Abstract
Background
Crizotinib has been approved for the treatment of non-small-cell lung cancer (NSCLC) with ROS proto-oncogene 1 (ROS1) gene fusion. This drug has also been granted breakthrough designation for NSCLCs with MET exon 14 alterations.
Objective
This systematic review and meta-analysis aimed to investigate the efficacy and safety of crizotinib in patients with these diseases.
Methods
We searched PubMed and Web of Science for relevant studies. Meta-analysis of proportions was conducted to calculate the pooled rate of complete response, partial response, stable disease, progressive disease, disease control rate (DCR), objective response rate (ORR), and drug adverse effects (AEs) of crizotinib in NSCLCs with ROS1 rearrangement or MET alterations.
Results
A total of 20 studies were included for meta-analysis. Among patients with ROS1-positive NSCLC, crizotinib exhibited a pooled DCR of 93.2% (95% confidence interval [CI] 90.8–95.5) and a pooled ORR of 77.4% (95% CI 72.8–82.1). The median progression-free survival (PFS) and overall survival (OS) of patients in this group was 14.5 and 32.6 months, respectively. For NSCLC with MET alterations, crizotinib was associated with a lower efficacy (DCR 78.9% [95% CI 70.3–87.4] and ORR 40.6% [95% CI 28.3–53.0]). The median PFS was 5.2 months, and median OS was 12.7 months. The most common drug AEs were vision impairment (43.7%), edema (42.9%), and fatigue (40.1%).
Conclusion
Our study highlighted and confirmed the efficacy of crizotinib in patients with NSCLC with ROS1 or MET genetic alterations. Crizotinib had remarkable effects on advanced NSCLC with ROS1 fusion, as previously reported. However, the role of this targeted therapy in MET-altered NSCLC remains investigational.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Crizotinib elicits a dramatic response in advanced non-small-cell lung cancer (NSCLC) with ROS proto-oncogene 1 (ROS1) rearrangements. |
The role of crizotinib in MET-deregulated NSCLC remains investigational. |
Drug-related adverse effects are common among patients with NSCLC treated with crizotinib. |
1 Introduction
Lung cancer continues to be the deadliest malignancy in the world. It caused 1.8 million deaths in 2018 and has a 5-year survival rate of only about 15% [1]. Lung cancer is classified into two types: small-cell and non-small-cell lung cancer (NSCLC). While the former accounts for 15% of lung cancers and is aggressive and mostly incurable at advanced stages, the latter accounts for about 85% of lung cancer and often has better prognosis because of its differing underlying biology.
Over the past several years, the emergence of genomics has led to the identification of specific driver mutations in NSCLC, which have become targets for more specific treatment [2,3,4,5,6,7]. Of those, the driver mutations of protein tyrosine kinase receptor MET encoded by gene MET, and tyrosine kinase receptor ROS proto-oncogene 1 (ROS1) encoded by gene ROS1, have been studied as treatment targets in NSCLC [8,9,10]. MET alterations, which have been shown to drive carcinogenesis, include MET copy number gains and amplification and MET exon 14 skipping mutations [11]. MET gene amplification has been seen in about 20% of patients with NSCLC who developed acquired resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) [12] and was rarely seen in EGFR TKI-untreated patients [13]. MET splice mutations did not concurrently occur in tumors with MET amplification [14]. The MET pathway dysregulations, including MET gene amplification and MET exon 14 skipping mutations, have been found in about 3% of NSCLCs [16,17,18]. This genetic alteration induces loss of ubiquitin-mediated degradation through the Casitas B-lineage lymphoma-negative regulatory mechanism and promotes tumorigenesis [19].
The ROS1 gene fusion occurs in approximately 2% of patients with NSCLC [6]. ROS1 is a type of tyrosine kinase insulin-receptor gene. ROS1 fusion causes uncontrolled downstream signal transduction, leading to carcinogenesis [15].
