Abstract
Advanced Merkel cell carcinoma (MCC) is a very aggressive, rare neuroendocrine tumor of the skin with a high frequency of locoregional recurrence and metastasis, and a high mortality rate. Surgical resection, sentinel lymph node biopsy, and radiotherapy represent the gold standard of treatment in patients with localized disease, while chemotherapy has a significant role in the treatment of advanced disease. However, no definitive evidence on the survival impact of radiotherapy in the advanced stages has been provided to date, and response to chemotherapy remains brief in the majority of cases, indicating an urgent need for alternative approaches. Biological and genome sequencing studies have implicated multiple molecular pathways in MCC, thus leading to the development of new agents that target angiogenic factors, anti-apoptosis molecules, poly-ADP ribose polymerase, intracellular signal proteins such as the PI3K/AKT/mTOR pathway, and peptide receptors such as somatostatin receptors. More recently, immunotherapy agents such as avelumab, pembrolizumab, and nivolumab, which act by blocking the programmed cell-death (PD)-1/PD-L1 immune checkpoint, have shown promising results, especially in the advanced setting, and should now be considered standard of care for metastatic MCC. Current research is focusing on developing new immunotherapeutic strategies, identifying predictive biomarker to aid in the selection of patients responsive to immunotherapy, and defining combination approaches to increase efficacy in refractory patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Merkel cell carcinoma (MCC) is a very aggressive disease, especially in the advanced setting, where currently available treatments such as radiotherapy, chemotherapy, and targeted therapy still only offer limited benefits to patients | |
Considering the strong association between MCC and immune functions, immunotherapy—both as monotherapy and in combination strategies—offers a potential for extended benefits in MCC patients, particularly in the advanced setting |
1 Introduction
Merkel cell carcinoma (MCC) is a rare neuroendocrine tumor (NET) of the skin with a very aggressive clinical course and high propensity for locoregional recurrence and metastasis [1]. Although still relatively rare—with an estimated 1500 new cases diagnosed in the USA each year [2]—the incidence of MCC has tripled over the past 20 years [3]. The Surveillance of Rare Cancers in Europe (RARECARE) project reported an incidence rate of 0.13 MCC cases per 100,000 between 1995 and 2002 [4]. The incidence of MCC increases with age [5, 6], and the mean age at diagnosis is approximately 75 years [7], although there are reports of MCC developing in children and adolescents [8, 9].
MCC was first described in 1972 by Toker [10] as “trabecular carcinoma of the skin”. Currently, pathological diagnosis of MCC using hematoxylin and eosin staining requires further confirmation by immunohistochemistry tests, including keratin-20 (CK-20), thyroid transcription factor (TTF)-1, CD56, chromogranin A, synaptophysin, and neurofilament protein (NFP) [11, 12]. In particular, CK-20 is a very sensitive marker for MCC, since CK-20-positive staining is found in 89–100% of cases [13].
In 2016, an updated staging system [14] was introduced following the analysis of prognostic factors in more than 9000 MCC cases. The new categorization is based on primary tumor size and disease extent at presentation, which is considered predictive of 5-year overall survival (OS), with estimates of 51, 35, and 14% for local, nodal, and distant disease, respectively. One of the main novelties of the updated staging system is the definition of distinct clinical and pathological prognostic stage groups, depending on how the disease is detected; moreover, patients with unknown primary tumors are regrouped into a separate substage, IIIA, which reflects improved prognosis compared with subjects with metastatic MCC and concurrent primary tumor [14].
Despite recent advances in therapeutic options, the clinical outcome for the majority of MCC patients remains poor. Progression to metastatic disease typically occurs within 3 years after diagnosis, with the most common sites being regional lymph nodes, distant skin, lung, central nervous system, bone, and liver [6].
1.1 Etiology
MCC development can be associated with integrated Merkel cell polyomavirus (MCPyV) [15] and/or with ultraviolet light (UV) exposure [16] or immunosuppression [17,18,19].
MCPyV is an abundant virus frequently detected on healthy human skin [20, 21] and in approximately 80% of MCC cases [15]. Serological evidence confirms that exposure to the virus is essentially ubiquitous in the general population [22, 23]. Human polyomavirus infection is usually asymptomatic, except in immunocompromised individuals, who can develop nephropathy (BK virus) or progressive multifocal leukoencephalopathy (JC virus) [24]. While the mechanism of MCPyV in the pathogenesis of MCC is still unclear, there is evidence that the virus plays a causal role in disease [25]. Recent studies have identified dermal fibroblasts as host cells supporting productive MCPyV infection [26]. In these cells, UV radiation and aging stimulate the Wnt signaling pathway and matrix metalloproteinase (MMP) expression, eventually promoting viral infection, and thus providing mechanistic insight into the role of these main risk factors [27].
Therefore, two distinct etiologies and two distinct pathological entities are currently being considered for MCC: virus-positive tumors, where MCPyV-encoded transforming genes (large and small T-antigens) support proliferation of neoplastic cells, and MCPyV-negative MCCs, where a complex pattern of UV-induced genetic changes act as driver mutations [28].
1.2 Genomic Landscape
The genomic landscape of MCC has recently been mapped, highlighting the presence of multiple and often unique distinct aberrations, as well as significant differences in the mutational profiles of tumors with and without MCPyV [29].
MCPyV-negative tumors are characterized by a higher overall mutation burden, presence of a UV-signature pattern (C > T transitions accounting for 85% of mutations), and a high number of predicted neoantigens [30,31,32]. Conversely, in MCPyV-positive tumors, the mutation burden is low and the UV signature is not observed [30, 31, 33].
Gene sequencing analysis conducted in the last few years has identified multiple potential driver mutations in MCPyV-negative tumors affecting several pathways, including TP53, the cell cycle pathway (RB1), genes involved in chromatin modification (ASXL1, MLL2, and MLL3), JNK (MAP3K1 and TRAF7), and DNA repair (ATM, MSH2, BAP1, BRCA1/2, CHEK2, FANCA, and MLH1) [31, 32, 34, 35]. Aberrations in the PI3K/AKT/mTOR pathway (AKT1, PIK3CG, AKT2, FBXW7, NF1, PIK3CA, PIK3R1, PTEN, or RICTOR), the MAPK pathway members (HRAS and NF1), and the receptor tyrosine kinase FGFR2 were also commonly seen in MCC [28, 34].
On the other hand, MCPyV-positive tumors harbor few somatic mutations and present more frequently with focal host genome amplifications and fusion transcripts caused by viral integration [33]. No TP53 mutations have been observed in these tumors, while some cases of mutations in the RB1 gene by mutated large T-antigen, or in the mTOR pathway by small T-antigen, have been reported [32]. Finally, according to a recent exome sequencing study, both types of tumors share some rare cancer-promoting mutations that can activate the PI3K pathway (HRAS, KRAS, PIK3CA, PTEN, and TSC1) and inactivate the Notch pathway (NOTCH1 and NOTCH2) [31].
A more comprehensive understanding of the etiology and mutagenic processes of viral and non-viral MCC provides an important framework for investigating potential new targets, and has profound implications for the effective treatment of these tumors.
In this paper, we review the current therapeutic options for patients with advanced MCC, including recent therapies under investigation in clinical trials, with a particular focus on the novel immunotherapy approach.
2 Methods
2.1 Search Strategy
Studies were identified by searching MEDLINE (PubMed), EMBASE and the ClinicalTrials.gov website.
Search terms included the following keywords: “Merkel cell carcinoma” associated with “Merkel cell polyomavirus”, “treatment”, “prognosis”; “chemotherapy”, “somatostatin analogues”; “peptide receptor radio-therapy”, “radiotherapy” and “targeted therapy”.
A specific focus was placed on current targeted immunotherapeutic agents for MCC using the following keywords: “Merkel cell carcinoma” and “immunotherapy”” associated or not with “T-cell antigen receptor specificity”, “antigens, neoplasm”, “tumor-infiltrating lymphocytes”, “next-generation sequencing”, “avelumab”, “pembrolizumab”, “nivolumab”, and “ipilimumab”.
