Abstract
This research presents estimates of HIV prevalence rates among injection drug users (IDUs) in large US metropolitan statistical areas (MSAs) during 1992–2002. Trend data on HIV prevalence rates in geographic areas over time are important for research on determinants of changes in HIV among IDUs. Such data also provide a foundation for the design and implementation of structural interventions for preventing the spread of HIV among IDUs. Our estimates of HIV prevalence rates among IDUs in 96 US MSAs during 1992–2002 are derived from four independent sets of data: (1) research-based HIV prevalence rate estimates; (2) Centers for Disease Control and Prevention Voluntary HIV Counseling and Testing data (CDC CTS); (3) data on the number of people living with AIDS compiled by the CDC (PLWAs); and (4) estimates of HIV prevalence in the US. From these, we calculated two independent sets of estimates: (1) calculating CTS-based Method (CBM) using regression adjustments to CDC CTS; and (2) calculating the PLWA-based Method (PBM) by taking the ratio of the number of injectors living with HIV to the numbers of injectors living in the MSA. We take the mean of CBM and PBM to calculate over all HIV prevalence rates for 1992–2002. We evaluated trends in IDU HIV prevalence rates by calculating estimated annual percentage changes (EAPCs) for each MSA. During 1992–2002, HIV prevalence rates declined in 85 (88.5%) of the 96 MSAs, with EAPCs ranging from −12.9% to −2.1% (mean EAPC = −6.5%; p < 0.01). Across the 96 MSAs, collectively, the annual mean HIV prevalence rate declined from 11.2% in 1992 to 6.2 in 2002 (EAPC, −6.4%; p < 0.01). Similarly, the median HIV prevalence rate declined from 8.1% to 4.4% (EAPC, −6.5%; p < 0.01). The maximum HIV prevalence rate across the 11 years declined from 43.5% to 22.8% (EAPC, −6.7%; p < 0.01). Declining HIV prevalence rates may reflect high continuing mortality among infected IDUs, as well as primary HIV prevention for non-infected IDUs and self-protection efforts by them. These results warrant further research into the population dynamics of disease progression, access to health services, and the effects of HIV prevention interventions for IDUs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Almost 30 years after the HIV epidemic among injection drug users (IDUs) was recognized, scientific understanding of the nature, causes, and consequences of geographic and temporal variability in HIV prevalence rates among IDUs remains somewhat limited. Earlier studies, such as the National AIDS Demonstration Research (NADR) project and the World Health Organization’s Multi-Center, revealed geographic variability in HIV prevalence and incidence rates among IDUs across cities.1–6 More recent studies have also found considerable geographic variation across US cities,7–9 as well as across cities internationally.10
Though many epidemiologists do not completely understand how epidemics among IDUs start, it is known that outbreaks can arise very quickly. Friedman and Des Jarlais11 showed this for a wide range of European cities as well as American and South Asian cities. Further, support for this is evident from the history of epidemics in places like New York City in the mid-1970s,12 , 13 Southeast Asia in the late 1980s and early 1990s,14 Vancouver in the 1990s,15 , 16 and, more recently, China, Vietnam,17 , 18 Iran,19 and Russia.20
We have nonetheless been hampered in our ability to understand the spread and prevalence of HIV among IDUs by the lack of comparable interurban data over time and space. The absence of such data limits our knowledge about processes associated with the rise and fall of epidemics among IDUs—a necessary basis for understanding the origins and paths of large IDU-associated HIV epidemics. The aim of this research is to estimate HIV prevalence rates among IDUs in 96 US metropolitan statistical areas (MSAs) from 1992 to 2002, and evaluate trends over time. Here, we present a method of calculating HIV prevalence rate estimates annually during an 11-year period. We examine changes in temporal trends in HIV prevalence rates by computing estimated annual percent change (EAPC) for each MSA. We also validate the findings and discuss the limitations of our data.
Geographic-specific data over time on HIV prevalence rates among IDUs are important as they may help direct policy makers and concerned public in allocating resources and establishing public policy. Such data can provide a foundation for the design and implementation of structural interventions for preventing the spread of HIV epidemics among IDUs.
Historical Data on Differences in HIV Prevalence among IDUs in MSAs
Holmberg21 developed a components model to estimate HIV prevalence and incidence rates in 1992 among IDUs, men who have sex with men (MSM), and high-risk heterosexuals in 96 MSAs in the US with population >500,000. Until then, data on HIV among IDUs had been limited to a relatively limited number of cities (e.g., New York City, San Francisco, Chicago, Miami).12 , 13 In addition to providing a useful description of the number of IDUs and of the HIV epidemic in US metropolitan areas, data of this kind provide the basis for comparative analysis to study what metropolitan area characteristics predict (a) the extent to which the local population injects drugs; (b) HIV prevalence among IDUs; and (c) HIV incidence among IDUs. Thus, Holmberg’s estimates allowed for broad geographic comparisons across MSAs and risk-populations.
His estimates were derived from a variety of published and unpublished studies, as well as data from a variety of drug treatment and publicly funded HIV counseling and testing sites. These were validated by comparison with a set of criteria specifying interval bounds for “reasonable” estimates. Estimates that approximately satisfied all inclusion criteria were averaged to provide an overall estimate.
Using these methods, Holmberg estimated that HIV prevalence rates among IDUs ranged from 1% to 41% (with median 5.9% and considerable right-skewing) across MSAs in 1992, and that incidence rates varied from 0.2 to 4.9 per 100 person-years at risk (Table 1).
Friedman et al.22 estimated 1998 HIV prevalence rates among IDUs within the same 96 large MSAs as Holmberg. These estimates were based on (1) adjusting Centers for Disease Control (CDC) Counseling and Testing data by regression imputation techniques with research study data, and (2) estimates of the ratio of the number of IDUs living with HIV to the number of IDUs living in an MSA. The validity of the resulting estimates was assessed, and mean values were used as best estimates. The estimates varied from 2.4% to 27.4% across MSAs, with a mean of 7.9% and a median of 5.9% for IDUs. Results from this study indicate that most MSAs continued to have HIV prevalence rates among IDUs less than 10%, and approximately 40% of MSAs had prevalence rates less than 5%.
