Abstract
The potential utility of natural volatiles in various essential oils (EOs) from plants as fumigants to control potato tuber (Solanum tuberosum L.) pathogens was assessed. The antifungal effects of the volatiles at various concentrations were studied at 10 °C both in vitro using conidial suspensions of Helminthosporium solani, Fusarium solani var. coeruleum and Phoma foveata plated on agar, and in vivo by inoculating potato tubers. The effects of the volatiles on the mycelial growth of Rhizoctonia solani were also studied, but only in vitro. Vapours of many of the EOs tested exhibited some fungicidal activity but volatiles of garlic, Allium sativum, were, with few exceptions, most effective on all four pathogens in all experiments. An exposure time of at least 2 weeks was usually required for good control of disease development in vivo. Vapours of A. sativum never stimulated conidial germination, as was observed with some other oils, or damaged tubers, but those of Armoracia rusticana caused tuber collapse. Volatiles from thyme (Thymus vulgaris) EO showed antifungal activity in vitro on all four pathogens, but did not control F. solani, P. foveata or H. solani in vivo. In contrast, sage (Salvia officinalis) EO was ineffective against P. foveata and H. solani in the in vitro system, but controlled disease development in vivo at similar doses. The sometimes conflicting results obtained in the two test systems show that screening in vitro only is insufficient for evaluation of potent antifungal substances to be used in practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In accordance with increasing global concerns for the protection of public health and the environment, in Sweden there is growing public and governmental pressure to minimise the use of synthetic pesticides in food production and to increase the acreage of organic farming. Consequently, alternative methods to control pests and diseases are urgently needed. Since antiquity, aromatic plants have been used to improve the storability of food and feed. For example, in the Andes, leaves of Muña plants (Minthostachys and Satureja spp.) have been traditionally placed in potato (Solanum tuberosum L.) tuber storage chambers to repel insects and prevent the tubers from sprouting (Aliaga and Feldheim 1985). More recently, the potential utility of natural volatile products in potato production has also been explored, but mainly as sprout suppressants of tubers (Meigh 1969; Vaughn and Spencer 1993; Vokou et al. 1993; Hartmans et al. 1995; Oosterhaven et al. 1995a, b, c). Their antimicrobial activities against potato pathogens have not been extensively studied to date. In an in vitro trial, the effects of caraway, cassia, cumin, dill and spearmint essential oils (EOs) at 160 ppm on the radial growth of the tuber pathogens Fusarium solani var. coeruleum, F. sulphureum (also known as F. sambucinum), Phoma exigua var. foveata and Helminthosporium solani were limited (Gorris et al. 1994). Mayton et al. (1996) have observed fungicidal actions of volatile compounds released from fresh, macerated Brassica juncea cv. Cutlass leaves in vitro on the mycelial growth of the potato pathogens Verticillium dahliae, V. albo-atrum, Pythium ultimum, Colletotrichum coccodes and Rhizoctonia solani. In vivo trials have generally focused solely on the effects of single compounds. For instance, Vaughn and Spencer (1994) found that tuber decay caused by two different strains of F. sambucinum (F. sulphureum) was reduced by vapours of both cineole and menthol. In addition, carvone purified from caraway seeds significantly reduced the silver scurf (H. solani) index in naturally infected potato tubers when they were exposed to vapours under semipractical storage conditions (Hartmans et al. 1995). Rotting was also prevented in tubers artificially inoculated with F. sulphureum or Phoma foveata, but not in those inoculated with F. solani, when exposed to carvone vapours in studies by Gorris et al. (1994) and Hartmans et al. (1995).
The aim of the work presented here was to study the potential utility of volatiles released from various EOs or macerated aromatic plant material, as natural postharvest fumigants to control potato tuber pathogens. Four pathogenic fungi, with varying infection strategies, were included in the experiments: H. solani Dur. et Mont, which actively penetrates the tuber skin and causes silver scurf; the two wound parasites F. solani var. coeruleum (Sacc.) Booth and P. foveata Foister, which cause dry rot and gangrene of potatoes, respectively; and R. solani Kühn, which produces superficial sclerotia, ‘black scurf’, on the surface of tubers. Preferably, one plant species would be identified that would be effective against all four pathogens and could be routinely and conveniently used to control infections in organic potato seed production. This is the first report of antifungal activity by volatiles of a broad array of plant EOs on conidia of H. solani, F. solani and P. foveata in vitro and diseases caused by these pathogens in vivo and, in addition, on mycelial growth of R. solani, most probably AG3, in vitro.
Materials and Methods
To resemble practical conditions during the first few weeks of tuber storage, the most critical period for disease development, a treatment temperature of 10 °C and relatively short exposure periods were used in all experiments. The effects of varying (1) the duration of the exposure to the EOs, (2) the doses of the EOs and (3) the number of applications of the EOs were studied both in vitro (using inoculated agar in Petri dishes) and in vivo (using artificially inoculated potato tubers) in successive experiments. As the aim was to find something that could be practically useful, the results of preceding experiments sometimes led to adjustments of the experimental plans in those that followed.
Essential Oils
Most of the EOs used were imported ones, bought from CreArom, Gammelstad, Sweden, although some were gifts from other sources listed in the tables and “Acknowledgements”. The plant species from which the EOs were derived are shown in the tables. According to the suppliers, all of the EOs were produced by steam distillation. All of the EOs were stored in the dark at 5 °C and handled in a fume cupboard.
The initially and subsequently added (as appropriate) liquid volume of the oils introduced into the treatment chambers is indicated as the dose used in the experiments. However, owing to leakage in the experimental systems the concentrations of the vapours during treatments were not constant. To facilitate comparisons between systems, the amounts of oil used are presented in ppm, i.e. the volume of EO added divided by the volume of the treatment chamber (the headspace above the agar surface in the Petri dishes or the volume of the empty boxes used in the in vivo studies).
