Abstract
This trial evaluated the effect of adding lapatinib to letrozole after clinical resistance to aromatase inhibitor (IA) treatment in hormone receptor-positive metastatic breast cancer. Postmenopausal women received daily letrozole plus lapatinib (1,500 mg). The primary end point was objective rate response (ORR) at week 12. Secondary objectives included time to response, duration of response, clinical benefit (CB), progression-free survival (PFS), overall survival, and safety. Twenty-four human epidermal growth factor receptor 2 (HER2)-negative patients were included with secondary resistance to IA. ORR at 12 weeks was 4 % (95 % confidence interval (CI), 0.7–20). Stable and progression diseases were reported in 25 % (95 % CI, 12–45) and 71 % (95 % CI, 51–85) of cases, respectively. At 24 weeks, the ORR increased to 8 %. CB was 21 % (95 % CI, 9–40). At a median follow-up of 27 months, median PFS was 3.4 months (95 % CI, 2.8–5.4). Grade 3 or 4 adverse events were rarely reported. No clinical cardiac toxicity was observed. Lapatinib was discontinued in two patients due to severe diarrhea. This trial was prematurely closed due to low recruitment. These preliminary results suggest that the addition of lapatinib to letrozole has a favorable safety profile and could overcome tumoral resistance to letrozole among HER2-negative tumors.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common malignancy and the second most common cause of cancer-related death in Western European and North American women [1]. The worldwide incidence of breast cancer in the year 2000 was estimated at 1,050,000 cases; mortality in the same year was estimated at 373,000 deaths [2]. In the USA, the incidence of breast cancer is increasing, although the mortality rate from breast cancer has decreased by approximately 1.9 % per year since 1990. Mortality incidence in France in 2008 was calculated at 11,700 patients and the same decrease mortality in the last 10 years [3]. Despite improvements in early diagnosis, almost all patients with metastatic disease and up to 40 % of patients receiving adjuvant hormonal therapy eventually relapse and die from their disease [4] which illustrates the current therapeutic limitations.
Breast carcinoma is a heterogeneous group of tumors, in their biology, clinical behavior, prognosis, and treatment. Hormonal therapy (HT) setting is an established standard in metastatic breast cancer (MBC) that expresses hormonal receptors. Three new generations of aromatase inhibitors (AIs) were developed during the 1990s, letrozole, anastrozole, and exemestane, which demonstrated better activity and survival than megesterol acetate or tamoxifen in MBC patients [5–10]. As a result, AI has become the standard first-line HT in MBC. Around 32 to 35 % of patients achieve an objective response after 3 months of treatment with a median time to progression of 8 months are observed.
A major therapeutic issue is represented by the outcome of treatment resistance and approximately one half of patients with estrogen (ER)- and/or progesterone receptor (PR)-positive tumors will respond to therapy. Several models have been proposed to explain the mechanisms of endocrine resistance including aberrant growth-signaling pathways and have led to the rational design of studies combining HT with signal transduction inhibitors in MBC.
Human epidermal growth factor receptor 2 (HER2) overexpression in MBC has been associated with resistance to endocrine tumors in preclinical and clinical work [11, 12], possibly due to an interaction between ER and HER2 signaling pathways. Upregulation and autocrine activation of these receptors appears to convey an increased resistance to HT and an increased risk for disease progression and death [13]. Additionally, increased expression of the epidermal growth factor or transforming growth factor alpha, which are ligands to epidermal growth factor receptor, is a poor prognostic factor in some cancer patients [14, 15]. Expression of these ligands appears to be responsible for maintaining HER receptors in an activated state even in the absence of receptor overexpression. Because HER2-containing heterodimers elicit such potent mitogenic signals, targeting both HER2 and EGFR simultaneously may provide therapeutic synergy [16].
ER-positive breast cancer cells initially inhibited by an antiestrogen can use an autocrine ErbB signaling network to reestablish their growth [13]. Estrogen acts to suppress EGFR expression in estrogen-responsive breast cancer cells, and conversely, estrogen depletion has been found to upregulate EGFR expression [17]. Additionally, it has been shown that ER-positive breast cancers associated with elevated serum HER2 levels are relatively resistant to AI as well as to tamoxifen [12, 18, 19], compared to breast cancers with normal serum HER2 levels.
There are data [20] showing that tyrosine kinase inhibitors in combination with AI are able to reverse in vitro resistance to hormonal therapy (tamoxifen) in breast cancer cell lines. It has been suggested that double inhibition EGFR/HER2 may be even more effective in further delaying the onset of resistance in HER2-overexpressing cell lines [21, 22].
Simultaneous interruption of the ER and HER pathways with an AI and dual EGFR and HER2 TKI, respectively, may provide greater growth inhibition than that achieved with either agent alone. Additionally, this combination therapy may also prevent or delay the development of hormone-resistant progressive disease that would occur with AI therapy alone.