Because the methods used by Holmberg were no longer feasible, Friedman et al. worked out modified ways to estimate HIV prevalence rates among IDUs. Dissimilarity in methods and problems of regression to (and from) the mean, limited our ability to study change in these estimates and makes interurban comparisons between Holmberg’s 199221 estimates and Friedman’s 199822 HIV prevalence estimates problematic. Developing our HIV prevalence rates among IDUs over time will enhance our capacity to study the determinants of changes in HIV epidemics. Annual data allow us to use longitudinal statistical methods to make inferences about causes of change in IDU-related HIV transmission and factors associated with program changes and design.
Methods
Overview
Our estimates of HIV prevalence rates among IDUs in the 96 MSAs of interest during 1992–2002 were derived from four independent sets of data: (1) research-based HIV prevalence rate estimates compiled from published literature, conference abstracts, web-based searches and inquiries of researchers to find HIV prevalence rate estimates among IDUs; (2) Centers for Disease Control and Prevention Voluntary HIV Counseling and Testing data (CDC CTS unpublished data 1992–2002); (3) data on the number of people living with AIDS (PLWAs unpublished data 1992–2002), compiled by the CDC; and (4) estimates of HIV prevalence in the US (Holtgrave personal communication 3/3/2008).( 23 )
Using these data sources, we developed two sets of estimates based on independent methodologies: (1) the CTS-based method (CBM) and (2) the PLWA-based method (PBM). The CBM modifies the approach of Friedman22 for longitudinal data. In calculating the CBM estimates, we used a regression model in which research-based estimates were regressed on CDC CTS estimates to correct for bias in CDC CTS data due to the fact that people testing positive for HIV tend not to get retested.24–26 In the CBM, we corrected for four types of missing data in the CDC CTS data set:
-
1.
Missing values (suppressed) known to be between 0 and 4 HIV-positive tests;
-
2.
Missing data for 1–3 years (but not all years on number of IDUs tested);
-
3.
Missing data for four or more years, or reporting a dramatic drop or increaseFootnote 1 in the number of IDUs tested, which accounted for nine MSAsFootnote 2. For these MSAs, prevalence rates could not be determined or imputed based on lack of trend data. As a result, we treated these MSAs as missing data for all 11 years
-
4.
Missing data for all 11 years which accounted for five MSAsFootnote 3 (i.e., CDC does not collect data in these areas)
PBM estimates22 , 27 , 28 were based on the annual number of living persons reported with AIDS in each MSA and the estimated total number of persons living with HIV or AIDS (PLWHAs) in the US and were adapted for longitudinal data.
CBM and PBM estimated HIV prevalence rates were smoothed and then averaged to create our final “best estimates.” In the following subsections, we describe each stage in calculating our HIV prevalence rates for all 96 MSAs in greater detail.
Unit of Analysis and Sample
We studied the 96 largest MSAs as defined by the 1993 census boundary file.29 MSAs are defined by the US Census Bureau as contiguous counties that contain a central city of 50,000 people or more and that form a socioeconomic unity as defined by commuting patterns and social and economic integration among the constituent counties. The MSA was chosen as the unit of analysis for three reasons. First, it allows continuity with 1992 HIV prevalence rates from Holmberg21 and 1998 HIV prevalence rates from Friedman et al.22 Second, health data are more available for the county units that comprise MSAs than for municipalities. Third, the economic, social, and commuting unity of metropolitan areas makes them a reasonable unit in which to study drug-related HIV and other epidemics.22
Research-based Estimates for CBM
-
Stage 1
Compiling research estimates
Our research estimates were based on a review of published literature and conference abstracts, as well as web-based searches and inquiries of researchers to find HIV prevalence rate estimates among IDUs in the 96 MSAs of interest. To be eligible, a study had to have been conducted during 1992–2002 and to have determined HIV serostatus through the testing of blood, urine, or saliva samples rather than through self-report. An additional inclusion criterion was that the study could not have been part of the CDC CTS system. We identified eligible research-based estimates from 33 of 96 metropolitan areas totaling 131 data points over time (Electronic Supplementary Material). The annual number of research-based estimates declined over time (i.e., after 1997), and were concentrated mainly within MSAs having substantial research institutions with an interest in drug users and/or tending to have highly populated central cities (i.e., New York City, Chicago, Baltimore, Seattle and San Francisco).
The research studies were categorized by setting: (1) drug treatment centers and methadone maintenance treatment programs (MMTP); (2) syringe exchange programs (SEPs); (3) sexually transmitted disease (STD) clinics; (4) prisons; (5) street outreach/network; and (6) all other settings, e.g., those where homeless persons are found). Analysis of variance showed there were no large differences in HIV prevalence rates across study settings. Accordingly, the regression model included all research study categories without adjustment for study category.
CTS-based Method (CBM)
-
Stage 2
Correcting for suppressed cell values as reported by the CDC CTS
CDC provided data on numbers of HIV-positive tests among IDUs in publicly funded counseling and testing sites (duplicate count, not unique individuals) for each MSA. However, all counts that were <5 were reported as missing by the CDC CTS. Where this occurred, and where data on HIV prevalence rate trends were sufficient to determine trends, imputation methods were applied to derive “best estimates” within an MSA. This accounted for 28 MSAs with 118 suppressed cells. Imputations considered both the fact that the number of people positive had to be an integer under 5 and temporal trends in the proportion of IDUs testing positive in the MSA could be determined. In some cases, it could not be determined whether data missing was based on a value <5, or based on the lack of temporal trends in testing. In these specific cases where this occurred we treated these data in the cell as missing and dealt with it in Stage 3 of our estimation model.
-
Stage 3
Regression adjustments to CDC CTS-based on research estimates
After imputing missing values <5, we then used a regression model to predict HIV prevalence rates based on our research estimates to correct for bias in CDC CTS data (i.e., positives tend not to get retested and duplicate counts).24–26 Included in our regression model was an adjustment for time to the advent of highly active antiretroviral therapies (HAART) in 1996. Since then, HAART has become the standard of care for the treatment of HIV-infected individuals. As HAART has become more widely used, AIDS researchers have seen an increase in the time from HIV infection to a diagnosis of AIDS, and an increase in survival time from a diagnosis of AIDS to death.23 Thus, as survival time increases prevalence rates in the research estimates we needed to adjust for this in our equation.
During our study period, access to HAART changed over time and took time to reach IDUs—thus, we assume January 1, 1998 as a uniform date for which IDUs had access to HAART. To adjust for this in our estimation model, we assume that IDUs in all MSAs have equal access to HAART, starting after 1997. The pre-HAART period was defined as 1992–1997, and the post-HAART period was defined as 1998–2002. The pre-HAART time variable was defined as equal to six in 1992 and to decrease by one each year until 1997; from 1998 to 2002, the pre-HAART time variable was set equal to zero. The post-HAART time variable was set equal to zero from 1992 to 1997; in 1998 the post-HAART variable was defined as one and set to increase by one each year until 2002.