The reference lists of the most important papers were also examined, and some authors were contacted by e-mail for further information about their work.
2.1.1 Inclusion/Exclusion Criteria
Inclusion was restricted to English-language articles published in peer-reviewed journals. Abstract communications from the most important conferences on NET were considered, while animal studies were excluded.
The review was limited to studies published through March 2018.
In the selection process, we favored randomized clinical trials and observational studies included or not in systematic reviews or meta-analyses. However, due to the limited evidence on certain topics, both case series and case reports were also included in this review.
2.1.2 Study Selection
Briefly, 1280 papers were identified. In the first stage, 1170 studies were excluded based on the publication title and abstract content. For the next stage, full text of all relevant scientific papers was analyzed; abstracts were considered when an extended version of the work was not available. Finally, a total of 42 papers were identified as eligible for this review.
A summary of the studies reviewed, including the type of study, treatment options, and reference or NCT number, can be found in Tables 1 and 2.
3 Current Therapeutic Options for Advanced MCC
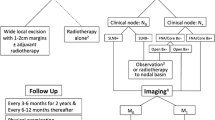
The selection of the best therapeutic approach is based on multiple factors including tumor stage, particularly with respect to nodal involvement, tumor location, and the patient’s general condition. In localized MCC (TNM stage I and II), surgery is the treatment of choice. A wide local excision with 1–2 cm resection margins up to the muscle fascia, associated with sentinel lymph node (SLN) biopsy, is recommended. If the SLN is involved, nodal dissection should be performed, or radiotherapy (RT) to the nodal basin in patients who may not tolerate a central neck lymph node dissection [49]. Adjuvant radiation therapy to the primary site and regional nodes in addition to lymph node dissection is generally recommended [49, 50].
3.1 Radiotherapy
Radiotherapy (RT) to the primary excision site and regional lymph node is most commonly used as adjuvant therapy after surgery in early-stage disease, to improve locoregional control and reduce the risk of recurrence. Radiotherapy may sometimes also have a role in advanced stages as palliative treatment when surgery is not feasible due to unresectable disease or in patients who are not candidates for surgical resection [51,52,53].
The effect of postoperative RT in the adjuvant setting has been investigated mainly in retrospective analyses, which have reported a decrease in the probability of local and regional recurrence after postoperative RT [54,55,56,57,58,59]. Moreover, adjuvant RT has been associated with improved survival, with a median OS of 45 months after surgery alone versus 63 months with surgery plus RT [55].
However, it is important to note that no definitive evidence on the survival impact of RT has been produced to date, and further studies are still needed to clarify whether patient SLN biopsy status should dictate the use of RT. A retrospective study conducted by Grotz et al. in 111 MCC patients suggested that nodal RT has no impact on recurrence rate or survival benefit in SLN biopsy-negative patients [60]. Conversely, a single-institution study conducted in 113 patients with non-metastatic MCC of the head and neck showed improved local and regional disease control after postoperative RT in both clinical node-negative and clinical/pathological node-positive patients [57].
According to the National Comprehensive Cancer Network (NCCN) 2017 guidelines, the recommended dosage of postoperative RT to the primary tumor site ranges from 50 to 66 Gy when the tumor is >1 cm, while in the case of widely excised small primary tumors (<1 cm) with negative margins and no other risk factors such as lymphovascular invasion or immunosuppression, the guidelines suggest only observation [50]. Radiotherapy (50–60 Gy) is also indicated after lymph node dissection in the presence of multiple involved lymph nodes or in cases of extracapsular extension [50].
Hypofractionated schedules (e.g., 30 Gy/10 fractions, 20 Gy/5 fractions) can be used in a palliative setting or in the case of patients with poor performance status [50]. It is also important to consider that to obtain the best outcomes, RT should be started within a few weeks after surgery [61].
When surgery is not feasible due to an unresectable tumor, patient refusal, or high risk of operative morbidity, RT may be used as single treatment. A recent systemic review suggests that in patients with locoregional macroscopic MCC who are not eligible for surgery, definitive RT may represent a potential cure, with clinically meaningful locoregional in-field control rates of 75–85% and 5-year OS ranging from 40 to 60% [52].
The recommended schedule for obtaining good local disease control is 55 Gy in 20–25 fractions. The use of doses >55 Gy is suggested for treatment of macroscopic disease, while a hypofractionated scheme of 25 Gy in 5 fractions or 8 Gy in a single fraction is suggested for patients with poor performance status and clinically significant comorbidities, or in palliative treatment of symptomatic or visceral lesions [51].
3.2 Chemotherapy
Chemotherapy in MCC is mainly reserved for patients with metastatic disease or as palliative therapy in symptomatic patients [62]. Most MCC chemotherapy regimens are extrapolated from those used for small cell lung cancer (SCLC), and comprise multiple agents such as cyclophosphamide, doxorubicin, vincristine, etoposide, cisplatin, and carboplatin [63].
MCC is generally considered a chemosensitive tumor; however, current literature data on the use of chemotherapy in the metastatic setting are still scant, with most studies being case series or case reports [62]. Due to the rarity of the disease, there are no randomized trials comparing different chemotherapy regimens, and the available data are still insufficient to completely assess the effect of chemotherapy on patient survival.
Overall, response to chemotherapy in metastatic disease ranges from 20 to 61% [62]: in the first-line or mostly first-line setting, response rates vary from 53 to 61% [39], while in the second-line setting they range from 23% in patients with known distant metastasis, to 45% in patients with unclear metastasis location (nodal and/or distant) [40]. Regardless of the line of therapy, durability of response is short, and most patients experience relapse within 8 months [62].
The impact of chemotherapy on OS is still unclear. A recent systematic literature review of 35 studies published up to January 2016 reported a median OS of 9 months [62].
In addition to the unclear benefit in OS, chemotherapy is associated with numerous toxicities, including fatigue, alopecia, gastrointestinal diseases, renal toxicity, and especially hematological effects such as febrile neutropenia and leukopenia [62]. In particular, regimens including platinum-based agents such as carboplatin or cisplatin, with or without etoposide or topotecan, are associated with significant adverse reactions [50, 64].
The current use of cytotoxic chemotherapies with unclear benefit and short duration of response highlights the need for alternative treatment options in MCC.
3.3 Targeted Therapies
The recent availability of novel targeted agents has provided new and promising opportunities for cancer care. Recent biological and genome sequencing studies have uncovered multiple molecularly targetable pathways implicated in MCC, including angiogenic growth factors and their receptors (e.g., vascular endothelial growth factor [VEGF]-A and VEGF-C, VEGF-R2, platelet-derived growth factor [PDGF]-α and PDGF-β), peptide receptors (e.g., somatostatin receptors [SSTR]1–5), intracellular signaling proteins (mTOR), and other target molecules (c-kit, Mcl-1, Bmi-1) [65].
3.3.1 Angiogenic Targets
Biological agents targeting angiogenesis (pazopanib, cabozantinib, and vatalanib) seem to be the most promising drugs in MCC and are currently under evaluation in phase I and II trials.
Pazopanib is a multi-tyrosine kinase inhibitor (TKI) that targets VEGFR, PDGFR, FGFR, and c-kit, and has shown clinical activity in a number of solid tumors, such as renal cell carcinoma [66]. Little information is available on the effects of pazopanib in MCC: so far, only one case report has been published, which reported a partial response (PR) in both the primary tumor and pulmonary metastases [41], and a phase II trial is ongoing in the UK (UKMCC-01) [42].
A similar phase II trial, which is currently in the active phase of recruitment, addresses the overexpression of VEGFR2 in MCC by testing the safety and efficacy of cabozantinib, a TKI with antiangiogenic activity against c-Met and VEGFR2 (NCT02036476) [65]. Lastly, a phase I trial already approved but not actively recruiting is evaluating dose-limiting toxicity (DLT) and maximum tolerated dose (MTD) for the combination of everolimus, an mTOR inhibitor, and vatalanib, a new oral antiangiogenic small molecule targeting all known VEGFRs, in patients with advanced solid tumors, including MCC (NCT00655655).