Pathogen Inocula
All isolates used to prepare inocula originated from naturally infected tubers and were cultured at room temperature (20–22 °C): F. solani, P. foveata and R. solani on a previously described sucrose agar medium amended with streptomycin sulphate (Bång 1989), and H. solani on a medium specifically developed to accelerate the growth rate of this slow-growing organism, consisting of 39 g l−1 potato dextrose agar from Oxoid with 2 g l−1 of both yeast extract and bacteriological peptone, from Difco, adjusted to pH 6.6–6.7.
Conidial suspensions of H. solani and F. solani, and pycnidiospore suspensions of P. foveata were mostly prepared from mixtures of strains, occasionally only one, by repeatedly washing cultures with sterile tap water and gently rubbing their surfaces with a sterile plastic loop, followed by filtration through a thin layer of sterile cotton wool. The P. foveata cultures were flooded with water 30 min prior to harvesting to stimulate the release of pycnidiospores from pycnidia. The age of the cultures used varied from 3 to 5 weeks for H. solani and from 4 to 16 weeks for F. solani and P. foveata, respectively. The suspensions were counted using a Bürker haemocytometer and diluted with sterile tap water so they contained appropriate amounts of conidia per ml. In experiments with R. solani in vitro mycelial plugs from 1–2-week-old cultures were used.
In Vitro Experiments: Inoculation and Treatment Procedures, Scoring of Fungal Growth
The technique used to assess the effects of the EO vapours on the pathogens in vitro was based on the microatmosphere method of Kellner and Kober, described by Benjilali et al. (1984). Suspensions of fungal propagules were evenly plated on agar in 9-cm Petri dishes. The suspensions were applied using sterile plastic 10-μl loops in amounts varying between 20 and 40 μl. After the agar had been allowed to absorb the suspensions for 1–2 h the plates were inverted and the EO to be tested was added to a 10-mm-diameter filter paper disc in the middle of the cover. In experiments with R. solani, agar plugs (4 mm diameter) with actively growing mycelia were cut out using a sterilised cork borer and inserted into holes of the same diameter made in new agar dishes. These inoculated dishes were handled as described above.
The dishes were sealed with Parafilm and replicates of the same treatment (the number of which varied among experiments; see later) were placed in a plastic bag, which was sealed. Dishes were stored at 10 °C in the dark during the EO treatments, the length of which varied among experiments; see later. The covers containing the EO-amended filter paper were then removed and replaced by new sterile covers, after which the plates were stored at room temperature without any sealing to permit ventilation of the residual volatiles. The numbers of colony-forming units (cfu) of H. solani, F. solani and P. foveata developing on the agar and the radial growth of R. solani were continuously recorded for at least 5 weeks, making assessments of the fungistatic versus fungicidal actions of the EOs possible. Registrations started from the time the EOs were removed and ended when there was no further colony development or when the number had reached 200, as the agar surface in the Petri dishes was then fully covered. The maximum radial growth of R. solani was 85 mm.
In Vivo Experiments: Disinfection of Tubers, Inoculation and Treatment Procedures
Tubers to be inoculated were gently washed in running tap water, surface-sterilised by immersion in 2% sodium hypochlorite for 2 min, rinsed for 5 min in running tap water, immersed in 0.5% sodium thiosulphate for 2 min to inactivate excess chlorite and then rinsed for 15 min in running tap water (Löschenkohl 1979). The tubers were subsequently air-dried and rested at 10 °C for 2–5 days in the dark and then inoculated.
Tubers to be inoculated with F. solani or P. foveata were wounded by swinging a pendulum bearing a circular metal disc with nine sharp 2-mm points at them from an angle of 60° (modified after Olsson 1988). Each tuber was hit twice, once on each side, and a single drop (approximately 30 μl) of conidial or pycnidiospore suspension (as appropriate) was applied at each wounding site using a Pasteur pipette.
In order to prevent movement of the tubers to be superficially inoculated with H. solani, their downward side was cut flat. On the upper surface, they were then inoculated with conidial suspension at sites, gently marked using a cork borer and waterproof ink to facilitate observations of pathogen development, by means of a sterile plastic loop dipped in the conidial suspension.
After inoculation, tubers were allowed to rest in darkness for 4 h at 10 °C before being placed in treatment chambers (3.25-l plastic boxes) that had been prepared by placing the bottom of a 9-cm glass Petri dish, containing the desired amount of the EO to be tested, on a paper towel that had been wetted by adding 50 ml of tap water to maintain high levels of relative humidity, on the floor of each chamber, and covering the dish with a centred 16 × 16 cm square of plexiglass. The plexiglass in each case was perforated with a 14-mm-diameter hole in its centre and eight peripheral 8-mm holes, each approximately 2 cm from its edges to promote even distribution of the vapours. The inoculated tubers were gently placed on the perforated plexiglass, avoiding covering the central hole, so that passage of vapours within the box would not be impeded. The boxes were closed with a plastic lid. During the treatment period, the boxes were ventilated by removing the lid once or twice a week for 2 min, to keep the oxygen and carbon dioxide concentrations at approximately atmospheric levels. Controls were handled and stored likewise, but without plant volatiles. All in vivo experiments were carried out at 10 °C in the dark. The plastic treatment boxes, which absorbed some components, were carefully marked and reused with the same EO in successive experiments.
After the EO treatments, tubers were gently transferred to plastic trays with wetted paper towels on the bottom and loosely covered with a plastic sheet. Trays were stored at 10 °C in the dark until pathogen development was scored.