The resulting predictor equation for research-based estimates of HIV prevalence rates (R 2 = 0.77) was
-
Stage 4
Interpolating and extrapolating where the estimate of HIV prevalence rate was missing for some years but not all years for a specified MSA
We next interpolated and extrapolated using generalized linear regression modeling for MSAs where specific years of HIV prevalence rates were missing from the CDC CTS. The model to correct for missing data included linear and quadratic effects of time measured as years since 1992. This gave a separate and independent regression equation for each MSA and year where values of rates were missing. There were seven MSAs that fell into this categoryFootnote 4, accounting for 12 missing values. Four of the seven MSAs each had one missing value, one MSA had two, and two MSA had three missing values.
After computing stages 1–3, our CBM provided HIV prevalence rates for 82 MSAs totaling 902 points for years 1992–2002.
-
Stage 5
Predicting missing estimates for CBM prevalence rates based on PBM
PBM HIV prevalence rates were available for all 11 years and all 96 MSAs, but there were only 82 MSAs for which we had CBM estimates based on CDC CTS. In stage 5 of computing our CBM estimates, we apply a linear regression equation to the remaining 14 MSAs. Thus, we utilize the PBM estimates to predict missing CDC CTS values in 14 MSAs that were missing 11 years of data from CDC CTS. Here, we apply the following generalized linear equation which predicts missing estimates for CBM prevalence rates based on PBM.
In the last stages of our estimation model, we smoothed the final CBM and PBM estimates for all MSAs and years, and then averaged the two smoothed final estimates.
PLWA-based Method (PBM)
Overview
The PBM was derived from an existing model which describes the relationship among estimates of IDU HIV prevalence rates, IDU HIV prevalence, and numbers of IDUs in a given population.27 This model has previously produced plausible estimates of HIV prevalence rates and HIV prevalence among risk-populations.22 , 27 , 28
In short, the estimated total number of HIV-infected IDUs residing in a given MSA and year, (i.e., the HIV prevalence estimate, or estimated number of PLWHAs), was designated as k. The estimated total number of IDUs (a) and the estimated HIV prevalence rate among IDUs (b) were related by the function, k = ab; thus, b = k / a. Year-specific values of the estimated number of IDUs, a, for the MSAs were taken from previous research by Brady et al.30 Values of k and b were estimated using two parameters.
-
Stage 1
Estimating Parameter1—an expansion factor equal to the annual ratio of US total HIV prevalence (PLWHAs) to US total number of persons living with AIDS (PLWAs).
The purpose of estimating parameter 1 is to extrapolate from the number of reported PLWAs to the estimated number of PLWHAs through the end of each year. Thus, the reservoir of all HIV-infected persons (PLWHAs, or the HIV prevalence estimate) includes the (known) number of reported persons living with AIDS (PLWAs), as well as the (unknown) numbers of persons alive and diagnosed with AIDS but not reported; the number of persons living with diagnosed HIV; and the number of undiagnosed HIV-infected persons. To obtain annual estimates of the number of PLWHAs (variable k), we used a multiplier (expansion factor) for each year for the US, applying it to the number of reported PLWAs.
We relied on two data sources for this first parameter: (1) reported annual data from CDC on PLWAs for the entire US by risk factor, and (2) annual estimates of HIV prevalence for the entire US, 1992–2002 (Holtgrave personal communication).23 We obtained annual data from CDC on the total number of PLWAs in the US and 96 MSAs by exposure category. For each year, we estimated the number of HIV-infected IDUs (HIV prevalence estimate, k) by multiplying the MSA-specific numbers of IDU PLWAs by the year-specific expansion factor.
-
(1)
Annual Expansion Factor = (Estimated total number of PLWHAs in the US in that year) / (Total number of reported PLWAs in the US in that year)
-
(2)
Estimated k = Number of IDU PLWHAsyear i, MSA j = (Number of IDU PLWAsyear i, MSA j )* (Expansion factoryear i )
This estimates values of k, the numerator of the model’s equation. Since the variable of interest, b (HIV prevalence rateyear i, MSA j ), equals k / a, we need values of the denominator, a (the estimated number of IDUs in year i, MSA j ), to solve for b. We rely on research by Brady30 for estimates of year- and MSA-specific numbers of IDUs.
Parameter 1 assumes that the year-specific expansion factor does not materially vary by MSA. It also assumes that the distribution of PLWHAs by HIV risk factor is similar to the distribution of the known PLWAs. Lastly, PLWA case data is defined as those injectors who have a history of injection drug use (i.e., any drug injection since 1977) following the CDC convention for classifying HIV exposure category.25
-
Stage 2
Estimating Parameter 2—an adjustment factor to account for differences in HIV prevalence rates via the CBM and the PBM
For the 85 MSAs where estimates were available by both methods, the annual MSA-specific PBM HIV prevalence rate estimates were highly correlated with the CBM estimates, but were systematically greater than the CBM estimates. We determined that the PBM estimates were most likely artificially inflated because they were partially based on national estimates of HIV prevalence and because the number of HIV-infected IDUs may be increasing over time in localities outside of the 96 MSAs—i.e., the epidemic is diffusing to small metropolitan areas and non-metropolitan areas.31–37 We adjusted for this systematic difference by computing Parameter 2 for each year, which equals the ratio of the overall PBM weighted average rates to the overall CBM weighted average rates. Weighting depended on the estimated size of the IDU populations,30 so that, for example, a large MSA like New York contributed more to the weighted average rate than a smaller MSA like San Francisco. The weighted average HIV prevalence rates for a given year were computed algebraically, equaling the sum of the HIV prevalence estimates (i.e., the estimated number of IDU PLWHAs) across the MSAs (k) divided by the sum of the estimated number of IDUs across the MSAs (a).