The US FDA approved the use of crizotinib, a TKI, as a treatment in patients with NSCLC with translocations involving the anaplastic lymphoma kinase (ALK) in 2011 [20] and has since also approved an additional expansion of crizotinib use in patients with NSCLC with positive ROS1 rearrangement [21]. Crizotinib has also been granted breakthrough designation for NSCLCs with MET exon 14 alterations [22]. Subsequently, a number of trials have been conducted to assess the efficacy and safety of crizotinib in patients with NSCLC with ROS1 fusion or MET alterations [7, 9, 23,24,25]. However, associations between a positive ROS1 fusion or MET alteration status and clinical adaptation and prognosis in patients with NSCLC receiving crizotinib remain inconsistent. This study aimed to summarize the efficacy and safety of crizotinib in patients with NSCLC with positive ROS1 gene fusion or MET deregulation.
2 Methods
2.1 Search Strategy and Study Identification
We searched for potential articles published from inception to May 2020 in electronic databases including PubMed, Web of Science, and clinicaltrials.gov. We used the following search terms: crizotinib AND (ROS1 OR MET) AND (lung OR pulmonary OR NSCLC). Our study protocol strictly followed the recommendations of the Preferred Reporting Items for Systemic Review and Meta-Analysis (PRISMA) statement [26].
2.2 Selection Criteria and Abstract Screening
All studies were imported into EndNote, and duplicates were deleted. Two reviewers then independently screened the titles and abstracts of all articles. Studies were eligible if they were studies or clinical trials reporting the efficacy of crizotinib as monotherapy in patients with NSCLC with MET alterations or ROS1 fusions. Studies were excluded if they were (1) studies on other lung cancer types (e.g., salivary gland type cancer, lymphoma); (2) studies reporting the efficacy of crizotinib in combination with other drugs; (3) case reports; (4) reviews; (5) conference/proceeding papers, posters, theses, books; and (6) duplicated results. Discrepancies between the two reviewers were resolved by discussion and consensus.
2.3 Full-Text Screening and Data Extraction
The following data were extracted from the included studies: institution, city, country, year of publication, study design, age, sex, smoking history, metastasis sites, histologic subtypes of NSCLC, Eastern Cooperative Oncology Group performance status, prior treatments, duration of follow-up and treatment, patient best response (complete response [CR], partial response [PR], stable disease [SD], progressive disease [PD]), disease control rate (DCR), objective response rate (ORR), progression-free survival (PFS), overall survival (OS), and drug adverse effects (AEs). To ensure accuracy, two reviewers read the full text of potential articles, and data were extracted into a predesigned worksheet. Disagreements, if any, were resolved by discussion and consensus.
2.4 Data Analysis
Statistical analyses were conducted using the JAMOVI (www.jamovi.org) and Comprehensive Meta-Analysis (Biostats Inc., Englewood, NJ, USA) software. Pooled proportions and corresponding 95% confidence intervals (CIs) were calculated using a random-effects model. Heterogeneity among the included studies was tested using the I2 statistic, which is the percentage of the total variation between studies that cannot be attributed to chance [27]. We classified the heterogeneity across the studies as low if 25% < I2 ≤ 50%, moderate if 50% < I2≤ 75%, and high if I2 > 75 [27]. Publication bias was analyzed using Egger’s regression test and funnel plots. A p value ≤ 0.05 was considered a statistically significant publication bias.
3 Results
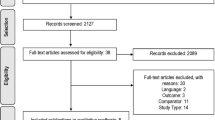
We found 711 results from PubMed and Web of Science. In total, 37 were selected for full-text reading, after which 20 studies comprising 719 patients with NSCLC were included for final analyses [9, 25, 28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44, 46] (Fig. 1). Characteristics of the included studies are shown in Table 1. All included studies recruited patients with advanced-stage NSCLC. The initial dose for crizotinib was 250 mg twice daily in all studies. Treatment efficacy and tumor response were assessed using Response Evaluation Criteria in Solid Tumor classifications.
Data from the studies by Li et al. [30] and Shen et al. [45] were from the same institution. Additionally, patients in the two studies by Shaw et al. [7, 46] were both recruited from the PROFILE 1001 trial. To avoid overlapping data, we selected from these only studies with the highest number of patients for analyses.
3.1 Efficacy of Crizotinib in Non-Small-Cell Lung Cancer (NSCLC) with ROS Proto-Oncogene 1 (ROS1) Fusion/Deletion
In total, 16 studies reported the efficacy of crizotinib in patients with ROS1 alterations. The pooled proportions for CR, PR, SD, and PD were 4.2% (95% CI 1.9–6.4), 71.2% (95% CI 65.3–77.1), 13.4% (95% CI 9.7–17.0), and 5.8% (95% CI 3.8–7.8), respectively. The pooled DCR was 93.2% (95% CI 90.8–95.5), and the pooled ORR was 77.4% (95% CI 72.8–82.1) (Fig. 2). Most analyses had a significant level of heterogeneity (I2 > 25%).