3.3.2 Anti-Apoptosis Targets
B-cell lymphoma 2 (BCL2) is an anti-apoptotic protein whose overexpression is associated with chemoresistance, aggressive clinical course, and poor survival [67]. Preclinical studies have also identified upregulation of the critical anti-apoptotic BCL2 gene in MCC [67, 68]. A multicenter phase II trial tested the safety and efficacy of BCL2 antisense therapy in patients with advanced MCC using oblimersen sodium, a novel BCL2 antisense agent (G3139, Genasense) designed to specifically bind to BCL2-mRNA, promoting its degradation and decrease in protein translation [38]. The study, conducted in 12 patients with histologically confirmed metastatic or regionally recurrent MCC, showed a good tolerability profile; however, no objective response for protocol-specified criteria was observed.
3.3.3 Poly-ADP Ribose Polymerase Inhibitors
A promising area of clinical research is the investigation of poly-ADP ribose polymerase (PARP) inhibitors in BRCA1/2-mutated NETs [69]. Veliparib is an oral PARP inhibitor currently under evaluation in a number of phase I–III clinical trials, including phase III studies in non-small cell lung cancer (NSCLC) and ovarian and breast cancer [70].
In the USA, a phase I trial is ongoing to evaluate DLT and MTD in a combination of veliparib plus capecitabine and temozolomide in patients with metastatic NET, including MCC (NCT02831179).
3.3.4 mTOR Inhibitors
Cohen et al. [34] reported abnormalities in the PI3K/AKT/mTOR pathway that can be targeted by specific inhibitors such as everolimus and temsirolimus in MCC. Since both MCPyV-positive and MCPyV-negative MCCs have shown activated PI3K/AKT signaling, PI3K and dual PI3K/mTOR inhibitors may be used as potential targeted therapies, although these agents have been proven to be ineffective in many tumors with pathway activation [71].
With regard to RICTOR amplification, recent studies have shown that this aberration may be targetable by investigational mTORC1/mTORC2 inhibitors such as AZD8055 and MLN0128. A phase I/II clinical trial is currently being conducted to assess the safety and efficacy of MLN012 in nine patients with recurrent/metastatic MCC (NCT02514824), while AZD8055 has been investigated in multiple phase I trials in advanced solid tumors (NCT00731263 and NCT00973076). Other phase I trials have investigated the possible synergistic effects of combination therapies including an mTOR inhibitor. Study NCT01155258 assessed the association between temsirolimus and vinorelbine ditartrate in 19 patients with unresectable or metastatic solid tumors, including MCCs, while study NCT00655655 investigated the possible synergistic effects of everolimus and vatalanib, as already mentioned above. Both trials have completed recruitment, but no results have yet been published.
3.4 Somatostatin Analogues and Peptide Receptor Radionuclide Therapy
Somatostatin receptors are up-regulated in MCC [72], thus providing a rationale for treatment with somatostatin analogues (SSAs) such as octreotide or lanreotide and peptide receptor radionuclide therapy (PRRT).
Some encouraging responses have been observed in case reports [44,45,46] in which a therapeutic radioisotope was coupled with a peptide of this class (90Y-DOTATOC), demonstrating clinical benefit with a favorable safety profile. A phase II trial evaluating the efficacy of 177Lutetium-DOTA-octreotate in NETs, including locally advanced or metastatic MCC, is currently ongoing (NCT01237457). Other non-radioactive SSAs, such as pasireotide (NCT01652547) and lanreotide (NCT02351128), are also being investigated for MCC treatment in clinical trials.
4 Immunotherapy
The discovery of MCPyV has revealed the existence of multiple interactions between the host immune system and MCC biology, improving our understanding of tumor pathogenesis and providing encouraging opportunities for immunotherapy [73].
Indeed, multiple studies have proven the existence of a spontaneous adaptive immune response against MCC tumor cells. This response is both humoral, with increased titers of virus-specific antibodies to MCPyV capsid protein (VP) and to T-antigen oncoproteins, and cellular, mediated by MCPyV-directed CD8+ and CD4+ T cells [73,74,75].
Intratumoral infiltration by MCPyV-specific T cells is particularly important in MCC, as it seems to be associated with improved survival and better prognosis [76]. Several studies have investigated the presence of tumor-infiltrating lymphocytes (TILs) in MCC patients and its association with response and survival, leading to the identification of some prognostic and predictive markers [76,77,78,79].
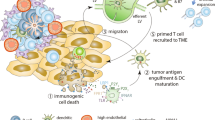
Despite the activation of an anti-tumor immune response, MCCs are often able to circumvent the host immune system, leading to tumor escape and progression [73]. One of the mechanisms of immune evasion employed by many MCCs is the downregulation of HLA class I expression on tumor cells, leading to a dysfunctional endogenous MCPyV-specific CD8+ T-cell response [80, 81]. Another crucial mechanism of evasion is related to the PD-1/PD-L1 pathway. The binding of PD-L1, an immune checkpoint protein, to its main receptor PD-1, expressed by activated T lymphocytes, leads to inhibition of kinase signaling pathways involved in T-cell proliferation, survival, and cytotoxic activity (including cytokine release), preventing overstimulation of the immune response [82]. In MCCs, PD-L1 can be expressed both by tumor cells and adjacent immune cell infiltrates, thus contributing to the emergence of immune escape [79, 83].
The discovery of immune-related inhibitory markers provides a rationale for investigating the therapeutic potential of immune checkpoint inhibitors in MCC [75]. Agents designed to restore, stimulate, or enhance the ability of the immune system to recognize and eliminate tumors such as anti PD-1/anti-PD-L1 antibodies have shown extremely promising results in a series of solid cancers [84], and have also achieved rapid and important progress in the treatment of MCC. Furthermore, the combination of immune stimulatory therapies (e.g., local and systemic immune therapies) seems to be a promising approach for overcoming immune evasion in MCC, especially in virus-driven cancers [85].
In summary, the immunotherapy approach in metastatic MCC is supported by:
-
The peculiar biology of this virus-induced tumor and its association with immunosuppression [86]. Patients affected by MCC present MCPyV-specific humoral and cellular immune responses, which are linked to the natural history of the disease [76]. Although immunogenic viral proteins are persistently expressed, MCC is eventually able to evade the immune system, leading to tumor progression. Understanding the mechanisms of MCC immune evasion is a crucial step for developing immune therapies to improve patient outcomes [73];
-
The expression of PD-L1 by MCPyV-specific CD8+ T cells, MCC tumor cells, and adjacent immune infiltrates, based on the clinical activity reported by anti-PD-L1 investigational drugs in tumors expressing PD-L1 on the cancer cell surface or in the tumor microenvironment (TME) [79].
-
The higher mutational burden of MCPyV-negative tumors [87, 88], in which a markedly elevated number of tumor-associated neoantigens are detected [88].
4.1 The Immune Contexture in MCC and Prognostic/Predictive Value of the Immune Infiltrate in MCPyV-Positive and MCPyV-Negative Tumors
A common theme emerging in most solid tumors, and also in MCC, is that the presence of a T-cell infiltrate and/or expression of inhibitory receptor ligands such as PD-L1 may have prognostic (e.g., improving survival) and/or predictive value for response to immunotherapy [76].
Patients with virus-positive MCC present a robust humoral and cellular immune response against MCPyV, which consists for example of a high hematic antibody titer, developed in response to immunogenic oncoproteins such as MCPyV T-antigens [89, 90] and by the presence of CD4+ and CD8+ T cells specific for viral oncoproteins within the tumor and in the peripheral blood [74, 75]. Chronic exposure to these oncoproteins in virus-positive MCC leads to the upregulation of inhibitory receptors such as PD-1 and T-cell immunoglobulin mucin (Tim)-3, with consequent development of an exhausted T-cell phenotype in the TME [79, 91]. Over time, this translates to the emergence of immune escape.
MCPyV-positive MCC not only presents TILs and frequent PD-L1 expression, but also circulating virus-specific T cells, which express different inhibitory receptors and whose frequency increases with progression of the disease [79]. Moreover, high levels of regulatory T cells have also been reported in several tumors [91]. Collectively, these immunosuppressive signals permit tumor evasion from cytotoxic T-cell attack.