In Vivo Experiments: Scoring of Disease Development
The number of H. solani conidiophores developing at each inoculated site on the tubers was non-destructively observed at 25–40-fold magnification on at least two occasions, the first within 15 days after the last EO treatment when conidia were already abundant on controls, and the last 3 weeks later. Infection was evaluated as the number of conidiophores per inoculation site and expressed as an index, ranging from 0 to10, as follows: 0, no conidiophores; 1, one to ten conidiophores; 2, 11–20 conidiophores; 3, 21–30 conidiophores; 4, 31–40 conidiophores; 5, 41–50 conidiophores; 6, 51–60 conidiophores; 7, 61–70 conidiophores; 8, 71–80 conidiophores; 9, 81–90 conidiophores; and 10, 91 or more conidiophores. Occasionally, some inoculation sites had more than 200 conidiophores, probably owing to spontaneous infections. They were also given a score of 10. Index scores from all inoculation sites in each replicate were summed and divided by the total number of inoculation sites to obtain the mean infection index per replicate.
Tubers inoculated with P. foveata or F. solani were scored 7 weeks after inoculation, a period encompassing exposure to the EOs and the following storage period. Each tuber was sliced through both inoculation sites and visually rated for both the width (w i ) and depth (d i ) of the rots. For w i a scale of 1 corresponding to no rotting and 2, 3, 4 and 5 corresponding to rots with diameters of 13 mm or less, 13–26 mm, 26–39 mm and more than 39 mm was used. Values for d i were assigned as follows: 1, no extension; 2, superficial lesion restricted by the vascular bundle; and 3, lesion extending deep into the tuber. An index of attack was calculated using the formula
where n is the number of inoculation sites in a sample. The rot index could thus have values between 1 and 15.
Treatments that were only initially significantly suppressive, and gave scores that were close to control values and not significantly different from them at the final reading, were deemed to be fungistatic, while those resulting in significantly fewer colony-forming units or significantly less disease on the final registration date were deemed to be fungicidal.
In Vitro and In Vivo Experiments Including Exposures of 1, 2 or 3 Weeks
In the first in vitro studies, 50 μl of EO, listed in Table 1, was added to the filter paper discs in the lids of each inoculated Petri dish, giving an initial concentration of around 800 ppm (volume of EO divided by volume of air). The treatments lasted for 1, 2 or 3 weeks, after which the oil-amended filter paper discs were removed as described earlier. In each of these experiments there were two dishes per pathogen and treatment duration. F. solani and H. solani experiments were repeated after some time, resulting in four replicates, but regarding P. foveata there was one experiment, i.e. two replicates. In a following in vitro experiment of a similar kind with H. solani, 10 μl oil was used in each Petri dish, resulting in an initial concentration of 160 ppm. EOs of Allium sativum, Carum carvi, Lavandula angustifolia, Mentha arvensis, M. piperita, M. spicata and Minthostachys verticillata received from Vincenzo De Feo, University of Salerno, Italy, Populus balsamifera received from Lars Björk, Swedish University of Agricultural Sciences, Balsgård, Sweden, Rosmarinus officinalis, Salvia officinalis and Thymus vulgaris were then included. There were two Petri dishes (replicates) per EO and treatment duration. Suspensions used for inoculations in these experiment contained between 7 × 103 and 12 × 103 conidia per ml.
The influence of treatment durations as described above was also studied in in vivo experiments with F. solani, P. foveata and H. solani, respectively. An amount of 250 μl was then added to the 3.35-l plastic treatment boxes, resulting in an initial concentration of 77 ppm. The same EOs as those used in in vitro studies, listed in Table 1, were used except for Tagetes mexicana, which was not included. In the F. solani experiment, performed in October, there were two replicates per EO treatment. Each treatment box contained nine inoculated (8.5 × 104 conidia per ml) tubers, cv. King Edward. Three tubers were picked out of the boxes after 1 and 2 weeks, respectively, when the tubers were ventilated, leaving three tubers for the 3-week treatment period. Consequently there were six inoculations (three tubers with two inoculations each) per replicate. In the P. foveata experiment, performed in March, there were six replicates per EO treatment, and the cultivar used was Sabina, inoculated using a suspension with 7.8 × 104 conidia per ml. Otherwise the procedure was as described for F. solani.
Two in vivo experiments were performed with H. solani, one in November, using cultivar Sabina, and one in January, using cultivar Bintje. In the November experiment, each tuber was inoculated (10 × 104 conidia per ml) at five marked sites as earlier described, and there were nine tubers in each treatment box, three for each exposure as described above. Consequently, each replicate consisted of 15 inoculations in this experiment. In the January experiment, there were three inoculation sites per tuber (7.5 × 104 conidia per ml). Instead of picking tubers out at intervals as in the previous experiment, there were two boxes, each containing ten tubers, for each EO and exposure. Consequently, each of the two replicates consisted of 30 inoculations in the January experiment.
In Vitro and In Vivo Experiments Including Single and Split Doses
The effects of single and split doses on fungal developments were compared in vitro and in vivo. In an H. solani in vitro experiment, the single doses were obtained by initially adding 5 or 10 μl oil to the filter paper disc in each Petri dish, giving 80 and 160 ppm, respectively. In a twofold split dose 5 μl was added initially and another 5 μl was added to the same filter paper disc after 1 week, and in a threefold split there was 4 μl (60 ppm) initially, 3 μl (50 ppm) was added after 1 week and an additional aliquot of 3 μl was added after 2 weeks. The total exposure lasted 5 weeks for all treatments (2 weeks after the last addition of oil in the threefold split). There were five dishes per dose.