Based on those results, we hypothesized that lapatinib, a reversible TKI that inhibits both EGFR and HER2 added to letrozole, may overcome resistance to first-line treatment with AI in patients with MBC. Therefore, this study is designed to evaluate the clinical effects, the efficacy, and tolerability of the addition of lapatinib administered in combination with letrozole, as treatment for hormone receptor-positive, advanced, or MBC with a primary or secondary resistance to AI.
Patients and methods
Study design
This phase II, open-label, multicenter, single-arm study aimed to assess the objective response rate of lapatinib and letrozole combination as first- or second-line treatment for locally advanced breast cancer or MBC after a primary or secondary resistance to AI. Two cohorts of patients were studied: patients with primary resistant tumor (cohort 1) who had a progressive disease as best response or patients with secondary resistant tumor (cohort 2) who had disease control followed by progression while they were receiving AI (exemestane, anastrozole, or letrozole) defined as recurrence after 24 months in adjuvant hormonal treatment or progression after response or clinical benefit in metastatic setting. Patients must have received IA during the last 12 weeks.
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice and approved by the Institutional Review Board/Independent Ethics Committee at the University Hospital of Besançon in Franche-Comté, France.
Eligibility
Postmenopausal women with histologically proven MBC or locally advanced breast cancer ER and/or PR positive who signed informed consent and had received adjuvant or first-line treatment with AI were eligible. Patients should have demonstrated tumor progression while they received AI and no longer be considered candidates for chemotherapy treatment. Measurable disease was required. Hormone and HER2 status were assessed locally by immunohistochemistry and/or fluorescence in situ hybridization. Other eligibility criteria included a performance status of 2 or less, left ventricular ejection fraction (LVEF) ≥50 %, and adequate hematologic, renal, and hepatic functions. Written informed consent was required prior to the enrolment.
Patients with a previous chemotherapy for MBC or prior therapy with any EGFR and/or HER2 inhibitor were not eligible. Premenopausal patients or prior endocrine therapy in the metastatic setting with tamoxifen or fulvestrant was not allowed. Other exclusion criteria included history of other prior malignancies, significant cardiac disease, known history of uncontrolled or symptomatic angina, arrhythmias, congestive heart failure, malabsorption syndrome, disease significantly affecting gastrointestinal function, or concurrent treatment with oral or intravenous steroids.
Treatment plan
All patients received lapatinib 1,500 mg once a day (six tablets dosed at 250 mg) in addition to letrozole (2.5 mg once a day). Subjects were carefully instructed by study staff as how to take therapy. A daily dose of lapatinib was taken at approximately the same time each day. Subjects were instructed to take therapy either 1 h (or more) before breakfast or 1 h (or more) after breakfast. Inhibitor of CYP3A4 has been prohibited like macrolides, antiretrovirals, protease inhibitors, systemic antifungals, calcium channel blockers, cimetidine, aprepitant, amiodarone, and grapefruit or its juice. A record of therapy administered to each subject has been maintained in the source documents.
Treatment was continued until progression, unacceptable toxicity, or consent withdrawal. Dose adjustments and treatment interruptions were planned according to adverse effects.
Tolerability and efficacy assessment
At baseline and before each cycle, vital signs and performance status were assessed. Adverse events were evaluated continuously and graded according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 2.0. Serum chemistry and hematology were evaluated before each cycle. Cardiac evaluation with LVEF determination by echocardiography was performed every 8 weeks.
Tumor evaluation (by physical examination and imaging studies) was performed every 12 weeks and was assessed by investigators at the site. For measurable lesions, the response was defined according to Response Evaluation Criteria in Solid Tumors (version 1).
Statistical design and methodology
The primary end point of this study was objective response rate at 12 weeks. Secondary end points included best objective response rate (ORR), duration of response (DR), progression-free survival (PFS), overall survival (OS), and safety.
DR was defined, for responding patients only, as the time from registration until disease progression. PFS was the time between registration and disease progression, death, or withdrawal of treatment due to adverse events or insufficient therapeutic response, whatever occurs first. OS was calculated from the date of registration to the date of death from any cause or to the date of loss to follow-up. Survival data were computed according to the Kaplan–Meier method and analyses were performed on treated population. Cox regression models have been fitted to the data, including covariates for type of AI resistance, site of disease, and prior adjuvant antiestrogen therapy. Other factors predictive of response and time to progression have been included in the models as appropriate. Hazard ratios and associated 95 % confidence limits and p values for each factor were presented.
For each cohort, the study leads to a decision between two pre-specified hypotheses about the probability of a complete or partial tumor response, p. The null hypothesis H0 (p = 10 %) reflects a response rate that would be of no clinical benefit, and the alternative hypothesis HA (p = 25 %) is a response rate that might lead to larger, confirmatory studies. An interim analysis based on an evaluation of confirmed tumor response from the first 38 recruited subjects from each cohort had been conducted by an independent data monitoring committee (IDMC).