Remarkably high numbers of tumor-associated neoantigens have been detected in virus-negative MCCs [88]. Even though the levels of PD-1/PD-L1 expression in virus-negative MCC seem to be lower than those found in virus-positive MCC [83], a subset of virus-negative tumors has shown high levels of TILs and PD-L1, matching the high mutational load in these tumors [28].
Additionally, patients with MCPyV-positive tumors seem to have better OS than patients with MCPyV-negative tumors, as already observed in other immunogenic tumors [92].
The high mutational burden observed in virus-negative MCC explains why T cells would easily respond to its neoantigens. In general, high infiltration by CD8+ T lymphocytes and upregulation of genes involved in immune responses correlate with a better prognosis [6, 14]. A recent study by Feldmeyer et al. showed that patients with higher CD3+ and CD8+ T-cell density at the tumor periphery had improved OS in MCPyV-positive, but not MCPyV-negative, MCC [93].
4.2 Current Immunotherapeutic Agents for MCC
In this section, current data on the activity and safety of immunotherapeutic agents in MCC are reviewed. Studies evaluating combinations of immunotherapeutic agents with other immunotherapies or targeted therapies are examined (Table 2).
4.2.1 Anti-PD-(L)1
Two anti-PD-(L)1 agents, pembrolizumab and avelumab, are in advanced stages of development for the treatment of MCC (Table 3). Nivolumab, a fully human IgG4 PD-1 immune checkpoint inhibitor, is also currently being evaluated for the treatment of MCC [94].
Pembrolizumab
Pembrolizumab (MK-3475) is a potent, highly selective IgG4-k humanized monoclonal antibody that inhibits the interaction between the PD-1 receptor and its ligands (PD-L1 and PD-L2). Pembrolizumab is characterized by a high affinity for PD-1, strong inhibition of PD-L1 and PD-L2, and robust activity in functionally modulating T-cell activity, as shown in an ex vivo assay conducted on human-donor blood cells [95].
A phase I study evaluated the safety, MTD, anti-tumor activity, pharmacokinetics, and pharmacodynamics of pembrolizumab in 30 patients with advanced solid tumors [96]. In this study, one complete response (CR) was observed after 90 weeks in a previously untreated MCC patient who received pembrolizumab 2 mg/kg every 3 weeks. Treatment-related adverse events (AEs), mainly fatigue, nausea, and pruritus, were observed in 70% of cases (21 patients), and were generally not serious and were easily manageable in clinical practice.
In a recent phase II trial [97], 26 previously untreated patients with stage IIIB or IV MCC and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 received at least one dose of pembrolizumab. The median age was 68 years (range: 57–91). Nine patients were classified as having MCPyV-negative and 17 (65%) MCPyV-positive tumors (35%). In this study, four patients achieved CR and ten patients had a partial response (PR), resulting in an overall response rate (ORR) of 56% (14 of 25 evaluable patients). Responses were observed in ten of 17 MCPyV-positive and in four of nine MCPyV-negative patients. Pretreatment PD-L1 expression was more frequent in virus-associated tumors, but did not predict response to pembrolizumab. Similarly, no relation was observed between the level of intratumoral CD8+ T-cell infiltration and patient response status (responder vs. non-responder).
Treatment-related AEs of any grade occurred in 77% of patients, with fatigue and laboratory abnormalities such as anemia, leukocytosis, and thrombocytopenia being the most common. Grade 3 or 4 treatment-related AEs were observed in four of the 26 patients (15%): one patient had G4 myocarditis after one dose of pembrolizumab, and another had G4 hypertransaminasemia after two doses of pembrolizumab. Both AEs resolved after discontinuation of pembrolizumab and initiation of steroids. Both also had durable tumor regression (one PR and one CR).
At the 2018 American Society of Clinical Oncology (ASCO) annual meeting, Nghiem et al. presented additional data on an expansion cohort of 24 patients, for a total of 50 patients followed for up to 2 years. The results, which represent the longest observation of anti-PD-1 therapy in patients with advanced MCC, confirmed the beneficial effect of pembrolizumab, with durable tumor control (ORR: 50%, CR: 19%, PR: 31%), a favorable survival rate (PFS not yet reached and 68% OS at 18 months), and a manageable safety profile [98].
Avelumab
Avelumab (MSB0010718C) is a fully human anti-PD-L1 IgG1 monoclonal antibody that in 2017 received accelerated approval by the US Food and Drug Administration (FDA) for the treatment of MCC, and was recently approved in Europe and in Japan [99,100,101]. Avelumab seems to stimulate both the adaptive and innate immune response—the former thanks to the inhibition of PD-L1/PD-1, which in turns leads to T-cell activation, and the latter by inducing antibody-dependent cell-mediated cytotoxicity (ADCC) through the native Fc region.
The JAVELIN Merkel 200 trial investigated the safety and efficacy of avelumab 10 mg/kg every 2 weeks in 88 patients previously treated for metastatic MCC [27]. The response rate after 10.4 months was 31.8% (20 PR and 8 CR), with stable disease in 10% of cases (SD). Seventy percent of patients suffered treatment-related AEs. In most cases these were grade 1–2 fatigue or infusion-related reactions. A limited number of patients suffered more serious adverse reactions (two grade 3 lymphopenia and three grade 3 laboratory abnormalities); however, no grade 4 treatment-related AEs or treatment-related deaths occurred. The results of this study suggest that avelumab might be beneficial in patients whose tumors are not driven by status of PD-L expression of MCPyV, since no correlation was detected between these characteristics and patient response rates.
The long-term results of the JAVELIN Merkel 200 trial were recently published, also confirming the efficacy of avelumab in this population with longer follow-up. After ≥1 year of follow-up, ORR was 33.0% (with 11.4% CR), responses were durable, with a median duration of response not reached at the time of the analysis, the median OS was 12.9 months, and 1-year PFS was 30%. According to subgroup analysis, patients with fewer prior lines of systemic treatment, lower disease burden at baseline, and PD-L1-positive tumors had a higher probability of response. The longer follow-up analysis further confirmed that PD-L1 expression and MCPyV status did not affect the duration of response to avelumab [102]. These results were further confirmed after ≥2 years of follow-up, as presented at the 2018 ASCO meeting [103].
A second part of the JAVELIN Merkel 200 trial is currently testing the safety and efficacy of avelumab as first-line treatment in patients with metastatic MCC [104]. The recently published interim analysis, which reported the results on 29 patients with at least 3 months of follow-up, showed high rates of response and a good tolerability profile for avelumab as first-line therapy. Specifically, the study reported an ORR of 62.1%, with 77.8% of the responses still ongoing at the time of the analysis; the rate of ongoing response was estimated at 93% at 3 months and 83% at 6 months. Moreover, avelumab was generally safe, with no treatment-related grade 4 AEs or deaths. These results further support approval of avelumab as standard of care for metastatic MCC [104].
Nivolumab
A recently published case report has highlighted the potential benefit of nivolumab in terms of durability of response in metastatic MCC [94]. Indeed, the case shows achievement of a PR after two cycles of nivolumab, and partial metabolic response (assessed by FDG-PET/CT) by cycle 5. After cycle 6, the patient was treated with intravenous steroids due to pneumonia and autoimmune hepatitis. He was subsequently discharged on prednisone 1 mg/kg per day, and after a slow steroid taper and no further treatment with nivolumab, he maintained an excellent PR in distant metastasis, with no new sites of disease and no recurrence on physical examination for 8 months.
The results of a phase I/II study (NCT02488759) recently presented at the ASCO meeting highlighted the good safety profile and the beneficial effects of nivolumab as neoadjuvant treatment in patients with resectable MCC. The study investigated the response to nivolumab, administered intravenously at a dose of 240 mg for 4 weeks before surgery, in 25 patients with resectable MCC, including 18 subjects with MCPyV-positive cancer. Substantial radiological and pathological tumor regression was observed in 45 and 65% of patients, respectively, obviating in some cases the need for a subsequent surgery, and after 12 months most of the subjects were free of tumor progression [105].