In a parallel in vivo experiment, potato tubers, cv. Bintje, were inoculated with H. solani at three marked sites as described above at the beginning of April. In the single doses, 260 μl or 520 μl was added to the treatment boxes, resulting in 80 and 160 ppm, respectively, the twofold split consisted of 260 μl (80 ppm) plus 260 μl that was added after 1 week, and in the threefold split 195 μl (60 ppm) plus 162.5 μl (50 ppm) after 1 week plus 162.5 μl after 2 weeks was used to obtain concentrations comparable to those used in the in vitro study. The total exposure lasted 5 weeks for all treatments (2 weeks after the last addition of oil in the threefold split). There were three boxes per EO and dose containing eight tubers each, resulting in 24 inoculations per replicate. The same original suspension was used for inoculations in both in vitro and in vivo experiments, starting the same day, having 4.5 × 103 and 10 × 103 conidia per ml, respectively.
In two other in vivo experiments, the effect of a single dose of 77 ppm was compared with a twofold split of 38.5 ppm initially plus 38.5 ppm after 11 days using Ukama tubers inoculated with F. solani in February and Bintje tubers, each inoculated at three marked sites with H. solani, in March. In addition to the EOs listed in Table 2, L. angustifolia, M. spicata and M. verticillata received from Vincenzo De Feo, P. balsamifera received from Lars Björk and T. vulgaris (H. solani experiment, only) were included. In the F. solani experiment there were five replicates per EO and dose, and in the H. solani experiment there were two replicates per EO and dose, each comprising eight tubers. Exposures were 3 weeks in both experiments.
In Vitro and In Vivo Experiments Including Various Doses in Single Applications
The effects on P. foveata of various oils, listed in Table 3, at concentrations between 10 and 320 ppm were investigated in an in vitro experiment, and in vivo at concentrations between 10 and 160 ppm. A. sativum EO was the only one used at 10 and 20 ppm and it was not included at 160 or 320 ppm. Appropriate amounts of oils were added as described earlier. In the in vitro experiment, there were five Petri dishes per EO and dose and the exposure lasted 2 weeks. In the in vivo experiment King Edward tubers were inoculated in February and exposures lasted 3 weeks. Per EO and dose there were three boxes, each containing eight tubers, leading to 16 inoculations per replicate. The same original conidial suspension was used for both experiments, which started the same day. For inoculation of tubers it contained 6.2 × 104 conidia per ml, and for plating on agar it was diluted tenfold.
Similar types of parallel in vitro and in vivo experiments were performed with F. solani at 40–160 ppm over 3 weeks. The conidial suspension contained 7.5 × 104 conidia per ml when inoculating tubers, cv. Escort, and it was diluted tenfold for use in in vitro experiments. A. sativum, C. carvi, M. piperita and S. officinalis oils were included.
In addition, two P. foveata in vitro experiments were repeated twice with the same EOs as those listed in Table 3, except that L. angustifolia, O. majorana and the Salvia species were not included and the lowest dose of A. sativum used was 20 ppm (included in one of these experiments). Also there were three more P. foveata in vivo experiments including various oils at concentrations between 40 and 277 ppm.
In Vitro R. solani Experiments Including Various Doses
The effects on the radial growth of R. solani of various oils, listed in Table 4, at concentrations between 40 and 320 ppm were investigated in two similar in vitro experiments. In each of these there were five Petri dishes per EO and dose and exposures lasted 2 weeks.
In Vivo P. foveata Experiment Including Delayed Start of EO Treatments After Inoculation
The effects of applying vapour treatments some time after inoculation with P. foveata were studied in an experiment starting in November using cv. Bintje. Various oils, indicated in Fig. 2, were added to the boxes 0, 4, 8 or 12 days after inoculation with a suspension having 9 × 104 conidia per ml. The amount of oil used was 300 μl, 92 ppm, and the exposure lasted 3 weeks. Per EO and time interval there were three boxes, each containing eight tubers, leading to 16 inoculations per replicate. Gangrene indexes were scored 8 weeks after inoculations in this experiment.
In Vivo P. foveata and F. solani Experiments Including Vapours of Macerated Plant Material
In two experiments the influence of vapours from garlic (A. sativum) or onion (A. cepa) bulbs or horseradish (Armoracia rusticana) roots on dry rot and gangrene development in artificially inoculated tubers was studied. Material was macerated using an electric household mincer (Fig. 3a). In the P. foveata experiment, tubers of cv. Bintje were inoculated in December (7.6 × 104 conidia per ml) and in the F. solani experiment, tubers of cv. Escort were inoculated in January (7.5 × 104 conidia per ml). Treatments lasted 3 weeks and were performed in 5.5-l glass desiccators wherein 50 g macerated plant material (in the F. solani experiment only 10 g of A. rusticana was used) was placed on the bottom and the inoculated tubers were rested above on the perforated ceramic disc. The dose thus achieved for the Allium species was 9.1 g l−1. There were three desiccators per treatment.
In the third of this type of experiments, performed in February, A. sativum bulbs were macerated manually using the finest side of a handhold grater, put in wide glass bowls at doses of 0.2, 0.04 or 0.02 g l−1 in 25-l plastic boxes for 3 weeks (Fig. 3b). There were three boxes per dose, each containing 20 King Edward tubers, inoculated with P. foveata (6.2 × 104 conidia per ml), which rested on a frame constructed of plastic mosquito netting.
Statistical Analyses
Statistical calculations were performed using GLM-ANOVA as implemented in NCSS, copyright Jerry L. Hintze, Kaysville, UT, USA. Since many treatments applied in the in vitro experiments resulted in zero values, while others resulted in large numbers of colony-forming units per dish, these skewed data sets were square-root-transformed according to the formula \(\surd \left( {x + 0.5} \right)\) before the calculations, where x is the number of colony-forming units per plate. The disease indexes obtained in the in vivo trials were used without further transformation. With very few exceptions, analysis of variance (ANOVA) revealed significant interactions between the factors oil and exposure time, or oil and concentration when all data from a trial were analysed by two-factorial ANOVA. Partly for this reason, and partly because the separate means for each treatment are of greater interest than the overall mean values, the data were partitioned and analysed by one-way ANOVA. In some cases, data from two experiments with the same experimental plan were combined and the experiment was then treated as for a random blocking factor. To facilitate the interpretation and comparisons of results from trials with differing units, treatment means of untransformed data are expressed relative to controls in all in vitro and most in vivo trials.