-
(1)
Parameter 2year i = (PBM weighted average HIV prevalence rateyear i) / (CBM weighted average HIV prevalence rateyear i)
The adjusted PBM rates then equal the unadjusted PBM rates divided by Parameter 2
-
(2)
PBM adjusted HIV prevalence rate in MSA j , year i = (PBM unadjusted rateyear i, MSA j ) / Parameter 2year i
Combined Final Best Estimates
Lastly, to minimize random error, we first smoothed the final CBM and PBM estimates for all MSAs and years, and then averaged the two smoothed final estimates. The end results are the “final best HIV prevalence rate estimates”—i.e., the smoothed average of the CBM and the PBM HIV prevalence rates by MSA for each year. The data were smoothed using loess regression, which fits curves to noisy data and smoothes data in a manner similar to computing a weighted moving average.38
“Sample” and its Implications for Statistical Analyses
This is a study of 96 metropolitan areas that were the largest MSAs in the United States in 1990. Thus, it is a study of a “population” rather than of a sample. This has several implications. First, it means that there is no sampling error (though there is measurement error). There is some debate over whether issues of statistical inference, (e.g., p-values), have any scientific applicability in this research. Second, statistical analyses are primarily descriptive rather than inferential. As a result, p-values generated in this analysis will be used mainly as a heuristic device.39
Lastly, there is no universe for which these findings can be generalized except the universe being studied. Since these 96 MSAs had a population of 159 million (62% of the U.S. population in 1993), and an estimated 1.5 million IDUs in the early 1990s, this universe is of great public health importance in its own right. It will nonetheless be possible to develop hypotheses about the implications of these findings for other localities. With appropriate caution, findings in this research can consider the extent to which findings are relevant to smaller urban areas in the U.S., or to metropolitan areas in other countries.
Validating the HIV Prevalence Rate Estimates
Several procedures were used to validate our estimates. First, we used a paired t-test and examined a single measure, absolute agreement intraclass correlation coefficient (ICC),40 which counts mean differences as a mismatch, between our 1992 best HIV prevalence rate estimates and Holmberg’s 199221 HIV prevalence rate estimates. We did the same for our 1998 best HIV prevalence rate estimates and the Friedman 199822 HIV prevalence rate estimates. We also use descriptive statistics to provide simple summaries about the sample and the measures of our HIV prevalence rates over time.
We further validated our estimates by correlating them with measures of theoretically-related constructs:1 hard-core drug arrests per capita;2 police protection expenditures per capita;3 corrections expenditures per capita;4,41 income inequality;5 poverty; and6 anti-over-the-counter (OTC) syringe laws.7 , 22 , 39 , 42 , 43
Hard-core drug arrests per capita; police protection expenditures per capita; and corrections expenditures per capita
Theoretically, these variables characterize a “criminal justice” approach to social problems, an approach consistent with hostility toward drug users in general and programs that help reduce harm. Friedman et al.41 found that higher rates of legal repressiveness (hard drug arrests; police employees per capita; and corrections expenditures per capita) are associated with higher HIV prevalence rates among injectors. Aggressive police tactics and/or stigmatization may lead IDUs to engage in hurried injection behaviors, to share syringes more often, and/or to inject in high-risk environments.44–48
The number of hard drug arrests (per 10,000 population) for possession of cocaine or heroin comes from the Uniform Crime Reporting Program: County-Level Detailed Arrest and Offense Data (1992–2002);49 the police protection expenditures per capita and corrections expenditures per capita are taken from United States Census Bureau data on Government Finances (1992;97;2002).50–52
Poverty and Income inequality
Both have been found to be related to a wide range of morbidity and mortality rates at the neighborhood and MSA level.53–57 Poverty index comes from the United States Bureau of the Census (2000);58 and income inequality is the ratio of income received by the top 10% to that received by the bottom 10% (Harper unpublished data, 7/18/2005 University of Michigan).
Anti-OTC laws
States regulate syringe access through OTC laws; these laws work against IDUs having access to clean syringes. Previous research has shown that OTC laws are related to HIV prevalence and incidence rates among IDUs.22 , 39 , 43 , 59 Data on OTC syringe laws were derived from Burris et al.60
Trend Analysis
We describe trends in HIV prevalence rates by computing estimated annual percent change (EAPC) and 95% confidence intervals (CIs) for each MSA based on the Surveillance, Epidemiology, and End Results (SEER) methods established by the National Cancer Act of 1971. The EAPC was calculated by regressing the log of the HIV prevalence rates against the year. We used the MSA- and year-specific averages of the unsmoothed CBM and PBM HIV prevalence rates as a basis for calculating the EAPC.
Statistical analyses were conducted using SAS version 9.1.61 EAPCs and 95% CIs were calculated using R software version 2.7.1.62
Results
Descriptive statistics
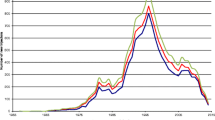
Across the 96 MSAs, collectively, the mean HIV prevalence rate declined from 11.2% in 1992 to 6.2% in 2002 (Table 2; EAPC, −6.4%; 95% CI, −7.0% to −5.7%; p < 0.001; Table 3). Similarly, the median HIV prevalence rates declined from 8.1% to 4.4% (EAPC, −6.5%; 95% CI, −7.3% to −5.6%; p < 0.001). The maximum HIV prevalence rate across the 11 years also showed a significant decline, ranging from 43.5% (1992) to 22.8% (2002) (EAPC, −6.7%; 95% CI, −7.6% to −5.8%; p < 0.001).
Correlations and Prevalence rates
The schedule of unadjusted HIV prevalence rates by MSA according to the CBM and the PBM were well correlated for each of the 11 years (Pearson r-squared ranged from 0.42 to 0.79; p < 0.01); (mean r-squared = 0.67; median r-squared = 0.71; SD = 0.11). The combined best HIV prevalence rates (i.e., averages of the smoothed adjusted PBM HIV prevalence rate estimates and the smoothed CBM HIV prevalence rate estimates) for each MSA and year are shown in Table 4.
Trend Analysis
Figure 1 shows point estimates and 95% confidence intervals for MSA-specific EAPCs in HIV prevalence rates over the study period. Most MSAs (n = 85) experienced a significant decrease in prevalence rate, with EAPCs ranging from −12.9% to −2.1% annually (mean = −6.5; SD = 2.1). The remaining eleven MSAs had either a non-significant increase (e.g., San Jose, CA; El Paso, TX; and Knoxville, TN) or a non-significant decrease (e.g., San Antonio, TX; Toledo, OH; Louisville, KY; Pittsburgh, PA; Columbus, OH; Bakersfield, CA; Fort Lauderdale, FL; and Buffalo, NY).