4.2.2 Ipilimumab
Ipilimumab inhibits the checkpoint molecule CTLA-4 and is approved as immunotherapy for metastatic melanoma [106]. This new drug seems to improve recurrence-free survival in the adjuvant setting, in patients with completely resected high-risk stage III melanoma [107]. The effects of ipilimumab as adjuvant therapy in patients with resected MCC were further investigated in comparison to close observation in a European phase II trial (NCT02196961). The preliminary results in 47 patients led to the early termination of the study, as no significant difference in disease-free survival was observed between the treatment and observation arms, and patients who received ipilimumab showed fourfold increased occurrence of AEs, including ≥ grade 3 AEs. Based on these results, adjuvant ipilimumab should not be considered in MCC patients [108].
4.2.3 Safety of Checkpoint Inhibitors
Despite clinical benefit [109, 110], many immune checkpoint inhibitors have been associated with immune-related AEs (irAEs). In general, toxicities related to immunotherapy may be divided into “on-target” and “off-target” [111]. The former occurs when the immune activation is directed against antigens present on healthy cells, while the latter is caused by the modulation of other targets [111], such as alterations in immunological tolerance.
Depending on the type of checkpoint inhibitors, AEs may range from diarrhea, colitis, hepatitis, and skin toxicities, to more serious endocrinopathies such as hypophysitis or thyroid dysfunction [112].
In particular, treatment with monoclonal antibodies that blocks the CTLA-4 and PD-1 immune checkpoints leads to activation of T cells specific to tumor antigens and potentially to self-antigens [113]. This may induce autoinflammatory irAEs, which are mostly grade 1–2 and are usually reversible [114], despite requiring attentive monitoring. The most common types of irAEs reported with checkpoint pathway inhibitors include gastrointestinal, dermatological, and endocrine side effects [114].
4.3 Potential Novel Immunotherapy Strategies for MCC
4.3.1 Combination Immunotherapy
Immunotherapy combinations have demonstrated superior efficacy over single agents in the treatment of various cancer types, including melanoma [115]. Combining immunotherapies may increase the risk of irAEs [116], even though these appear to be manageable and in most cases resolve with the use of immune-modulatory agents [117].
A phase I/II study (NCT02488759) is currently recruiting participants to investigate the safety and effectiveness of nivolumab, alone or in combination therapy, in patients with virus-associated tumors, such as Epstein-Barr virus (EBV)-positive gastric and nasopharyngeal cancer; human papillomavirus-positive cervical, vaginal, and vulvar cancer, and squamous cell cancer of the head and neck (SCCHN); and MCPyV-positive MCC.
Another research strategy in this field includes the use of cellular adoptive immunotherapy to stimulate the immune system and stop tumor cells from growing. Aldesleukin is a recombinant IL-2 which may stimulate white blood cells to kill tumor cells. A phase I/II trial (NCT01758458) testing the side effects, best administration schedule, and efficacy of laboratory-treated autologous T cells together with aldesleukin in patients with metastatic MCC was recently terminated.
Lastly, an open-label multicenter phase I/II study (NCT02643303) is now under way that is investigating an anti-CTLA-4 antibody, tremelimumab, and the anti-PD-L1 antibody durvalumab (MEDI4736) in combination with the TME modulator poly-ICLC, a TLR3 agonist, in subjects with advanced, measurable, biopsy-accessible cancers, including MCC.
4.3.2 Other Immunotherapy Strategies
A natural killer cell line, activated NK-92 natural killer cells (aNK; formerly Neukoplast), recovered from a patient with a large granular lymphoma is now under evaluation in a multi-center, non-randomized, open-label phase II trial (NCT02465957) in patients with unresectable stage III (IIIB) or metastatic (stage IV) MCC.
Talimogene laherparepvec is a genetically modified herpes simplex virus type 1 that replicates within tumor cells and produces granulocyte–macrophage colony-stimulating factor (GM-CSF) to enhance anti-tumor immune response [118]. A phase II trial (NCT02978625) investigating talimogene laherparepvec and nivolumab in patients with lymphomas or non-melanoma skin cancers that did not respond to standard treatment is about to start recruitment.
5 Expert Commentary
MCC is a very aggressive disease with a high rate of mortality. Surgical resection, SLN biopsy, and RT remain the gold standard treatment in patients with localized disease. Chemotherapy plays a significant role in the treatment of advanced disease, but in the majority of cases remission is brief [119], indicating an urgent need for alternative approaches.
Incidence and prognosis of MCC are strongly related to immune function in both virus-positive and virus-negative MCCs [120]. Immunotherapy offers a potential for extended benefits in MCC, particularly in advanced disease. Further research is needed to identify predictive biomarkers capable of selecting patients responsive to immunotherapy. On the other hand, combination therapies, such as nivolumab and ipilimumab, may demonstrate some efficacy in patients whose disease proves refractory to immune checkpoint monotherapy [121]. However, this combination may be associated with increased toxicity, which should be carefully considered, especially given the advanced age and the overall frail status of MCC patients.
Furthermore, the successful treatment of patients on immunotherapy depends on timely identification and correct management of immune-related AEs. Serious irAEs, if not adequately managed, can lead to treatment discontinuation and life-threatening sequelae. For this reason, a multidisciplinary comprehensive approach within a team of experts is certainly the first step in the management of patients with MCC experiencing toxicities with immunotherapies.
6 Future Perspectives
MCC is a rare and aggressive skin cancer, with overall 5-year survival rates of 51, 35, and 14% for local, nodal, and distant disease, respectively [14]. Given the poor prognosis, interdisciplinary management and effective strategies to improve survival are needed.
In the last few years, tremendous advances have been made in the treatment of NETs, thanks to the development of immunotherapy. Despite the positive results, however, more work still needs to be done.
The identification of predictive biomarkers and clinical factors is needed to help predict response in MCPyV-positive versus MCPyV-negative patients, and in PD-L1-positive versus PD-L1-negative MCC. Significant advances in cancer immunobiology, including whole-exome sequencing, RNA expression evaluation, T-cell receptor sequencing, proteomics, multiplex immunohistology, flow cytometry, epigenetics, and microbiome composition, will play an important role in identifying future biomarkers to guide treatment selection for each patient [122].
Despite the emergence of extremely encouraging clinical results regarding immunotherapy in solid tumors thanks to the inhibition of CTLA-4 and PD-1 (and especially of their combination), there are still numerous non-responder patients, or patients whose survival could be greatly prolonged. How can we improve outcomes in this setting? The challenge for researchers is to obtain a response even from patients whose tumors do not seem to have the immunogenic characteristics initially required. The main strategy would be to enhance access of lymphocytes to the TME. First of all, it is believed that some potential approaches (such as chemotherapy, radiotherapy, surgery, targeted therapy, and antiangiogenic and hormone therapy), when combined (in association or in sequence) with immunotherapeutic agents capable of modulating the immune system (blocking [CTLA-4, PD-1/PD-L1, LAG3, TIM3] or stimulating [ICOS, vaccines, cytokines, oncolytic viruses] agents), could achieve this goal through greater release of endogenous tumor antigens, reduced influence of immunosuppressive mechanisms, and greater activation or specificity of T lymphocytes. It is also well known that many of the conventional treatments, including targeted therapies and antiangiogenic therapies, are able to reduce the negative influence of the suppressor cells contributing to the secondary improvement in tumor immunity [123].
Another future challenge for researchers is represented by individualized immunotherapy for each patient, which could be achieved thanks to the development of adoptive T-cell therapy or the identification and exploitation of single cancer neoantigens.
Lastly, it is important to emphasize that new molecules of the immune checkpoint family (both inhibitors and activators) are in the initial phase of investigation and could enrich the armamentarium of oncological drugs based on immunomodulation in the near future.
References
Lemos BD, Storer BE, Iyer JG, Phillips JL, Bichakjian CK, Fang LC, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63:751–61.