Results
In Vitro and In Vivo Experiments Including Exposures of 1, 2 or 3 Weeks
The results from in vitro experiments including exposures at 800 ppm of F. solani, P. foveata and H. solani conidial suspensions plated on agar for 1, 2 or 3 weeks are presented in Table 1. Many treatments significantly reduced the number of colony-forming units, in most cases more efficiently so with increasing treatment periods. None of the pathogens grew after any of the treatment periods using vapours of A. sativum or P. balsamifera. After a minimum of 2 weeks of exposure, M. spicata completely prevented growth of all three fungi too, while T. vulgaris and Anethum graveolens were then only effective against P. foveata and H. solani. After a treatment period of at least 3 weeks using C. carvi none of the organisms produced any colonies; for H. solani only 2 weeks was needed for complete control. Significantly more colonies of P. foveata developed when conidia were exposed to an atmosphere of M. piperita for 1 week, but not if the treatment was longer.
In an in vitro experiment with H. solani at a lower dose, 160 ppm, for 1, 2 or 3 weeks only a few colonies developed after the shortest treatment time with A. sativum, which was otherwise completely fungicidal, as was T. vulgaris oil in 3-week treatments (data not shown). Most of the other oils used were merely fungistatic under these experimental conditions, but the C. carvi oil significantly stimulated conidial germination in exposures of 1 week (relative value 213, P ≤ 0.001) or 2 weeks (relative value 194, P ≤ 0.001).
In Fig. 1 selected results from three in vivo experiments with F. solani, P. foveata and H. solani, respectively, examining the effects of exposures for 1–3 weeks at 77 ppm, are presented. F. solani and P. foveata disease developments were significantly reduced when A. sativum treatments lasted at least 2 weeks, while the H. solani disease index was reduced after a treatment period of 1 week. P. balsamifera EO significantly reduced the P. foveata disease index after just 1 week of exposure and the F. solani index after at least 2 weeks, but it only transiently reduced H. solani conidiophore development after 2 weeks (data not shown). M. piperita EO reduced the rot incidence of F. solani after 2 and 3 weeks of treatment and the disease index of H. solani after 3 weeks (data not shown). All oils included in the P. foveata experiment except A. sativum and P. balsamifera increased the gangrene incidence significantly (data not shown).
Relative values of disease caused by Fusarium solani, Phoma foveata and Helminthosporium solani, compared with untreated controls, having mean disease indexes of 6.2, 9.7 and 4.0, respectively, in artificially inoculated potato tubers after exposure to volatiles of Allium sativum essential oil at 77 ppm for 1, 2 or 3 weeks. Data selected from three experiments, one pathogen each, performed on different occasions, using different cultivars of potato
In Vitro and In Vivo Experiments Including Single and Split Doses
The results from in vitro and in vivo experiments with H. solani, in which the effects of single and split doses were compared, are presented in Table 2. A. sativum vapours completely prevented growth of the fungus in vitro and significantly reduced disease development in vivo at all doses. C. carvi significantly reduced disease in vivo at the split doses and the number of colony-forming units in vitro at single and split doses summing up to 160 ppm. M. piperita oil significantly controlled H. solani development in vivo at all 160-ppm doses and in vitro at split doses. For the threefold split, S. officinalis EO significantly reduced the H. solani disease index in vivo but had merely a fungistatic action in vitro for the 160 and 80 + 80 ppm applications. M. arvensis var. sachalinensis had a fungistatic action in vitro and was not effective in vivo, whereas the fungus was indifferent to all R. officinalis treatments.
The two in vivo F. solani and H. solani experiments including 77 ppm or 38.5 + 38.5 ppm applications again confirmed that A. sativum effectively controlled disease development of both pathogens, the relative infections compared with control values after single and split applications being significantly reduced to 41 and 37, and 11 and 17, for the two pathogens, respectively (data not shown). Also, S. officinalis significantly reduced the F. solani rot index to 79% of the control value at a single dose and the H. solani index to 30% for the split application, whereas P. balsamifera significantly increased the H. solani infection index to relative values of 227 and 167 for single and split applications (data not shown).
In Vitro and In Vivo Experiments Including Various Doses in Single Applications
The results from P. foveata experiments with EO doses between 10 and 320 ppm (in vitro) or 10 and 160 ppm (in vivo) are presented in Table 3. A. sativum EO completely prevented growth of the fungus in vitro at all doses (also at 20 ppm; data not shown) and significantly reduced gangrene development in vivo, even at the lowest dose of 10 ppm. Both Salvia species controlled disease development in tubers significantly although their actions in vitro were merely fungistatic or non-significant. T. vulgaris and Ocimum basilicum EOs on the other hand were not fungicidal in vivo but greatly and significantly reduced the numbers of colony-forming units in vitro. The Mentha species reduced gangrene to 70–77% of the index in control tubers at 160 ppm.
In parallel in vitro and in vivo F. solani experiments at 40–160 ppm, A. sativum oil was nearly completely fungicidal in vitro and significantly reduced dry rot development in vivo (data not shown). O. basilicum oil caused significantly increased dry rot development but had no visible stimulatory effect on conidia (data not shown). Across all additional in vivo P. foveata experiments of the single-dose type, A. sativum was most effective and significantly reduced rot indexes at all doses included, while C. carvi, O. basilicum, P. balsamifera and T. vulgaris showed inconsistent and weak, although sometimes significant, gangrene reduction (data not shown).