Validating HIV prevalence rates
The ICC between Holmberg’s 199221 HIV prevalence rate estimates and our 1992 best HIV prevalence rate estimates is 0.941, which indicates very good consistency between the two estimates. The high ICC shows that the overwhelming majority of the variance is within data sources rather than between. We also ran a paired t-test, which indicates that our best 1992 estimates are about 1.75 percentage points higher than Holmberg’s21 on average. The ICC between our 1998 best HIV prevalence rates and Friedman et al. 199822 HIV prevalence rates is 0.955. A paired t-test indicates that our 1998 best HIV estimates are about 0.36 percentage points less on average as compared with Friedman et al. 199822 HIV prevalence rates. These results indicate that our 1992 and 1998 best estimates correlated extremely well with both Holmberg’s 199221 HIV prevalence rates and Friedman et al. 199822 HIV prevalence rates.
The correlations between MSA-specific HIV prevalence rates and hard-core drug arrests were significant (at least p < 0.05) for each year, 1992–2002 (Table 5). The estimated HIV prevalence rates for 2000 and the 2000 income inequality were significantly correlated (r = 0.467; p < 0.0001). Police expenditures and the estimated HIV prevalence rates were highly correlated for 1992 (r = 0.504); 1997 (r = 0.476); and 2002 (r = 0.473) (all p < 0.0001). Correlations between the HIV prevalence rates and corrections expenditure per capita for 1992 (r = 0.285); 1997 (r = 0.232); and 2002 (r = 0.297) were somewhat lower than those for the police expenditure, but all were significant at p < 0.05. The estimated HIV prevalence rates for 2000 and the 2000 poverty were also low (r = 0.268) but significant at p < 0.05. Overall, correlations between anti-OTC laws and HIV prevalence rates for three years (1992–1994) ranged from 0.280 to 0.300 (all p < 0.05).
Discussion
Despite best efforts in computing our estimates of HIV prevalence rates, limitations exist concerning accuracy. The research-based estimates compiled for 33 MSAs varied in the duration of injection periods used to classify an individual as an “IDU” (e.g., some people were classified as IDUs if they reported injecting in their lifetimes, while others were classified as an IDU only if they reported injecting in the past 30 days). Related biases would affect the accuracy of the regression adjustments made to the CDC CTS data, as would variations in Counseling and Testing site locations; access to testing; and other social factors associated with why IDUs do and do not get tested.
As previously noted by Friedman et al.,22 the CDC CTS data underestimate HIV prevalence rates. This bias increases over time, mainly because people who have tested positive once (or twice, as a confirmation) tend to have no reason to be tested again. These errors are likely to be greater in MSAs with higher HIV prevalence rates and those with long-lasting epidemics of stable (or declining) prevalence rates.
Further, the CDC CTS data include duplicate counts, reflecting number of HIV-positive tests, not number of HIV-positive individuals. Thus, it is not known how many IDUs may get tested more than once in a given year. These data are further limited by social desirability biases on the part of people being tested when they report on whether they have ever injected drugs and/or when they last injected. Consistent with CDC’s behavioral risk classification scheme, we thus consider IDUs to be those that injected drugs at anytime since the beginning of the HIV/AIDS epidemic (e.g., 1977) and thus capture experimenters and infrequent users.
There are a number of limitations to the PBM that we need to consider. First, this method relies on PLWA data provided by CDC, which represent estimates of reported cases, adjusted for reporting delays and redistribution of cases for which no risk is reported. It is likely that in redistributing such cases, some are misclassified as high-risk heterosexual or MSM that could actually be IDUs. This would result in undercounts in the numerators of the HIV prevalence rates, causing underestimates. Secondly, the proportional distribution of PLWAs was posited to be similar to that of all PLWHAs with respect to MSA, year and risk factor. The validity of this assumption could not be determined. Thus, we make an assumption of little proportional variation, and assume that Parameter 2 in the PBM is a constant for each year, and that all of a given year's PBM MSA-specific HIV prevalence rates can be adjusted by this constant. The adjustments could vary by MSA.
Despite limitations, developing HIV prevalence rate estimates among IDUs over time is useful to: (1) assist in implementing effective social and cultural interventions and public policy aimed at strengthening drug injectors’ health; (2) guide discussions regarding urban policing policy and other social policies that may shape HIV among IDUs; (3) aid research on how place-based processes (e.g. socio-political or economic factors) are associated with HIV prevalence rates among IDUs, and (4) serve as supporting data in seeking funding for harm reduction programs and prevention and care for IDU-related HIV.
In the past, we have been hampered in our ability to understand the spread and prevalence of HIV among IDUs by the lack of temporal and spatial data on HIV prevalence rates. Well-structured HIV public policy along with effective allocation of prevention resources require valid and current data on trends in HIV prevalence rates (and incidence rates) in the US and in major metropolitan areas. These current HIV prevalence rates may help shed light on how future IDU-related HIV epidemics geographically diffuse, as well as provide a foundation for the design and implementation of structural interventions for preventing the spread of HIV among IDUs.
Summation
The high degree of correlation between the unadjusted HIV prevalence rates according to the two estimation methods suggests a strong association between the two independent data sources, which appear to be measuring the same underlying factor. The results of our validation tests indicate a relatively high correlation between Holmberg21 and Friedman et al.22 and our best HIV prevalence rate estimates. In addition, we examined cross-sectional correlations in other factors that could be associated with HIV prevalence rate trends, e.g., hard-core drug arrests, income inequality, and police expenditures, and found significant correlations. These factors support findings by Friedman et al.41 that suggest that legal repressiveness (i.e., drug arrests; police and corrections expenditures) may have a high cost with regard to shaping IDU-related HIV transmission rates and perhaps other disease among injectors and their partners.
Our findings suggest an overall decreasing trend of HIV prevalence rates among IDUs in the 96 MSAs during 1992–2002. During this period, HIV prevalence rate estimates declined in 85 (88.5%) of the 96 MSAs. Possible reasons for this trend at the aggregate and individual MSA level include program efforts to increase users’ access to clean syringes both through syringe exchange programs and pharmacies;63 , 64 efforts to promote safer injection practices; effects of antiretroviral therapies on infectivity of IDUs;65 , 66 deaths from HIV not being matched by new infections;67 , 68 and possible changes in risk networks and other social mixing patterns which vary from place to place.67 , 69 , 70 Differences in HIV prevalence rates may also reflect differences in availability, accessibility and effectiveness of HIV prevention and treatment programs across metropolitan areas.71–73 Recent trends regarding the effects of HAART in keeping IDUs alive exert an upward pressure on prevalence rates. Combinations of these other factors seem to outweigh the effects of HAART producing a downward trend in HIV prevalence rates among IDUs over time.