The median PFS of ROS1-positive NSCLC treated with crizotinib ranged from 5.5 to 22.8 months, and the pooled median PFS was 14.5 months. The median OS was not reached in most of the included studies. In studies in which these data were available, the median OS was 32.6 months (range 17.2–51.4) (Table 2). Six studies reported the survival of cluster of differentiation (CD)-74 versus non-CD74 ROS1-positive patients [30, 35, 41,42,43, 46]. Our analysis showed no statistical difference in patient survival between these two subgroups.
3.2 Efficacy of Crizotinib in NSCLC with MET Alterations
Six studies reported the treatment response to crizotinib in patients with NSCLC with MET deregulations (Table 1). The pooled proportions for CR, PR, SD, and PD were 3.1% (95% CI 0.5–5.7), 39.3% (95% CI 25.8–52.7), 36.9% (95% CI 28.6–45.1), and 17.5% (95% CI 7.4–27.7). The pooled DCR and ORR were lower than in the ROS1 alteration group: 78.9% (95% CI 70.3–87.4) and 40.6% (95% CI 28.3–53.0), respectively (Fig. 3). There was a considerable amount of heterogeneity among the included studies (I2 > 25%). Sensitivity analysis did not detect the source of heterogeneity among the included studies (data not shown).
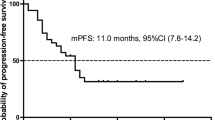
All six studies reported data for OS and PFS. The median PFS was 5.2 months (range 2.4–7.3) and median OS was 12.7 months (range 5.4–31.0) (Table 2). Survival data for MET deregulation subgroups (mutation vs. amplification) were insufficient for further analysis.
3.3 Crizotinib-Related Adverse Effects
The most common crizotinib-related AEs, regardless of grade, were vision impairment (43.7%), edema (42.9%), and fatigue (40.1%), followed by gastrointestinal symptoms (nausea, vomiting, diarrhea) (Table 3). Neutropenia (5.7%) and elevated transaminase (4.2%) were the most commonly seen severe AEs (grade 3 or higher). Data for all AEs are presented in Table 3.
3.4 Publication Bias
Egger’s regression test and observation of funnel plots did not suggest any evidence of publication bias (data not shown).
4 Discussion
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer death for people of all genders [1]. Effective treatments for advanced-stage NSCLC are desperately needed because of the poor prognosis and lack of effective targeted therapies for the majority of patients. Crizotinib has shown promising results in treating patients with ALK-positive, ROS1-positive, and MET-deregulated NSCLCs [7, 16, 47].
Patients treated with crizotinib had improved outcomes compared with patients treated with platinum-pemetrexed chemotherapy in ROS1-fused NSCLC [41, 45]. Our data showed that crizotinib was highly effective and had a significantly improved response rate in ROS1-rearranged NSCLCs. CD74 is the most common variant among patients with ROS1-positive NSCLC [7, 35]. Survival outcomes between ROS1-positive subgroups treated with crizotinib have been reported in several studies [30, 35, 41,42,43, 46], and we further confirmed that there was no statistical difference among different ROS1-positive subgroups. In most of the series, responses to treatment occurred early: about 50% of patients had an objective response after 2 cycles of treatment. Although the initial clinical response rate to ROS1 protein TKIs is dramatic, it is almost always temporary because acquired resistance to these drugs invariably develops. Nearly 50% of patients later developed disease progression or had died at the end of the follow-up [7]. A few distinct mechanisms of resistance to crizotinib among ROS1-positive NSCLC have been discussed previously [48, 49]. Capizzi et al. [28] reported that patients with ROS1 deletion had a high chance of response to crizotinib. However, it should be noted that half of the patients with ROS1 5′ deletion detected by fluorescent in situ hybridization also had a ROS1 rearrangement upon next-generation sequencing. As a result, 5′ deletion might not represent a biologically relevant genetic event since most of the responders in that study harbored ROS1 or ALK gene fusions [28]. We observed a considerable amount of heterogeneity among some analyses of the ROS1 group. The variations in the mutation baseline of selected cohorts might be a potential explanation. ROS1 rearrangement may occur concurrently with other genetic events in patients with NSCLC, such as EGFR, ALK, or TP53. Exclusive ROS1 fusion was associated with a better prognosis than were concomitant mutations [50]. Concomitant ROS1 fusion and TP53 mutations conferred a poorer outcome than ROS1 alone [35]. Additionally, variations in previous treatment modalities may have also crucially affected the treatment outcome of targeted therapies.