In Vitro R. solani Experiments Including Various Doses
The effects of 40–320 ppm of a range of EOs on mycelial growth of R. solani in vitro are presented in Table 4. A. sativum volatiles almost completely prevented the radial growth of R. solani mycelium; limited outgrowth occurred only at the lowest dose used. At the highest dose, 320 ppm, the C. carvi, M. arvensis var. sachalinensis, M. spicata, O. basilicum and T. vulgaris oils were fungicidal and at lower doses most of them were fungistatic. The domestic thyme oil was less effective than the imported one. A. graveolens and M. piperita EOs strongly prevented normal growth of the fungus at the highest dose and were fungistatic at lower ones. R. officinalis, L. angustifolia, S. officinalis and S. lavandulafolia EOs were merely fungistatic at higher doses, while Pogostemon patchouli EO did not influence the growth of R. solani at all.
In Vivo P. foveata Experiment Including Delayed Start of EO treatments After Inoculation
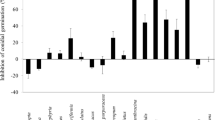
The results of an experiment in which tubers were inoculated with P. foveata and immediately exposed to vapour (equivalent to 92 ppm) or where exposure was delayed for up to 12 days after inoculation are presented in Fig. 2. When A. sativum, C. carvi and S. officinalis oils were used, the greatest reductions in gangrene severity occurred when tubers were concurrently inoculated with P. foveata and exposed to EO vapour. However, volatiles of M. piperita oil were as effective as A. sativum and S. officinalis oils if tubers were exposed 4–8 days after inoculation, allowing wounds to first heal for that period. The O. basilicum oil treatments resulted in slight, but significant reductions in gangrene development, compared with the controls, if tubers were exposed 4 or 12 days after inoculation. The T. vulgaris oil treatments did not decrease rotting of tubers at all.
Gangrene (Phoma foveata) index in tubers, cv. Bintje, after exposure to volatiles from essential oils for 3 weeks at 92 ppm, added 0, 4, 8 or 12 days after inoculation. Means of three replicates. Asterisks indicate that means are significantly different from the control mean (index 14.3) at P ≤ 0.05
In Vivo P. foveata and F. solani Experiments Including Vapours of Macerated Plant Material
Figure 3a illustrates the results of experiments in which tubers were inoculated with P. foveata or F. solani and exposed to the volatiles from macerated plant material at 9.1 g l−1 of Allium sativum and A. cepa. The mean disease indexes of unexposed control tubers were 13.5 and 5.2, respectively. Macerated A. sativum significantly reduced the severity of the diseases caused by both pathogens, while vapours of macerated A. cepa also greatly reduced the extent of rotting by both pathogens, but only gangrene significantly. Tubers exposed to the vapours of macerated A. rusticana at 9.1 g l−1 completely collapsed, precluding observations of their potential antifungal disease activities.
Relative incidence of gangrene caused by Phoma foveata, and dry rot caused by Fusarium solani var coeruleum, compared with unexposed controls, in artificially inoculated potato tubers after being exposed to vapours of macerated bulbs of onion (Allium cepa) or garlic (A. sativum) at 9.1 g l−1 (a) and the doses indicated (b) for 3 weeks. Data from three experiments, each with three replicates. Asterisks indicate that the means are significantly different from the control mean at P ≤ 0.05
Figure 3b illustrates the effect of macerated A. sativum at doses of 0.02, 0.04 and 0.2 g l−1 on gangrene development. The mean disease index of the unexposed control was 9.1. Only the highest dose caused a significant reduction in gangrene severity.
Discussion
The effects of EO volatiles on fungal potato tuber pathogens and disease development were investigated. Information on the main constituents of the oils used was provided by the suppliers. However, the major components of the oil may not be the principal substances in the headspace (Benjilali et al. 1984), and they may consequently not be those responsible for the effects observed in an experiment using volatiles. As the headspace in the treatment chambers was not analysed in the experiments reported here, the discussion will focus on the observed potential of EOs (rather than specific constituents) of various plant species for use as antifungal agents.
The results of the in vitro experiments clearly show that vapours of many plant extracts have fungicidal action on the pathogens studied provided that there is a sufficiently high concentration and the duration of exposure is sufficiently long. Even at a very high dose, 800 ppm, a 1-week treatment was too short for many of the EOs used to exert fungicidal effects on F. solani, P. foveata and H. solani (Table 1), and at a lower dose (160 ppm) 2- and 3-week treatments with the most potent oils (A. sativum and T. vulgaris) were, respectively, needed to completely prevent conidial germination of H. solani (data reported but not shown). These results were corroborated by data from in vivo experiments, showing that an exposure for 1 week is too short for good control of disease development, even when using the very effective A. sativum EO (Fig. 1).
In some of the in vitro experiments, a short exposure to EOs resulted in increases in the numbers of colony-forming units at the final reading; for instance a 1-week exposure of P. foveata to volatiles of M. piperita oil at 800 ppm (Table 1), and either 1-week or 2-week exposures of H. solani to caraway vapours at 160 ppm (data reported but not shown). Over a longer exposure period (3 weeks) this stimulatory influence was not observed and in another experiment with a similar dose and an exposure period of 5 weeks the caraway oil strongly reduced the numbers of H. solani colony-forming units (Table 2). In these cases stress on the fungi caused by a sublethal dose seems to have stimulated the subsequent germination of conidia. Similarly, initial inhibition of growth and toxin production of toxin-producing moulds was followed by increased fungal activity after treatment with citral, citronellal and eugenol in studies by Mahmoud (1994). In addition, increases in the incidence of disease caused by P. foveata, H. solani and F. solani were observed after vapour treatments of inoculated tubers using several EOs (data reported but not shown).