Thus, declining HIV prevalence rates may reflect high continuing mortality among infected IDUs, as well as primary HIV prevention for non-infected IDUs and self-protection efforts by them. These results warrant further research into the population dynamics of disease progression, access to health services, and the effects of HIV prevention interventions for IDUs.
Notes
e.g., number of IDUS tested in 1992 N = 673; number tested in 1997 N = 88.
Akron, OH; Charleston, SC; Gary, IN; Greenville, SC; Kansas City, KS; Minneapolis–St. Paul, MN-WI; Syracuse, NY; Ventura, CA; Youngstown, PA.
Birmingham, AL; Little Rock, AR; Norfolk, VA; San Juan, PR; Wichita, KS.
Albany-Schenectady, NY; Ann Arbor, MI; Atlanta, GA; Buffalo-Niagara Falls, NY; Nassau–Suffolk, NY; Providence–Fall River–Warwick, RI–MA; Rochester, NY.
References
Brown BS, Beschner GM. Handbook on Risk of AIDS: Injection Drug Users and Sexual Partners. Westport, CT: Greenwood; 1993.
Friedman SR, Jose B, Deren S, Des Jarlais DC, Neaigus A. Risk factors for HIV seroconversion among out-of-treatment drug injectors in high- and low-seroprevalence cities. Am J Epidemiol. 1995;142:864–874.
Hurley SF, Jolley DJ, Kaldor JM. Effectiveness of needle-exchange programs for prevention of HIV infection. Lancet. 1997;21:1797–800. doi:10.1016/S0140-6736(96)11380-5.
Kral AH, Bluthenthal RN, Booth RE, Watters JK. HIV seroprevalence among street-recruited injection drug and crack cocaine users in 16 US municipalities. Am J Public Health. 1998;88:108–113.
Malliori M, Zunzunegui MV, Rodriguez-Arenas A, Goldberg D. Drug injecting and HIV-1 infection: major findings from a multi-city study. In: Stimson GV, Des Jarlais DC, Ball A, eds. Drug Injecting and HIV Infection: Global Dimensions and Local Responses. London: Taylor and Francis; 1998.
Stimson GV, Des Jarlais DC, Ball A. Drug Injecting and HIV Infection: Global Dimensions and Local Responses. London: UCL; 1998.
Bluthenthal RN, Malik MR, Grau LE, Singer M, Marshall P, Heimer R. Sterile syringe access conditions and variations in HIV risk among drug injectors in three cities. Soc Study Addict. 2004;99:1136–1146. doi:10.1111/j.1360-0443.2004.00694.x.
Ciccarone D, Bourgois P. Explaining the geographic variation of HIV among injection drug users in the United States. Subst Use Misuse. 2003;38:2049–63. doi:10.1081/JA-120025125.
Kral AH, Page-Shafer K, Kellogg T, et al. Persistent HIV incidence among injection drug users in San Francisco during the 1990s: results of five studies. J Acquir Immune Defic Syndr. 2004;37:1667–1669. doi:10.1097/00126334-200412150-00025.
MacDonald M, Law M, Kaldor J, Hales J, Dore G. Effectiveness of needle and syringe programmes for preventing HIV transmission. Int J Drug Policy. 2003;14:351–352. doi:10.1016/S0955-3959(03)00133-6.
Friedman SR, Des Jarlais DC. HIV among drug injectors: the epidemic and the response. AIDS Care. 1991;3:239–250. doi:10.1080/09540129108253069.
Des Jarlais DC, Friedman SR, Novick DM, et al. HIV-1 infection among intravenous drug users in Manhattan, New York City, from 1977 through 1987. JAMA. 1989;126:1008–1012. doi:10.1001/jama.261.7.1008.
Novick DM, Trigg HL, Des Jarlais DC, Friedman SR, Vlahov D, Kreek MJ. Cocaine injection and ethnicity in parenteral drug users during the early years of the human immunodeficiency virus (HIV) epidemic in New York City. J Med Virol. 1989;29:181–185. doi:10.1002/jmv.1890290307.
Wodak A, Crofts N, Fisher R. HIV infection among injecting drug users in Asia: an evolving public health crisis. AIDS Care. 1993;5:313–320. doi:10.1080/09540129308258614.
Strathdee SA, Patrick DM, Currie SL, et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. AIDS. 1997;11:F59–65. doi:10.1097/00002030-199708000-00001.
Tyndall MW, Currie S, Spittal P, et al. Intensive injection cocaine use as the primary risk factor in the Vancouver HIV-1 epidemic. AIDS. 2003;17:887–93. doi:10.1097/00002030-200304110-00014.
Joint Assessment of HIV/AIDS Prevention. Treatment and Care in China. State Council HIV/AIDS Working Committee Office and UN Theme Group on HIV/AIDS in China. 2004.
Des Jarlais DC, Johnston P, Friedmann P, et al. Patterns of HIV prevalence among injecting drug users in the cross-border area of Lang Son Province, Vietnam, and Ning Ming County, Guangxi Province, China. BMC Public Health. 2005;5:89. http://www.biomedcentral.com/content/pdf/1471-2458-5-89.pdf. Accessed May 23, 2007. doi:10.1186/1471-2458-5-89.
Ohiri K, Claeson M, Razzaghi E, Nassirimanesh B, Afshar P, Power R. HIV/AIDS prevention among injection drug users: Learning from harm reduction in Iran. 2006. http://siteresources.worldbank.org/INTSAREGTOPHIVAIDS/Resources/LancetHarmReductionIran.pdf. Accessed Jan. 15, 2007.
Rhodes T, Platt L, Maximova S, et al. Prevalence of HIV, hepatitis C and syphilis among injecting drug users in Russia: a multi-city study. Addiction. 2006;101:252–266. doi:10.1111/j.1360-0443.2006.01317.x.
Holmberg SD. The estimated prevalence and incidence of HIV in 96 large US metropolitan areas. Am J Public Health. 1996;86:642–654.
Friedman SR, Lieb S, Tempalski B, et al. HIV among injection drug users in large US metropolitan areas, 1998. J Urban Health. 2005;82:434–445. doi:10.1093/jurban/jti088.