American Cancer Society. Key statistics for Merkel cell carcinoma. 2016. http://www.cancer.org/cancer/skincancer-Merkelcell/detailedguide/skin-cancer-Merkel-cell-carcinoma-key-statistics. Accessed 22 April 2017.
Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1–4.
van der Zwan JM, Trama A, Otter R, Larranaga N, Tavilla A, Marcos-Gragera R, et al. Rare neuroendocrine tumours: results of the surveillance of rare cancers in Europe project. Eur J Cancer. 2013;49:2565–78.
Banks PD, Sandhu S, Gyorki DE, Johnston ML, Rischin D. Recent insights and advances in the management of Merkel cell carcinoma. J Oncol Pract. 2016;12:637–46.
Schadendorf D, Lebbé C, Zur Hausen A, Avril MF, Hariharan S, Bharmal M, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017;71:53–69.
Albores-Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, Henson DE. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population-based study. J Cutan Pathol. 2010;37:20–7.
Koksal Y, Toy H, Talim B, Unal E, Akcoren Z, Cengiz M. Merkel cell carcinoma in a child. J Pediatr Hematol Oncol. 2009;31:359–61.
Marzban S, Geramizadeh B, Farzaneh MR. Merkel cell carcinoma in a 17-year-old boy, report of a highly aggressive fatal case and review of the literature. Rare Tumors. 2011;3:e34.
Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105:107–10.
Kotteas EA, Pavlidis N. Neuroendocrine Merkel cell nodal carcinoma of unknown primary site: management and outcomes of a rare entity. Crit Rev Oncol Hematol. 2015;94:116–21.
Johansson L, Tennvall J, Akerman M. Immunohistochemical examination of 25 cases of Merkel cell carcinoma: a comparison with small cell carcinoma of the lung and oesophagus, and a review of the literature. APMIS. 1990;98:741–52.
Scott MP, Helm KF. Cytokeratin 20: a marker for diagnosing Merkel cell carcinoma. Am J Dermatopathol. 1999;21:16–20.
Harms KL, Healy MA, Nghiem P, Sober AJ, Johnson TM, Bichakjian CK, et al. Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann Surg Oncol. 2016;23:3564–71.
Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–100.
Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832–41.
Lanoy E, Dores GM, Madeleine MM, Toro JR, Fraumeni JF Jr, Engels EA. Epidemiology of nonkeratinocytic skin cancers among persons with AIDS in the United States. AIDS. 2009;23:385–93.
Lanoy E, Costagliola D, Engels EA. Skin cancers associated with HIV infection and solid-organ transplantation among elderly adults. Int J Cancer. 2010;126:1724–31.
Koljonen V, Kukko H, Tukiainen E, Böhling T, Sankila R, Pukkala E, et al. Incidence of Merkel cell carcinoma in renal transplant recipients. Nephrol Dial Transplant. 2009;24:3231–5.
Foulongne V, Sauvage V, Hebert C, Dereure O, Cheval J, Gouilh MA, et al. Human skin microbiota: high diversity of DNA viruses identified on the human skin by high throughput sequencing. PLoS One. 2012;7:e38499.
Schowalter RM, Pastrana DV, Pumphrey KA, Moyer AL, Buck CB. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7:509–15.
Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5:e1000363.
Tolstov YL, Pastrana DV, Feng H, Becker JC, Jenkins FJ, Moschos S, et al. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;125:1250–6.
Wiedinger K, Bitsaktsis C, Chang S. Reactivation of human polyomaviruses in immunocompromised states. J Neuro-Oncol. 2014;20:1–8.
Chang Y, Moore PS. Merkel cell carcinoma: a virus-induced human cancer. Annu Rev Pathol. 2012;7:123–44.
Liu W, Yang R, Payne AS, Schowalter RM, Spurgeon ME, Lambert PF, et al. Identifying the target cells and mechanisms of Merkel cell polyomavirus infection. Cell Host Microbe. 2016;19:775–87.
Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–85.
Wong SQ, Waldeck K, Vergara IA, Schröder J, Madore J, Wilmott JS, et al. UV-associated mutations underlie the etiology of MCV-negative Merkel cell carcinomas. Cancer Res. 2015;75:5228–34.
Cassler NM, Merrill D, Bichakjian CK, Brownell I. Merkel Cell Carcinoma Therapeutic Update. Curr Treat Options in Oncol. 2016;17:36.
Harms PW, Vats P, Verhaegen ME, Robinson DR, Wu YM, Dhanasekaran SM, et al. The Distinctive mutational spectra of polyomavirus-negative Merkel cell carcinoma. Cancer Res. 2015;75:3720–7.
Goh G, Walradt T, Markarov V, Blom A, Riaz N, Doumani R, et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget. 2016;7:3403–15.
Mauzo SH, Ferrarotto R, Bell D, Torres-Cabala CA, Tetzlaff MT, Prieto VG, et al. Molecular characteristics and potential therapeutic targets in Merkel cell carcinoma. J Clin Pathol. 2016;69:382–90.
Starrett GJ, Marcelus C, Cantalupo PG, Katz JP, Cheng J, Akagi K, et al. Merkel cell polyomavirus exhibits dominant control of the tumor genome and transcriptome in virus-associated Merkel cell carcinoma. MBio. 2017;8:e02079–16.
Cohen P, Tomson BN, Elkin SK, Marchlik E, Carter JL, Kurzrock R. Genomic portfolio of Merkel cell carcinoma as determined by comprehensive genomic profiling: implications for targeted therapeutics. Oncotarget. 2016;7:23454–67.
Harms PW, Collie AM, Hovelson DH, Cani AK, Verhaegen ME, Patel RM, et al. Next generation sequencing of cytokeratin 20-negative Merkel cell carcinoma reveals ultraviolet-signature mutations and recurrent TP53 and RB1 inactivation. Mod Pathol. 2016;29:240–8.
Tai PT, Yu E, Winquist E, Hammond A, Stitt L, Tonita J, et al. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases. J Clin Oncol. 2000;18:2493–9.
Tai P, Yu E, Assouline A, Lian JD, Joseph K, Miale T, et al. Multimodality management for 145 cases of Merkel cell carcinoma. Med Oncol. 2010;27:1260–6.
Shah MH, Varker KA, Collamore M, Zwiebel JA, Coit D, Kelsen D, et al. G3139 (Genasense) in patients with advanced Merkel cell carcinoma. Am J Clin Oncol. 2009;32:174–9.
Satpute SR, Ammakkanavar NR, Einhorn LH. Role of platinum-based chemotherapy for Merkel cell tumor in adjuvant and metastatic settings. J Clin Oncol. 2014;32:9049.
Iyer JG, Blom A, Doumani R, Lewis C, Tarabadkar ES, Anderson A, et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med. 2016;5:2294–301.
Davids MS, Charlton A, Ng SS, Chong ML, Laubscher K, Dar M, et al. Response to a novel multitargeted tyrosine kinase inhibitor pazopanib in metastatic Merkel cell carcinoma. J Clin Oncol. 2009;27:e97–100.
Nathan PD, Gaunt P, Wheatley K, Bowden SJ, Savage J, Faust G, et al. UKMCC-01: A phase II study of pazopanib (PAZ) in metastatic Merkel cell carcinoma. J Clin Oncol. 2016;34:9542.
Samlowski WE, Moon J, Tuthill RJ, Heinrich MC, Balzer-Haas NS, Merl SA, et al. A phase II trial of imatinib mesylate in Merkel cell carcinoma (neuroendocrine carcinoma of the skin): A Southwest Oncology Group study (S0331). Am J Clin Oncol. 2010;33:495–9.
Fakiha M, Letertre P, Vuillez JP, Lebeau J. Remission of Merkel cell tumor after somatostatin analog treatment. J Cancer Res Ther. 2010;6:382–4.
Meier G, Waldherr C, Herrmann R, Maecke H, Mueller-Brand J, Pless M. Successful targeted radiotherapy with 90Y-DOTATOC in a patient with Merkel cell carcinoma. Oncology. 2004;66:160–3.