Interpretation of data from the in vivo system is, however, less straightforward. Increases or decreases in the severity or frequency of attacks, compared with controls, could be due to influences of volatiles on the pathogen, or on the host, or interactions between these variables. If the wound healing or defence activities of the host are impeded in any way by the volatiles, the ability of the pathogens to cause diseases would be expected to be higher in their presence than in unexposed controls. Figure 2 shows data from an experiment in which tubers were exposed to EOs up to 12 days after inoculation with P. foveata. The results from this experiment do not indicate that the defence or wound healing reactions were impaired in the prevailing experimental conditions. None of the treatments increased the gangrene index in this experiment. However, with an interval of at least 4 days between inoculation and start of exposure, the M. piperita oil at 92 ppm, which had not previously displayed strong activity against P. foveata in vivo in other experiments at similar doses, was as effective as A. sativum and S. officinalis oils applied concurrently with inoculation, although both the wound healing and the infection processes had progressed for 4 days before the exposure. Such responses might occur if a very volatile antifungal compound is accumulated in the host tissues at the site of pathogen attack, thus becoming more effective. In support of this hypothesis, Oosterhaven et al. (1995b) found higher amounts of (S)-carvone, and its metabolic products, in tissues that had been wounded and allowed to heal for 2 days or more before exposure than in tissues exposed directly after wounding. Split applications of M. piperita EO (Table 2) had stronger antifungal effects on H. solani in vitro than single initial application of the same total amount, indicating that the biologically active compounds in the steam phase of this oil is very volatile, quickly escaping from the experimental systems used.
T. vulgaris oil showed strong antifungal potential in the in vitro system against all pathogens included (Tables 1, 3, 4) but repeatedly failed to control gangrene development in inoculated tubers in vivo (Fig. 2, Table 3). Different results may be due to the use of different batches of EO since the composition of the oils may depend (inter alia) on the cultivation site and the cultivar of the source plants, harvest dates and handling procedures (Hay 1993). Differences may also be due to variations in the sensitivity of the isolates used, as shown for F. sambucinum by Vaughn and Spencer (1994). However, in the parallel P. foveata experiments (Table 3), similar doses, the same oil batches and the same original conidial suspensions were used. In accordance with results presented here, T. vulgaris EO has been reported to give diverging results in vitro and in vivo against Phytophthora infestans (Quintanilla et al. 2002), and Guynot et al. (2003) found it to have potent fungicidal activity on bread spoilage fungi in vitro, but only weak effects in tests with sponge cake analogues. In addition, Arras and Usai (2001) found the effects of T. capitatus oil against Penicillium digitatum on orange fruits to be weaker than expectations based on in vitro results. Some of the cited authors have argued that the lipophilic compounds in EOs may interact with food components, such as proteins and lipids, thereby decreasing their activity. This seems to be a plausible explanation, especially if the substrate is not a living host. If so, however, another possibility is that the biologically active compounds may be converted to less toxic substances by the host. Bioconversion of volatiles has been demonstrated in studies with strawberries (Hamilton-Kemp et al. 1996; Archbold et al. 1997), and Oosterhaven et al. (1995b, c) found that (S)-carvone was converted (mainly to neoisodihydrocarveol) by wounded potatoes.
In contrast to T. vulgaris oil, oils from Salvia species did not appear to be an effective natural fungicide against P. foveata when evaluated in the in vitro system, but significantly reduced gangrene in vivo at similar doses in parallel experiments using the same batch of oil and inoculum source (Table 3). Also, in vivo S. officinalis was as effective as A. sativum oil at 92 ppm (Fig. 2). Similarly, conflicting data were obtained in investigations of the activity of S. officinalis oil on H. solani. Neither single nor split doses showed fungistatic activity in vitro, but a threefold split used in vivo significantly reduced disease development in parallel experiments (Table 2). It also reduced dry rot development, although not significantly so, but had no visible effect in vitro on F. solani (data not shown). Such disparities could occur if the host converts volatiles in the EO into substances with greater toxicity to the pathogen, or if the treatment triggers an effective defence reaction in the host. Stronger antifungal effects of an EO in vivo than in vitro have also been observed by Quintanilla et al. (2002), who found that hyssop (Hyssopus officinalis) oil had very limited antifungal activity in plate assays against P. infestans, but strongly inhibited disease when sprayed as a dilute solution on potato plants that were challenge-inoculated with a zoospore suspension of P. infestans 3 days later. Quintanilla et al. (2002) speculate that the differences in results between in vivo and in vitro tests might be due to induced resistance of the host.
A. sativum EO showed the strongest effects against all four pathogens in vitro and in vivo, consistently giving significant reductions in pathogen development and disease compared with controls. Gangrene development was significantly reduced at 10 ppm (Table 3). Vapours from A. sativum EO have also been found to have potential against bread spoilage fungi in a study by Nielsen and Rios (2000), although they were less effective than mustard oil at 16 ppm (applications of 1 μl per Petri dish). In addition, volatiles from macerated bulbs controlled gangrene development (Fig. 3), indicating a possible practical use in small-scale organic farming systems. Clearly, a potential disease control agent should not inflict damage on the host itself. In contrast to the strong reaction of potato tubers to horseradish (A. rusticana) volatiles, A. sativum EO did not cause any visible damage to tubers in any of the experiments presented here. Thus, it stands out as the most promising plant EO for use as a natural fungicide of tuberborne diseases. The effects of treating seed tubers with vapours of A. sativum and other EOs on sprouting, disease development and yield have been studied in field trials, the results of which will be presented elsewhere.