Holtgrave DR. Estimation of annual HIV transmission rates in the United States, 1978–2000. J Acquir Immune Defic Syndr. 2004;35:89–92. doi:10.1097/00126334-200401010-00013.
Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2004. Atlanta: US Department of Health and Human Services. Centers Dis Contr Prev 2005;16:1–46.
Centers for Disease Control and Prevention. HIV counseling and testing at CDC-supported sites—United States, 1999–2004. 2006. 1–33. http://www.cdc.gov/hiv/topics/testing/resources/reports/pdf/ctr04.pdf. Accessed July 2, 2008.
Centers for Disease Control and Prevention. Trends in HIV/AIDS diagnoses among men who have sex with men—33 states, 2001–2006. Morb Mort Wkly Rep. 2008;57:681–716.
Lieb S, Friedman SR, Zeni MB, et al. An HIV prevalence-based model for estimating urban risk-populations of injection drug users and men who have sex with men. J Urban Health. 2004;81:401–415. doi:10.1093/jurban/jth126.
Lieb S, Trepka MJ, Thompson DR, et al. Men who have sex with men: estimated population sizes and mortality rates, by race/ethnicity, Miami–Dade County, Florida. J Acquir Immune Defic Syndr. 2007;46:485–490.
Office of Management and Budget. Standards for defining metropolitan and micropolitan statistical areas. Fed Regist. 2000;65:8228–82238.
Brady JE, Friedman SR, Cooper HL, Flom PL, Tempalski B, Gostnell K. Estimating the prevalence of injection drug users in the US and in large US metropolitan areas from 1992 to 2002. J Urban Health. 2008;85:323–351. doi:10.1007/s11524-007-9248-5.
Centers for Disease Control and Prevention. HIV/AIDS Surveillance Supplemental Report: AHIV/AIDS in Urban and Nonurban Area of the United States. Atlanta: US Department of Health and Human Services. Centers Dis Contr Prev 2000;6:1–15.
Cronk CE, Sarvela PD. Alcohol, tobacco, and other drug use among rural/small town and urban youth: A secondary analysis of the monitoring the future data set. Am J Public Health. 1997;80:760–764.
Havens JR, Walker R, Leukefeld CG. Prevalence of opioid analgesic injection among rural nonmedical opioid analgesic users. Drug Alcohol Depend. 2007;87:98–102. doi:10.1016/j.drugalcdep.2006.07.008.
Hutchison L, Blakely C. Substance Abuse Trends in Rural Areas. Rural Healthy People 2010: A companion document to Healthy People 2010. Volume 1. College Station, TX: The Texas AM University System Health Science Center, School of Rural Public Health, Southwest Rural Health Research Center; 2003.
Kline A, Mammo A, Culleton R, et al. Trends in injection drug use among persons entering addiction treatment—New Jersey, 1992–1999. Morb Mort Wkly Rep. 2001;50:378–381.
Substance Abuse and Mental Health Services Administration, Office of Applied Studies. The NSDUH Report: Demographic and Geographic Variations in Injection Drug Use. Rockville, MD; 2007.
Steel E, Fleming PL, Needle R. The HIV rates of injection drug users in less-populated areas. Am J Public Health. 1993;83:286–287.
Cleveland WS, Grosse E, Shyu WM. Local regression models. Chapter 8 of Statistical Models in Social Sciences. Chambers JM, Hastie TJ, eds. Brooks/Cole; 1992.
Friedman SR, Perlis T, Des Jarlais DC. Laws prohibiting over-the-counter syringe sales to injection drug users: relations to population density, HIV prevalence, and HIV incidence. Am J Public Health. 2001;91:791–793.
Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing reliability. Psychol Bull. 1979;86:420–428. doi:10.1037/0033-2909.86.2.420.
Friedman SR, Cooper HL, Tempalski B, et al. Relationships of deterrence and law enforcement to drug-related harms among drug injectors in US metropolitan areas. AIDS. 2006;20:93–99.
Bluthenthal RN, Kral AH, Erringer EA, Edlin BR. Drug paraphernalia laws and injection-related infectious disease risk among drug injectors. Drug Issues. 1999a;22:1–16.
Friedman SR, Perlis T, Lynch J, Des Jarlais DC. Economic inequality, poverty, and laws against syringe access as predictors of metropolitan area rates of drug injection and HIV infection. Proceedings of the Global Research Network Meeting, Durban, South Africa. July 2000.
Aitken C, Moore D, Higgs D, Kelsall J, Kerger M. The impact of a police crackdown on a street drug scene: evidence from the street. Int J Drug Policy. 2002;13:189–198. doi:10.1016/S0955-3959(02)00075-0.
Bluthenthal RN, Lorvick J, Kral AH, Erringer EA, Kahn JG. Collateral damage in the war on drugs: HIV risk behaviors among injection drug users. Int J Drug Policy. 1999;10:25–38. doi:10.1016/S0955-3959(98)00076–0.
Cooper H, Moore L, Gruskin S, Krieger N. The impact of a police drug crackdown on drug injectors’ ability to practice harm reduction: a qualitative study. Soc Sci Med. 2005;61:673–68. doi:10.1016/j.socscimed.2004.12.030.
Maher L, Dixon D. Policing and public health: law enforcement and harm minimization in a street-level drug market. Br J Criminol. 1999;39:488–512. doi:10.1093/bjc/39.4.488.
Rhodes T, Kimber J, Small W, et al. Public injecting and the need for ‘safer environment interventions’ in the reduction of drug-related harm. Addition. 2006;101:1384–1393. doi:10.1111/j.1360-0443.2006.01556.x.
Federal Bureau of Investigation. US Uniform Crime Reporting Program Data: County-Level Detailed Arrest and Offense Data. 1992–2002.
United States Census Bureau. United States Census Annual Surveys of Government Finances. 1992.
United States Census Bureau. United States Census Annual Surveys of Government Finances. 1997.
United States Census Bureau. United States Census Annual Surveys of Government Finances. 2002.
Lynch JW, Smith GD, Harper S, et al. Income inequality a determinant of population health? Part 1. A systemic review. Milbank Q. 2004;82:5–99. doi:10.1111/j.0887-378X.2004.00302.x.
Kaplan GA, Pamuk ER, Lynch JW, Cohen RD, Balfour JL. Inequality in income and mortality in the United States: analysis of mortality and potential pathways. BMJ. 1996;312:999–1003.