Salavati A, Prasad V, Schneider C-P, Herbst R, Baum RP. Peptide receptor radionuclide therapy of Merkel cell carcinoma using 177lutetium-labeled somatostatin analogs in combination with radiosensitizing chemotherapy: a potential novel treatment based on molecular pathology. Ann Nucl Med. 2012;26:365–9.
Luke JJ, Chmura SJ, Allred JB, Salama JK, Al-Hallaq HA, Hsu C, et al. A randomized phase II study of anti-PD1 antibody [MK-3475 (pembrolizumab)] alone versus anti-PD1 antibody plus stereotactic body radiation therapy in advanced Merkel cell carcinoma (Alliance A091605). J Clin Oncol. 2018;36:TPS9599.
Walker J, Kasturi V, Lebbe C, Sandhu SK, Grignani G, Hennessy MG, et al. Second-line avelumab treatment of patients (pts) with metastatic Merkel cell carcinoma (mMCC): Experience from a global expanded access program (EAP). J Clin Oncol. 2018;36:9537.
Tai P. A practical update of surgical management of Merkel cell carcinoma of the skin. ISRN Surg. 2013;2013:850797.
Bichakjian CK, Olencki T, Aasi SZ, Alam M, Anderson JS, Blitzblau R, et al. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Merkel Cell Carcinoma. Version 1. 2018. 2017. https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf. Accessed 20 May 2018.
Veness M, Foote M, Gebski V, Poulsen M. The role of radiotherapy alone in patients with Merkel cell carcinoma: reporting the Australian experience of 43 patients. Int J Radiat Oncol Biol Phys. 2010;78:703–9.
Gunaratne DA, Howle JR, Veness MJ. Definitive radiotherapy for Merkel cell carcinoma confers clinically meaningful in-field locoregional control: A review and analysis of the literature. J Am Acad Dermatol. 2017;77:142–8.
Mortier L, Mirabel X, Fournier C, Piette F, Lartigau E. Radiotherapy alone for primary Merkel cell carcinoma. Arch Dermatol. 2003;139:1587–90.
Lewis KG, Weinstock MA, Weaver AL, Otley CC. Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol. 2006;142:693–700.
Mojica P, Smith D, Ellenhorn JD. Adjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skin. J Clin Oncol. 2007;25:1043–7.
Kim JA, Choi AH. Effect of radiation therapy on survival in patients with resected Merkel cell carcinoma: a propensity score surveillance, epidemiology, and end results database analysis. JAMA Dermatol. 2013;149:831–8.
Strom T, Naghavi AO, Messina JL, Kim S, Torres-Roca JF, Russell J, et al. Improved local and regional control with radiotherapy for Merkel cell carcinoma of the head and neck. Head Neck. 2017;39:48–55.
Jouary T, Leyral C, Dreno B, Doussau A, Sassolas B, Beylot-Barry M, et al. Adjuvant prophylactic regional radiotherapy versus observation in stage I Merkel cell carcinoma: a multicentric prospective randomized study. Ann Oncol. 2012;23:1074–80.
Bichakjian CK, Harms KL, Schwartz JL. Selective use of adjuvant therapy in the management of Merkel cell carcinoma. JAMA Oncol. 2015;1:1162–3.
Grotz TE, Joseph RW, Pockaj BA, Foote RL, Otley CC, Bagaria SP, et al. Negative sentinel lymph node biopsy in Merkel cell carcinoma is associated with a low risk of same-nodal-basin recurrences. Ann Surg Oncol. 2015;22(12):4060–6.
Tsang G, O’Brien P, Robertson R, Hamilton C, Wratten C, Denham J. All delays before radiotherapy risk progression of Merkel cell carcinoma. Australas Radiol. 2004;48:371–5.
Nghiem P, Kaufman HL, Bharmal M, Mahnke L, Phatak H, Becker JC. Systematic literature review of efficacy, safety and tolerability outcomes of chemotherapy regimens in patients with metastatic Merkel cell carcinoma. Future Oncol. 2017;13:1263–79.
Desch L, Kunstfeld R. Merkel cell carcinoma: chemotherapy and emerging new therapeutic options. J Skin Cancer. 2013;2013:327150.
Cowey CL, Mahnke L, Espirito J, Helwig C, Oksen D, Bharmal M. Real-world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA. Future Oncol. 2017;13:1699–710.
Brunner M, Thurnher D, Pammer J, Geleff S, Heiduschka G, Reinisch CM, et al. Expression of VEGF-A/C, VEGF-R2, PDGF-alpha/beta, c-kit, EGFR, Her-2/Neu, Mcl-1 and Bmi-1 in Merkel cell carcinoma. Mod Pathol. 2008;21:876–84.
Welsh SJ, Fife K. Pazopanib for the treatment of renal cell carcinoma. Future Oncol. 2015;11:1169–79.
Mita M, Tolcher AW. Novel apoptosis inducing agents in cancer therapy. Curr Probl Cancer. 2005;29:8–32.
Schlagbauer-Wadl H, Klosner G, Heere-Ress E, Waltering S, Moll I, Wolff K, et al. Bcl-2 antisense oligonucleotides (G3139) inhibit Merkel cell carcinoma growth in SCID mice. J Invest Dermatol. 2000;114:725–73.
Scarpa A, Chang DK, Nones K, Corbo V, Patch AM, Bailey P, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543:65–71.
Isakoff SJ, Puhalla S, Domchek SM, Friedlander M, Kaufman B, Robson M, et al. A randomized Phase II study of veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in BRCA1/2 metastatic breast cancer: design and rationale. Future Oncol. 2017;13:307–20.
Nardi V, Song Y, Santamaria-Barria JA, Cosper AK, Lam Z, Faber AC, et al. Activation of PI3K signaling in Merkel cell carcinoma. Clin Cancer Res. 2012;18:1227–36.
Papotti M, Macri’ L, Pagani A, Aloi F, Bussolati G. Quantitation of somatostatin receptor type 2 in neuroendocrine (Merkel cell) carcinoma of the skin by competitive RT-PCR. Endocr Pathol. 1999;10:37–46.
Bhatia S, Afanasiev O, Nghiem P. Immunobiology of Merkel cell carcinoma: implications for immunotherapy of a polyomavirus-associated cancer. Curr Oncol Rep. 2011;13:488–97.
Lyngaa R, Pedersen NW, Schrama D, Thrue CA, Ibrani D, Met O, et al. T-cell responses to oncogenic Merkel cell polyomavirus proteins distinguish patients with Merkel cell carcinoma from healthy donors. Clin Cancer Res. 2014;20:1768–78.
Iyer JG, Afanasiev OK, McClurkan C, Paulson K, Nagase K, Jing L, et al. Merkel cell polyomavirus-specific CD8+ and CD4+ T-cell responses identified in Merkel cell carcinomas and blood. Clin Cancer Res. 2011;17:6671–80.
Miller NJ, Church CD, Dong L, Crispin D, Fitzgibbon MP, Lachance K, et al. Tumor-infiltrating Merkel cell polyomavirus-specific T cells are diverse and associated with improved patient survival. Cancer Immunol Res. 2017;5:137–47.
Behr DS, Peitsch WK, Hametner C, Lasitschka F, Houben R, Schönhaar K, et al. Prognostic value of immune cell infiltration, tertiary lymphoid structures and PD-L1 expression in Merkel cell carcinomas. Int J Clin Exp Pathol. 2014;7:7610–21.
Walsh NM, Fleming KE, Hanly JG, Dakin Hache K, Doucette S, Ferrara G, et al. A morphological and immunophenotypic map of the immune response in Merkel cell carcinoma. Hum Pathol. 2016;52:190–6.
Afanasiev OK, Yelistratova L, Miller N, Nagase K, Paulson K, Iyer JG, et al. Merkel polyomavirus specific T cells fluctuate with Merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markers. Clin Cancer Res. 2013;19:5351–60.
Paulson KG, Tegeder A, Willmes C, Iyer JG, Afanasiev OK, Schrama D, et al. Downregulation of MHC-I expression is prevalent but reversible in Merkel cell carcinoma. Cancer Immunol Res. 2014;2:1071–9.