Recent phase III randomized clinical trials with MET inhibitors in NSCLC have shown discouraging results [51,52,53]. However, it should be noted that those trials did not specifically target tumors with MET exon 14 alterations. Our results indicated that crizotinib demonstrated a considerably lower response rate and shorter PFS/OS in patients with MET alterations than in those with ROS1-positive disease. The 95% CIs of DCR and ORR in the patients with ROS1-rearranged and MET-deregulated NSCLC were sufficient to indicate statistical significance. Given the high rate of drug AEs (Table 3), this factor might limit the use of crizotinib in MET-positive NSCLC. There are several potential explanations for the discrepancies in efficacy between ROS1-positive and MET-positive groups. First, the activation mechanisms of ROS1 fusion protein and MET mutation differ. ROS1 fusion may signal tumorigenesis and promote cell growth and survival through mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), phosphoinositide 3 kinase/protein kinase B (PI3K/AKT), Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3), and Src homology region 2 domain-containing phosphatase-1 and 2 [54,55,56]. On the other hand, MET exon 14 mutation prevents ubiquitination and further promotes stabilization of MET protein [57]. In addition, the clinicopathological characteristics of patients with NSCLC with MET exon 14 mutations or amplifications have been demonstrated as distinct from those with ROS1-positive disease [6, 18, 58].
We observed a significant level of heterogeneity regarding response rate and survival among studies investigating the efficacy of crizotinib in patients with NSCLC with MET genetic alterations. Moro-Sibilot et al. [36] reported that patients with a high level of MET amplification were more likely to respond to crizotinib than those with low amplification. MET-amplified NSCLC without MET mutation is a heterogeneous group that is more likely associated with concurrent driver mutations such as NRAS, KRAS, and TP53 mutations [59]. In a phase I trial, those with NSCLC with a high MET/centromere ratio and gene copy number had a higher response rate to capmatinib than those with a lower level [60]. Possible underlying reasons for these heterogeneities are differences in patient selection, MET deregulation types of tumors (mutations, amplification, or copy number change), and different follow-up durations. It should also be noted that the MET TKI capmatinib has just been approved by the FDA to treat advanced NSCLC with MET exon 14 skipping [61]. In the phase II GEOMETRY mono-1 trial, capmatinib elicited a high response rate and relatively durable responses in advanced NSCLC with MET exon 14 mutations [62].
Although this meta-analysis demonstrated the promising efficacy of crizotinib in ROS1-positive and MET-positive NSCLC, a few limitations must be addressed. The first is an inevitable selection bias caused by the inclusion of retrospective studies, which were the most predominant type among the included studies. As we have stated, there was significant existing heterogeneity among the included studies, which might stem from differences in patient baseline characteristics, prior treatment regimens, and underlying genetic events.
5 Conclusion
Our meta-analysis confirmed remarkable results with crizotinib in advanced NSCLC with ROS1 fusion. However, the role of this targeted therapy in MET-altered NSCLC remains investigational. Additional trials with other TKIs (e.g., capmatinib) and longer follow-ups can further optimize the therapeutic treatment of advanced-stage NSCLCs with MET alterations.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. https://doi.org/10.1056/nejmoa040938.
Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786–92. https://doi.org/10.1056/nejmoa044238.
Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–6. https://doi.org/10.1038/nature05945.
Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94. https://doi.org/10.1056/nejmoa1214886.
Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–70. https://doi.org/10.1200/jco.2011.35.6345.
Shaw AT, Ou SHI, Bang YJ, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371(21):1963–71. https://doi.org/10.1056/nejmoa1406766.
