Phys 47:157–163
Kepka L, DeLaney TF, Suit HD, Goldberg SI (2005) Results of radiation therapy for unresected soft-tissue sarcomas. Int J Radiat Oncol Biol Phys 63:852–859
Paumier A, Le Pechoux C, Beaudre A et al (2011) IMRT or conformal radiotherapy for adjuvant treatment of retroperitoneal sarcoma? Radiother Oncol 99:73–78
Heslin MJ, Lewis JJ, Nadler E et al (1997) Prognostic factors associated with long-term survival for retroperitoneal sarcoma: implications for management. J Clin Oncol 15:2832–2839
Swanson EL, Indelicato DJ, Louis D et al (2012) Comparison of three-dimensional (3D) conformal proton radiotherapy (RT), 3D conformal photon RT, and intensity-modulated RT for retroperitoneal and intra-abdominal sarcomas. Int J Radiat Oncol Biol Phys 83:1549–1557
Yoon SS, Chen YL, Kirsch DG et al (2010) Proton-beam, intensity-modulated, and/or intraoperative electron radiation therapy combined with aggressive anterior surgical resection for retroperitoneal sarcomas. Ann Surg Oncol 17:1515–1529
Schmitt G, Mills EE, Levin V et al (1989) The role of neutrons in the treatment of soft tissue sarcomas. Cancer 64:2064–2068
Schonekaes KG, Prott FJ, Micke O et al (1999) Radiotherapy on adult patients with soft tissue sarcoma with fast neutrons or photons. Anticancer Res 19:2355–2359
Schwartz DL, Einck J, Bellon J, Laramore GE (2001) Fast neutron radiotherapy for soft tissue and cartilaginous sarcomas at high risk for local recurrence. Int J Radiat Oncol Biol Phys 50:449–456
Kamada T, Tsujii H, Tsuji H et al (2002) Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol 20:4466–4471
Imai R, Kamada T, Tsuji H et al (2010) Effect of carbon ion radiotherapy for sacral chordoma: results of Phase I–II and Phase II clinical trials. Int J Radiat Oncol Biol Phys 77:1470–1476
Imai R, Kamada T, Tsuji H et al (2004) Carbon ion radiotherapy for unresectable sacral chordomas. Clin Cancer Res 10:5741–5746
Kanai T, Matsufuji N, Miyamoto T et al (2006) Examination of GyE system for HIMAC carbon therapy. Int J Radiat Oncol Biol Phys 64:650–656
Lewis JJ, Leung D, Woodruff JM, Brennan MF (1998) Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 228:355–365
Youssef E, Fontanesi J, Mott M et al (2002) Long-term outcome of combined modality therapy in retroperitoneal and deep-trunk soft-tissue sarcoma: analysis of prognostic factors. Int J Radiat Oncol Biol Phys 54:514–519
Gronchi A, Casali PG, Fiore M et al (2004) Retroperitoneal soft tissue sarcomas: patterns of recurrence in 167 patients treated at a single institution. Cancer 100:2448–2455
Pierie JP, Betensky RA, Choudry U et al (2006) Outcomes in a series of 103 retroperitoneal sarcomas. Eur J Surg Oncol 32:1235–1241
Dziewirski W, Rutkowski P, Nowecki ZI et al (2006) Surgery combined with intraoperative brachytherapy in the treatment of retroperitoneal sarcomas. Ann Surg Oncol 13:245–252
Tzeng CW, Fiveash JB, Popple RA et al (2006) Preoperative radiation therapy with selective dose escalation to the margin at risk for retroperitoneal sarcoma. Cancer 107:371–379
van Dalen T, Plooij JM, van Coevorden F et al (2007) Long-term prognosis of primary retroperitoneal soft tissue sarcoma. Eur J Surg Oncol 33:234–238
White JS, Biberdorf D, DiFrancesco LM et al (2007) Use of tissue expanders and pre-operative external beam radiotherapy in the treatment of retroperitoneal sarcoma. Ann Surg Oncol 14:583–590
Caudle AS, Tepper JE, Calvo BF et al (2007) Complications associated with neoadjuvant radiotherapy in the multidisciplinary treatment of retroperitoneal sarcomas. Ann Surg Oncol 14:577–582
Conflict of interest
Jeffrey Tuan, Viviana Vitolo, Barbara Vischioni, Alberto Iannalfi, Maria Rosaria Fiore, Piero Fossati, Roberto Orecchia declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tuan, J., Vitolo, V., Vischioni, B. et al. Radiation therapy for retroperitoneal sarcoma. Radiol med 119, 790–802 (2014). https://doi.org/10.1007/s11547-013-0350-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-013-0350-3