Diez-Roux AV, Nieto FJ, Muntaner C, et al. Neighborhood environments and coronary heart disease: a multilevel analysis. Am J Epidemiol. 1997;146:48–63.
Diez-Roux AV, Nieto FJ, Caulfield L, Tyroler HA, Watson RL, Szklo M. Neighborhood differences in diet: the Atherosclerosis in Communities (ARIC) Study. J Epidemiol Community Health. 1999;53:55–63.
Sanmartin C, Ross NA, Tremblay S, Wolfson M, Dunn JR, Lynch J. Labour market income inequality and mortality in North American metropolitan areas. J Epidemiol Community Health. 2003;57:792–797. doi:10.1136/jech.57.10.792.
United States Census Bureau. Housing and Household Economic Statistics Division. Poverty Index; 2000.
Des Jarlais DC, Hagan H, Friedman SR, Ward TP. HIV among injecting drug users: epidemiology and emerging public health perspectives. In: Merigan TC Jr, Bartlett JG, Bolognesi D, eds. Textbook of AIDS Medicine, 2nd edn. Baltimore: Williams Wilkins; 1999.
Burris S, Vernick J, Ditzler A, Strathdee S. The legality of selling or giving syringes to injection drug users. In: Jones TS, Coffin PO, eds. Preventing blood-borne infections through pharmacy syringe sales and safe community syringe disposal. J Am Pharm Assoc 2002;42(Suppl. 2):S13–S17.
SAS Institute. SAS/STAT® 9.1 User’s Guide. Cary, NC: SAS Institute; 2004.
R Development Core Team. (2008). R: A language and environment for statistical computing. Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org.
Des Jarlais D, Marmor M, Paone D, Titus S, Shi Q, Perlis T, et al. HIV incidence among syringe exchange participants in New York City. Lancet. 1996;348:987–991. doi:10.1016/S0140-6736(96)02536-6.
Pouget ER, Deren S, Fuller C, et al. Receptive syringe sharing among injection drug users in Harlem and the Bronx during the New York State expanded syringe access program. J Acquir Immune Defic Syndr. 2005;39:471–477. doi:10.1097/01.qai.0000152395.82885.c0.
Wood E, Hogg RS, Lima D, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300:550–554. doi:10.1001/jama.300.5.550.
Morris JD, Golub ET, Mehta SH, Jacobson L, Gange SJ. Injection drug use and patterns of highly active antiretroviral therapy use: an analysis of ALIVE, WIHS, and MACS cohorts. AIDS Res Ther. 2007;4:1–8. doi:10.1186/1742-6405-4-12.
Des Jarlais DC, Perlis TE, Friedman SR, et al. Declining seroprevalence in a very large HIV epidemic: Injecting drug users in New York City, 1991 to 1996. Am J Public Health. 1998;88:1801–1806.
Des Jarlais DC, Marmor M, Friedmann P, et al. HIV incidence among injection drug users in New York City, 1992–1997: evidence for a declining epidemic. Am J Public Health. 2000;90:352–359.
Friedman SR, Curtis R, Neaigus A, Jose B, Des Jarlais DC. Social networks, drug injectors’ lives, and HIV/AIDS. New York: Kluwer/Plenum; 1999.
Morris M. Sexual networks and HIV. AIDS. 1997;11(Suppl A):S209–S216. doi:10.1097/00002030-199705000-00012.
Friedman SR, Tempalski B, Brady J, et al. Predictors of the degree of drug treatment coverage for injection drug users in 94 metropolitan areas in the United States. Int J Drug Policy. 2007;18:475–485. doi:10.1016/j.drugpo.2006.10.004.
Tempalski B, Flom PL, Friedman SR, et al. Social and political factors predicting the presence of syringe exchange programs in 96 Metropolitan areas in the United States. Am J Public Health. 2007;97:437–447. doi:10.2105/AJPH.2005.065961.
Tempalski B, Cooper H, Friedman SR, Des Jarlais DC, Brady J. Correlates of syringe coverage for heroin injection in 35 large metropolitan areas in the US in which heroin is the dominant injected drug. Int J Drug Policy. 2008;19S:S47–S58. doi:10.1016/j.drugpo.2007.11.011.
Acknowledgments
This project was supported by the National Institute of Drug Abuse (R01 DA13336; Community Vulnerability and Response to IDU-Related HIV).
We would like to thank Dr. Peter L. Flom, Daniel R. Thompson and Enrique Pouget for their statistical advice and Dr. David Holtgrave for his insight regarding the PLWHA data. We further thank the Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for HIV, STD, and TB Prevention, specifically Michael Fanning, David Hurst and Renee R. Stein for providing the HIV Counseling & Testing data and Andrew Mitsch from the CDC’s HIV Incidence and Case Surveillance Branch for providing PLWA data for which these analyses are based. We also thank Ms. Makini Booth for assembling the research on HIV prevalence studies and related literature reviews for which the research estimates are based.
We would further like to thank the following State and Local Health Departments and researchers for their assistance:
Alabama: Anthony Merriweather
Florida: Melinda Waters, Marlene LaLota, Lorene Maddox
Hawaii: Don C. Des Jarlais, Roy Ohye, Peter, M. Whiticar
Kansas: Jennifer VandeVelde, Karl V. Milhon
Massachusetts: Drew Hanchett, Deborah Isenberg, Teresa Anderson
New Mexico: Andrew Gans, Kathleen Rooney, Lily N. Foster, Bruce G. Trigg
New York: Mara San Antonio-Gaddy, Punkin Stevens, Daniel O’Connell, Thomas Chesnut
Pennsylvania: Kenneth McGarvey, Benjamin Muthambi, Brenda Doucette
Puerto Rico: José Toro-Alfonso, Rafaela R. Robles, Sherry Deren
Virginia: Chris Delcher, Jeff Stover, Jennifer Bissette, Theresa Henry
Washington: Frank Chaffee, Hanne Thiede, Leslie Pringle, Keith Okita, Michael Hanrahan, Mark Doescher
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material
Appendix
HIV Research Articles for Estimating HIV Prevalence Rates Among Injection Drug Users 1992-2002 (by MSA and Year) (PDF 141kb)
Rights and permissions
About this article
Cite this article
Tempalski, B., Lieb, S., Cleland, C.M. et al. HIV Prevalence Rates among Injection Drug Users in 96 Large US Metropolitan Areas, 1992–2002. J Urban Health 86, 132–154 (2009). https://doi.org/10.1007/s11524-008-9328-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11524-008-9328-1