Caparica R, Yen CT, Coudry R, Ignatius SH, Varella-Garcia M, Camidge DR, et al. Responses to crizotinib can occur in high-level MET-amplified non-small cell lung cancer independent of MET exon 14 alterations. J Thorac Oncol. 2017;12(1):141–4. https://doi.org/10.1016/j.jtho.2016.09.116.
Landi L, Chiari R, Tiseo M, D’Inca F, Dazzi C, Chella A, et al. Crizotinib in MET-deregulated or ROS1-rearranged pretreated non-small cell lung cancer (METROS): a phase II, prospective, multicenter, two-arms trial. Clin Cancer Res. 2019;25(24):7312–9. https://doi.org/10.1158/1078-0432.ccr-19-0994.
Ou SH, Tan J, Yen Y, Soo RA. ROS1 as a ‘druggable’ receptor tyrosine kinase: lessons learned from inhibiting the ALK pathway. Expert Rev Anticancer Ther. 2012;12(4):447–56. https://doi.org/10.1586/era.12.17.
Sadiq AA, Salgia R. MET as a possible target for non-small-cell lung cancer. J Clin Oncol. 2013;31(8):1089–96. https://doi.org/10.1200/jco.2012.43.9422.
Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science (NY NY). 2007;316(5827):1039–43. https://doi.org/10.1126/science.1141478.
Bean J, Brennan C, Shih J-Y, Riely G, Viale A, Wang L, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci. 2007;104(52):20932. https://doi.org/10.1073/pnas.0710370104.
Onozato R, Kosaka T, Kuwano H, Sekido Y, Yatabe Y, Mitsudomi T. Activation of MET by gene amplification or by splice mutations deleting the juxtamembrane domain in primary resected lung cancers. J Thorac Oncol. 2009;4(1):5–11. https://doi.org/10.1097/jto.0b013e3181913e0e.
Lin JJ, Shaw AT. Recent advances in targeting ROS1 in lung cancer. J Thorac Oncol. 2017;12(11):1611–25. https://doi.org/10.1016/j.jtho.2017.08.002.
Awad MM, Oxnard GR, Jackman DM, Savukoski DO, Hall D, Shivdasani P, et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J Clin Oncol. 2016;34(7):721. https://doi.org/10.1200/jco.2015.63.4600.
Tong JH, Yeung SF, Chan AWH, Chung LY, Chau SL, Lung RWM, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res. 2016;22(12):3048–56. https://doi.org/10.1158/1078-0432.ccr-15-2061.
Vuong HG, Ho ATN, Altibi AMA, Nakazawa T, Katoh R, Kondo T. Clinicopathological implications of MET exon 14 mutations in non-small cell lung cancer—a systematic review and meta-analysis. Lung Cancer. 2018;123:76–82. https://doi.org/10.1016/j.lungcan.2018.07.006.
Kong-Beltran M, Seshagiri S, Zha J, Zhu W, Bhawe K, Mendoza N, et al. Somatic mutations lead to an oncogenic deletion of met in lung cancer. Cancer Res. 2006;66(1):283–9. https://doi.org/10.1158/0008-5472.can-05-2749.
Kazandjian D, Blumenthal GM, Chen H-Y, He K, Patel M, Justice R, et al. FDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist. 2014;19(10):e5.
FDA expands use of Xalkori to treat rare form of advanced non-small cell lung cancer. US Food & Drug Administration. 2016. https://www.fda.gov/news-events/press-announcements/fda-expands-use-xalkori-treat-rare-form-advanced-non-small-cell-lung-cancer. Accessed 15 July 2020.
PFIZER’S XALKORI® (CRIZOTINIB) RECEIVES FDA BREAKTHROUGH THERAPY DESIGNATION IN TWO NEW INDICATIONS. Pfizer. 2018. https://www.pfizer.com/news/press-release/press-release-detail/pfizer_s_xalkori_crizotinib_receives_fda_breakthrough_therapy_designation_in_two_new_indications-0. Accessed 15 July 2020.
Wiesweg M, Schuler M, Schildhaus HU. Crizotinib in ROS1 and MET deregulated NSCLC-letter. Clin Cancer Res. 2020;26(7):1774. https://doi.org/10.1158/1078-0432.ccr-19-3740.