Ritter C, Fan K, Paschen A, Reker Hardrup S, Ferrone S, Nghiem P, et al. Epigenetic priming restores the HLA class-I antigen processing machinery expression in Merkel cell carcinoma. Sci Rep. 2017;7:2290.
Zitvogel L, Kroemer G. Targeting PD-1/ PD-L1 interactions for cancer immunotherapy. Oncoimmunology. 2012;1:1223–5.
Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, Wang H, et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1:54–63.
Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011;16:5–24.
Chapuis AG, Afanasiev OK, Iyer JG, Paulson KG, Parvathaneni U, Hwang JH, et al. Regression of metastatic Merkel cell carcinoma following transfer of polyomavirus-specific T cells and therapies capable of re-inducing HLA class-I. Cancer Immunol Res. 2014;2:27–36.
Arora R, Chang Y, Moore PS. MCV and Merkel cell carcinoma: a molecular success story. Curr Opin Virol. 2012;2:489–98.
Veija T, Sarhadi VK, Koljonen V, Bohling T, Knuutila S. Hotspot mutations in polyomavirus positive and negative Merkel cell carcinomas. Cancer Genet. 2016;209:30–5.
Vandeven N, Nghiem P. Rationale for immune-based therapies in Merkel polyomavirus-positive and negative Merkel cell carcinomas. Immunotherapy. 2016;8:907–21.
Paulson KG, Lewis CW, Redman MW, Simonson WT, Lisberg A, Ritter D, et al. Viral oncoprotein antibodies as a marker for recurrence of Merkel cell carcinoma: A prospective validation study. Cancer. 2017;123:1464–74.
Samimi M, Molet L, Fleury M, Laude H, Carlotti A, Gardair C, et al. Prognostic value of antibodies to Merkel cell polyomavirus T antigens and VP1 protein in patients with Merkel cell carcinoma. Br J Dermatol. 2016;174:813–22.
Dowlatshahi M, Huang V, Gehad AE, Jiang Y, Calarese A, Teague JE, et al. Tumor-specific T cells in human Merkel cell carcinomas: a possible role for Tregs and T-cell exhaustion in reducing T-cell responses. J Invest Dermatol. 2013;133:1879–89.
Sihto H, Kukko H, Koljonen V, Sankila R, Böhling T, Joensuu H. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:938–45.
Feldmeyer L, Hudgens CW, Ray-Lyons G, Nagarajan P, Aung PP, Curry JL, et al. Density, distribution, and composition of immune infiltrates correlate with survival in Merkel cell carcinoma. Clin Cancer Res. 2016;22:5553–63.
Walocko FM, Scheier BY, Harms PW, Fecher LA, Lao CD. Metastatic Merkel cell carcinoma response to nivolumab. J Immunother Cancer. 2016;4:79.
Shimizu T, Seto T, Hirai F, Takenoyama M, Nosaki K, Tsurutani J, et al. Phase 1 study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced solid tumors. Investig New Drugs. 2016;34:347–54.
Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody in patients with advanced solid tumors). Clin Cancer Res. 2015;21:4286–93.
Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374:2542–52.
Nghiem P, Bhatia S, Lipson EJ, Sharfman WH, Kudchadkar RR, Friedlander PA, et al. Durable tumor regression and overall survival (OS) in patients with advanced Merkel cell carcinoma (aMCC) receiving pembrolizumab as first-line therapy. J Clin Oncol. 2018;36:9506.
Bavencio (avelumab) injection [package insert]. Darmstadt, Germany: Merck KGaA. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761049s000lbl.pdf. Accessed 15 July 2018.
European Commission approves Bavencio (avelumab) for metastatic Merkel cell carcinoma. Darmstadt: Merck KGaA and Pfizer Inc. 2017. https://www.pfizer.com/news/press-release/press-release-detail/european_commission_approves_bavencio_avelumab_for_metastatic_merkel_cell_carcinoma. Accessed 15 July 2018.
Bavencio (avelumab) approved for Merkel cell carcinoma in Japan. Darmstadt, Germany: Merck KGaA and Pfizer Inc; September 21, 2017. https://www.pfizer.com/news/press-release/press-release-detail/bavencio_avelumab_approved_for_merkel_cell_carcinoma_in_japan. Accessed 15 July 2018.
Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer. 2018;6:7.
Nghiem P, Bhatia S, Brohl AS, Hamid O, Mehnert JM, Terheyden P, et al. Two-year efficacy and safety update from JAVELIN Merkel 200 part A: A registrational study of avelumab in metastatic Merkel cell carcinoma progressed on chemotherapy. J Clin Oncol. 2018;36:9507.
D’Angelo SP, Russell J, Lebbé C, Chmielowski B, Gambichler T, Grob JJ, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol. 2018; https://doi.org/10.1001/jamaoncol.2018.0077.
Topalian SL, Bhatia S, Kudchadkar RR, Amin A, Sharfman WH, Lebbe C, et al. Nivolumab (Nivo) as neoadjuvant therapy in patients with resectable Merkel cell carcinoma (MCC) in CheckMate 358. J Clin Oncol. 2018;36:9505.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23.
Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–30.
Becker JC, Hassel JC, Menzer C, Kähler KC, Eigentler TK, Meier FE, et al. Adjuvant ipilimumab compared with observation in completely resected Merkel cell carcinoma (ADMEC): A randomized, multicenter DeCOG/ADO study. J Clin Oncol. 2018;36:9527.
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65.
Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–64.
Rudmann DG. On-target and off-target-based toxicologic effects. Toxicol Pathol. 2013;41:310–4.
Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64.
Davies M. New modalities of cancer treatment for NSCLC: focus on immunotherapy. Cancer Manag Res. 2014;6:63–75.
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33.
Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of Immunotherapy for the Practitioner. J Clin Oncol. 2015;33:2092–9.
Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:1270–1.
Harrington KJ, Puzanov I, Hecht JR, Hodi FS, Szabo Z, Murugappan S, et al. Clinical development of talimogene laherparepvec (T-VEC): a modified herpes simplex virus type-1-derived oncolytic immunotherapy. Expert Rev Anticancer Ther. 2015;15:1389–403.
Poulsen MG, Rischin D, Porter I, Walpole E, Harvey J, Hamilton C, et al. Does chemotherapy improve survival in high-risk stage I and II Merkel cell carcinoma of the skin? Int J Radiat Oncol Biol Phys. 2006;64:114–9.
Triozzi PL, Fernandez AP. The role of the immune response in Merkel cell carcinoma. Cancers (Basel). 2013;5:234–54.
Vandeven NA, Nghiem P. Merkel cell carcinoma: An unusually immunogenic cancer proves ripe for immune therapy. J Oncol Pract. 2016;12:649–50.
Melero I, Navarro B, Teijeira A, Coukos G. Cancer immunotherapy full speed ahead. Ann Oncol. 2017;28:xii1–2.
Liu D, Li G, Avella DM, Kimchi ET, Kaifi JT, Rubinstein MP, et al. Sunitinib represses regulatory T cells to overcome immunotolerance in a murine model of hepatocellular cancer. Oncoimmunology. 2017;7:e1372079.
Acknowledgments
Editorial assistance in the preparation of this manuscript was provided by Aashni Shah, on behalf of Polistudium (Milan, Italy); this assistance was supported by internal funds. We also thank Lilia Biscaglia, PhD, for useful discussion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of Interest
Daniela Femia, Natalie Prinzi, Andrea Anichini, Roberta Mortarini, Federico Nichetti, Francesca Corti, Martina Torchio, Giorgia Peverelli, Filippo Pagani, Andrea Maurichi, Ilaria Mattavelli, Massimo Milione, Nice Bedini, Ambra Corti, Maria Di Bartolomeo, Filippo de Braud, and Sara Pusceddu declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Rights and permissions
About this article
Cite this article
Femia, D., Prinzi, N., Anichini, A. et al. Treatment of Advanced Merkel Cell Carcinoma: Current Therapeutic Options and Novel Immunotherapy Approaches. Targ Oncol 13, 567–582 (2018). https://doi.org/10.1007/s11523-018-0585-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-018-0585-y