Abbreviations
- cfu:
-
colony-forming units
- EO:
-
essential oil
References
Aliaga TJ, Feldheim W (1985) Hemmung der Keimbildung bei gelagerten Kartoffeln durch das ätherische Öl der südamerikanischen Munapflanze (Minthostachys spp.). Ernahrung 9:254–256
Archbold DD, Hamilton-Kemp TR, Bart MM, Langlois BE (1997) Identifying natural volatile compounds that control gray mold (Botrytis cinerea) during postharvest storage of strawberry, blackberry and grape. J Agric Food Chem 45:4032–4037
Arras G, Usai M (2001) Fungitoxic activity of 12 essential oils against four postharvest citrus pathogens: chemical analysis of Thymus capitatus oil and its effect in subatmospheric pressure conditions. J Food Prot 64:1025–1029
Bång U (1989) Effect of haulm treatment and harvest time on incidence of tuber rots of potato (Solanum tuberosum L.) after standard wounding and on frequency of stem lesions caused by Phoma foveata Foister. Potato Res 32:101–112
Benjilali B, Tantaoui-Elaraki A, Ayadi A, Ihlal M (1984) Method to study antimicrobial effects of essential oils: application to the antifungal activity of six Moroccan essences. J Food Prot 47:748–752
Gorris LGM, Oosterhaven K, Hartmans KJ, De Witte Y, Smid EJ (1994) Control of storage diseases of potato by use of plant essential oil components. In: Brighton crop protection conference—pests and diseases—1994, 3D-20, pp 307–312
Guynot ME, Ramos AJ, Setó L, Purroy P, Sanchis V, Marín S (2003) Antifungal activity of volatile compounds generated by essential oils against fungi commonly causing deterioration of bakery products. J Appl Microbiol 94:893–899
Hamilton-Kemp TR, Archbold DD, Loughrin JH, Collins RW, Byers ME (1996) Metabolism of natural volatile compounds by strawberry fruit. J Agric Food Chem 44:2802–2805
Hartmans KJ, Diepenhorst P, Bakker W, Gorris LGM (1995) The use of carvone in agriculture: sprout suppression of potatoes and antifungal activity against potato tuber and other plant diseases. Ind Crops Prod 4:3–13
Hay RKM (1993) Physiology. In: Hay RKM, Waterman PG (eds) Volatile oil crops. Longman, Harlow, pp 23–43
Löschenkohl B (1979) Phoma exigua var. foveata på kartoffelknolde, en anatomisk beskrivelse af patogenese og knoldresistens. (Phoma exigua var. foveata infection of potato tubers, a description of pathogen development and tuber resistance) Dissertation, Kongelige Veterinaer- og Landbohöjskole, Copenhagen
Mahmoud ALE (1994) Antifungal action and antiaflatoxigenic properties of some essential oil constituents. Lett Appl Microbiol 19:110–113
Mayton HS, Olivier C, Vaughn SF, Loria R (1996) Correlation of fungicidal activity of Brassica species with allyl isothiocyanate production in macerated leaf tissue. Phytopathology 86:267–271
Meigh DF (1969) Suppression of sprouting in stored potatoes by volatile organic compounds. J Sci Food Agric 20:159–164
Nielsen PV, Rios R (2000) Inhibition of fungal growth on bread by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. Int J Food Microbiol 60:219–229
Olsson K (1988) Resistance to gangrene (Phoma exigua var. foveata) and dry rot (Fusarium solani var. coeruleum) in potato tubers. 1. The influence of pectin-bound magnesium and calcium. Potato Res 31:413–422
Oosterhaven K, Poolman B, Smid EJ (1995a) S-Carvone as a natural potato sprout inhibiting, fungistatic and bacteriostatic compound. Ind Crops Prod 4:23–31
Oosterhaven K, Hartmans KJ, Scheffer JJC, van der Plas LWH (1995b) Inhibitory effect of S-carvone on wound healing of potato tuber tissue. Physiol Plant 93:225–232
Oosterhaven K, Hartmans KJ Scheffer JJ, van der Plas LHW (1995c) S-carvone inhibits phenylalanine ammonia lyase (PAL) activity and suberization during wound healing of potato tubers. J Plant Physiol 146:288–294
Quintanilla P, Rohloff J, Iversen TH (2002) Influence of essential oils on Phytophthora infestans. Potato Res 45:225–235
Vaughn SF, Spencer GF (1993) Naturally-occurring aromatic compounds inhibit potato tuber sprouting. Am Potato J 70:527–533
Vaughn SF, Spencer GF (1994) Antifungal activity of natural compounds against thiabendazole-resistant Fusarium sambucinum strains. J Agric Food Chem 42:200–203
Vokou D, Vareltzidou S, Katinakis P (1993) Effects of aromatic plants on potato storage: sprout suppression and antimicrobial activity. Agric Ecosyst Environ 47:223–235
Acknowledgements
These investigations were supported by the Swedish Farmers Foundation for Agricultural Research, SLF and the Swedish Board of Agriculture. The EO of M. verticillata was kindly provided by Vincenzo De Feo, University of Salerno, Italy, those of P. balsamifera, Tagetes mexicana and T. rosola by Lars Björk, Swedish University of Agricultural Sciences, Balsgård, Sweden, and that of Mentha arvensis var. sachalinensis by T.H. Iversen, University of Trondheim, Norway. The technical assistance of Britt-Marie Andersson, Malin Barrlund and Lars Wallgren is also greatly appreciated, as is the language editing by John Blackwell.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bång, U. Screening of Natural Plant Volatiles to Control the Potato (Solanum tuberosum) Pathogens Helminthosporium solani, Fusarium solani, Phoma foveata and Rhizoctonia solani . Potato Res. 50, 185–203 (2007). https://doi.org/10.1007/s11540-008-9044-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11540-008-9044-y