Shimokawa M, Nosaki K, Seto T, Ohashi K, Morise M, Horinouchi H, et al. Phase II, open-label, multicenter trial of crizotinib in Japanese patients with advanced non-small cell lung cancer harboring a MET gene alteration: Co-MET study. Trials. 2020;21(1):298. https://doi.org/10.1186/s13063-020-4221-7.
Drilon A, Clark JW, Weiss J, Ou SHI, Camidge DR, Solomon BJ, et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat Med. 2020;26(1):47. https://doi.org/10.1038/s41591-019-0716-8.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Capizzi E, Dall’Olio FG, Gruppioni E, Sperandi F, Altimari A, Giunchi F, et al. Clinical significance of ROS1 5′ deletions in non-small cell lung cancer. Lung Cancer. 2019;135:88–91. https://doi.org/10.1016/j.lungcan.2019.07.017.
Joshi A, Pande N, Noronha V, Patil V, Kumar R, Chougule A, et al. ROS1 mutation non-small cell lung cancer-access to optimal treatment and outcomes. Ecancermedicalscience. 2019;13:900. https://doi.org/10.3332/ecancer.2019.900.
Li Z, Shen L, Ding D, Huang J, Zhang J, Chen Z, et al. Efficacy of crizotinib among different types of ROS1 fusion partners in patients with ROS1-rearranged non-small cell lung cancer. J Thorac Oncol. 2018;13(7):987–95. https://doi.org/10.1016/j.jtho.2018.04.016.
Liu C, Yu H, Chang J, Chen H, Li Y, Zhao W, et al. Crizotinib in Chinese patients with ROS1-rearranged advanced non-small-cell lung cancer in routine clinical practice. Target Oncol. 2019;14(3):315–23. https://doi.org/10.1007/s11523-019-00636-6.
Masuda K, Fujiwara Y, Shinno Y, Mizuno T, Sato J, Morita R, et al. Efficacy and safety of crizotinib in patients with ROS1 rearranged non-small cell lung cancer: a retrospective analysis. J Thorac Dis. 2019;11(7):2965–72. https://doi.org/10.21037/jtd.2019.07.44.
Mazières J, Zalcman G, Crinò L, Biondani P, Barlesi F, Filleron T, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol. 2015;33(9):992–9. https://doi.org/10.1200/jco.2014.58.3302.
Mehta A, Saifi M, Batra U, Suryavanshi M, Gupta K. Incidence of ROS1-rearranged non-small-cell lung carcinoma in india and efficacy of crizotinib in lung adenocarcinoma patients. Lung Cancer-Targets Therapy. 2020;11:19–25. https://doi.org/10.2147/lctt.s244366.
Michels S, Massutí B, Schildhaus HU, Franklin J, Sebastian M, Felip E, et al. Safety and efficacy of crizotinib in patients with advanced or metastatic ROS1-rearranged lung cancer (EUCROSS): a European phase II clinical trial. J Thorac Oncol. 2019;14(7):1266–76. https://doi.org/10.1016/j.jtho.2019.03.020.
Moro-Sibilot D, Cozic N, Pero M, Mazieres J, Otto J, Souquet PJ, et al. Crizotinib in c-MET- or ROS1-positive NSCLC: results of the AcSe phase II trial. Ann Oncol. 2019;30(12):1985–91. https://doi.org/10.1093/annonc/mdz407.
Song ZB, Wang H, Yu ZY, Lu PH, Xu CW, Chen G, et al. De novo MET amplification in Chinese patients with non-small-cell lung cancer and treatment efficacy with crizotinib: a multicenter retrospective study. Clin Lung Cancer. 2019;20(2):E171–6. https://doi.org/10.1016/j.cllc.2018.11.007.
Wang SXY, Zhang BM, Wakelee HA, Koontz MZ, Pan MG, Diehn M, et al. Case series of MET exon 14 skipping mutation-positive non-small-cell lung cancers with response to crizotinib and cabozantinib. Anticancer Drugs. 2019;30(5):537–41. https://doi.org/10.1097/cad.0000000000000765.
Wang WX, Wang H, Lu PH, Yu ZY, Xu CW, Zhuang W, et al. Crizotinib with or without an EGFR-TKI in treating EGFR-mutant NSCLC patients with acquired MET amplification after failure of EGFR-TKI therapy: a multicenter retrospective study. J Transl Med. 2019;17:1–9. https://doi.org/10.1186/s12967-019-1803-9.
Wu YL, Yang JC, Kim DW, Lu S, Zhou J, Seto T, et al. Phase II study of crizotinib in East Asian patients with ROS1-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(14):1405–11. https://doi.org/10.1200/jco.2017.75.5587.
Xu H, Zhang Q, Liang L, Li J, Liu Z, Li W, et al. Crizotinib vs platinum-based chemotherapy as first-line treatment for advanced non-small cell lung cancer with different ROS1 fusion variants. Cancer Med. 2020;9(10):3328-36. https://doi.org/10.1002/cam4.2984.
Zeng L, Li Y, Xiao L, Xiong Y, Liu L, Jiang W, et al. Crizotinib presented with promising efficacy but for concomitant mutation in next-generation sequencing-identified ROS1-rearranged non-small-cell lung cancer. Onco Targets Ther. 2018;11:6937–45. https://doi.org/10.2147/ott.s176273.
Zhang L, Jiang T, Zhao C, Li W, Li X, Zhao S, et al. Efficacy of crizotinib and pemetrexed-based chemotherapy in Chinese NSCLC patients with ROS1 rearrangement. Oncotarget. 2016;7(46):75145–54. https://doi.org/10.18632/oncotarget.12612.
Zhu YC, Zhang XG, Lin XP, Wang WX, Li XF, Wu LX, et al. Clinicopathological features and clinical efficacy of crizotinib in Chinese patients with ROS1-positive non-small cell lung cancer. Oncol Lett. 2019;17(3):3466–74. https://doi.org/10.3892/ol.2019.9949.
Shen L, Qiang T, Li Z, Ding D, Yu Y, Lu S. First-line crizotinib versus platinum-pemetrexed chemotherapy in patients with advanced ROS1-rearranged non-small-cell lung cancer. Cancer Med. 2020;9(10):3310-18. https://doi.org/10.1002/cam4.2972.
Shaw AT, Riely GJ, Bang YJ, Kim DW, Camidge DR, Solomon BJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30(7):1121–6. https://doi.org/10.1093/annonc/mdz131.
Hoang T, Myung SK, Pham TT, Park B. Efficacy of crizotinib, ceritinib, and alectinib in ALK-positive non-small cell lung cancer treatment: a meta-analysis of clinical trials. Cancers (Basel). 2020;12(3):526. https://doi.org/10.3390/cancers12030526.
Awad MM, Engelman JA, Shaw AT. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med. 2013;369(12):1173. https://doi.org/10.1056/nejmc1309091.
Davies KD, Mahale S, Astling DP, Aisner DL, Le AT, Hinz TK, et al. Resistance to ROS1 inhibition mediated by EGFR pathway activation in non-small cell lung cancer. PLoS One. 2013;8(12):e82236. https://doi.org/10.1371/journal.pone.0082236.
Zeng L, Li YZ, Xiao LL, Xiong Y, Liu L, Jiang WJ, et al. Crizotinib presented with promising efficacy but for concomitant mutation in next-generation sequencing-identified ROS1-rearranged non-small-cell lung cancer. Oncotargets Ther. 2018;11:6937–45. https://doi.org/10.2147/ott.s176273.
Scagliotti G, von Pawel J, Novello S, Ramlau R, Favaretto A, Barlesi F, et al. Phase III multinational, randomized, double-blind, placebo-controlled study of tivantinib (ARQ 197) plus erlotinib versus erlotinib alone in previously treated patients with locally advanced or metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33(24):2667–74. https://doi.org/10.1200/jco.2014.60.7317.
Yoshioka H, Azuma K, Yamamoto N, Takahashi T, Nishio M, Katakami N, et al. A randomized, double-blind, placebo-controlled, phase III trial of erlotinib with or without a c-Met inhibitor tivantinib (ARQ 197) in Asian patients with previously treated stage IIIB/IV nonsquamous nonsmall-cell lung cancer harboring wild-type epidermal growth factor receptor (ATTENTION study). Ann Oncol. 2015;26(10):2066–72. https://doi.org/10.1093/annonc/mdv288.
Spigel DR, Edelman MJ, O’Byrne K, Paz-Ares L, Shames DS, Yu W, et al. Onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIb or IV NSCLC: Results from the pivotal phase III randomized, multicenter, placebo-controlled METLung (OAM4971g) global trial. J Clin Oncol. 2014;32(15_suppl):8000. https://doi.org/10.1200/jco.2014.32.15_suppl.8000.
Davies KD, Le AT, Theodoro MF, Skokan MC, Aisner DL, Berge EM, et al. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin Cancer Res. 2012;18(17):4570–9. https://doi.org/10.1158/1078-0432.ccr-12-0550.
Charest A, Wilker EW, McLaughlin ME, Lane K, Gowda R, Coven S, et al. ROS fusion tyrosine kinase activates a SH2 domain-containing phosphatase-2/phosphatidylinositol 3-kinase/mammalian target of rapamycin signaling axis to form glioblastoma in mice. Cancer Res. 2006;66(15):7473–81. https://doi.org/10.1158/0008-5472.can-06-1193.
Jun HJ, Johnson H, Bronson RT, de Feraudy S, White F, Charest A. The oncogenic lung cancer fusion kinase CD74-ROS activates a novel invasiveness pathway through E-Syt1 phosphorylation. Cancer Res. 2012;72(15):3764–74. https://doi.org/10.1158/0008-5472.can-11-3990.
Lu X, Peled N, Greer J, Wu W, Choi P, Berger AH, et al. MET exon 14 mutation encodes an actionable therapeutic target in lung adenocarcinoma. Cancer Res. 2017;77(16):4498–505. https://doi.org/10.1158/0008-5472.can-16-1944.
Schildhaus HU, Schultheis AM, Rüschoff J, Binot E, Merkelbach-Bruse S, Fassunke J, et al. MET amplification status in therapy-naïve adeno- and squamous cell carcinomas of the lung. Clin Cancer Res. 2015;21(4):907–15. https://doi.org/10.1158/1078-0432.ccr-14-0450.
Castiglione R, Alidousty C, Holz B, Wagener S, Baar T, Heydt C, et al. Comparison of the genomic background of MET-altered carcinomas of the lung: biological differences and analogies. Mod Pathol. 2019;32(5):627–38. https://doi.org/10.1038/s41379-018-0182-8.
Schuler M, Berardi R, Lim WT, de Jonge M, Bauer TM, Azaro A, et al. Molecular correlates of response to capmatinib in advanced non-small-cell lung cancer: clinical and biomarker results from a phase I trial. Ann Oncol. 2020;S0923-7534(20)36380-8. https://doi.org/10.1016/j.annonc.2020.03.293.
FDA approves first targeted therapy to treat aggressive form of lung cancer. US Food & Drug Administration. 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-treat-aggressive-form-lung-cancer. Accessed 15 July 2020.
Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJ, et al. Capmatinib (INC280) in METΔex14-mutated advanced non-small cell lung cancer (NSCLC): efficacy data from the phase II GEOMETRY mono-1 study. J Clin Oncol. 2019;37(15 suppl):9004. https://doi.org/10.1200/jco.2019.37.15_suppl.9004.
Acknowledgements
The authors thank Dr. Michael Magguilli (Oklahoma University Health Sciences Center) for his helpful advice that improved this paper.
Author information
Authors and Affiliations
Contributions
HGV contributed to the study conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, validation, supervision, writing-review, and editing. TQN contributed to the data curation, formal analysis, investigation, software, supervision, writing-review, and editing. HCN contributed to the data curation, formal analysis, investigation, validation, supervision, writing-review, and editing. PTN and ATNH contributed to the data curation, formal analysis, investigation, software, methodology, validation, supervision, writing-review, and editing. LH contributed to the data curation, formal analysis, investigation, software, methodology, project administration, validation, supervision, writing-review, and editing.
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflicts of Interest
HGV, TQN, HCN, PTN, ATNH, and LH have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Rights and permissions
About this article
Cite this article
Vuong, H.G., Nguyen, T.Q., Nguyen, H.C. et al. Efficacy and Safety of Crizotinib in the Treatment of Advanced Non-Small-Cell Lung Cancer with ROS1 Rearrangement or MET Alteration: A Systematic Review and Meta-Analysis. Targ Oncol 15, 589–598 (2020). https://doi.org/10.1007/s11523-020-00745-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-020-00745-7