The safety population included all treated patients. Adverse events (AEs)/signs and symptoms of disease observed by the investigator or reported by the patients were recorded and graded according to the NCI-CTC version 3 whenever possible; otherwise, the Medical Dictionary for Regulatory Activities version 6 was used.
Results
Patient’s characteristics
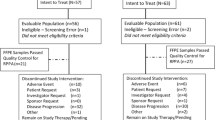
From January 2006 to November 2008, 28 patients were enrolled in four centers in France. Based on the results published by Johnston et al., the IDMC recommends an early assessment of the BES06 trial.
One patient was not evaluated due to consent withdrawn before treatment initiation. Three patients were excluded because of HER2-overexpressing tumors. All subsequent analyses are performed in a population of 24 patients with HER2-negative tumors. Patient demographic characteristics and baseline disease characteristics are reported in Table 1. At baseline median age of 67.2 years (range 34–93), all tumors were ER positive, 20 % of patients had non-visceral metastases (bone, effusion, skin, and node), 35 % had lung metastases, and 56 % presented with at least two metastatic sites. Nine (36 %) of patients included in the analysis had received prior adjuvant anthracycline-containing chemotherapy. Three of them (12 %) were also exposed to a taxane as part of their adjuvant regimen. The median disease-free interval was 5.8 years (range 0.75–15.6). A total of six patients presented a metastatic relapse while receiving adjuvant treatment by AI, 18 (75 %) patients had a progressive disease under the first line of HT by AI for MBC, and all cases analyzed were included in cohort 2.
Dosing
Patients received treatment for a median of 18 weeks with compliance (pill count agreement >80 %) higher than 90 %. Treatment was discontinued before 12 weeks due to disease progression in 17 patients (70 %). Lapatinib was decreased at 1,250 mg in three patients (12 %) and discontinued in two (8 %) patients due to severe diarrhea.
Safety
Non-hematological AEs probably or possibly related to study medication are listed by patient in Table 2.
Non-hematologic non-cardiac toxicity
Severe AE (grade ≥3) were infrequent with 8 % diarrhea, 4 % rash, and 4 % anorexia. No severe AE occurred for myalgia, arthralgia, mucositis, nausea, vomiting, and eye disorders. No patient died due to study treatment. One patient was withdrawn from the study due to severe diarrhea. Toxicities were reversible after reduction or permanent/transitory interruption of lapatinib.
The most frequent non-hematological AEs of any grade were diarrhea (53 %), asthenia (33 %), rash (32 %), mucositis (25 %), anorexia (12 %), nausea (12 %), vomiting (12 %), watery eyes (8 %), myalgia, articular disorders, abnormal liver function test, rhinorrhea, and alopecia, which were observed in only 4 % of patients.
Surprisingly, a case of asymptomatic grade 1 hypertriglyceridemia was observed. This event might be related to treatment because this toxicity recovered after drug discontinued due to progression; even if this situation has never been described in literature for both drugs.
Hematologic toxicity
No hematological toxicity was observed.
Cardiac toxicity
No clinical cardiac toxicity was observed in our study. Only three (12 %) patients experienced an asymptomatic LVEF reduction between 10 and 15 %.
Efficacy
The investigator-determined ORR at 12 weeks was 4 % (95 % CI, 0.7–20) in the treated patient population (Table 3), six patients (25 %) (95 % CI, 12 %–45 %) had stable disease, and 17 patients (71 %) (95 % CI, 51 % to 85 %) experienced a progression under therapy (Table 3). At 24 weeks, 2 of the 24 assessed patients presented a complete response with an increase of the ORR to 8 % (95 % CI, 2–26).
The median DR was 6 months among response-assessable patients. In addition to the objective responses, three patients (12 %) had tumor stabilization longer than 6 months. It results in 21 % (95 % CI, 9–40) of patients experiencing a clinical benefit (responsive or stable disease ≥6 months).
At a median follow-up of 27 months, median PFS was 3.4 months (95 % CI, 2.8 to 5.6 months) (Fig. 1) and the median overall survival was not reached with 11 deaths at the moment of evaluation.
Discussion
Despite promising initial responses to HT in a majority or patients, a subset of patients fails to benefit from treatment, displaying primary or de novo resistance. Even within the responders, acquisition of resistance during the course of treatment (secondary resistance) is an additional challenge. Therefore, intense investigations to understand the factors that contribute to the resistance and to identify therapeutic strategies to overcome the resistance are underway at various levels. Recently, BOLERO 2, a phase 3 study that evaluated the association of everolimus and exemestane compared to IA alone in an IA-resistant population, showed a significant benefit in PFS, reducing the risk of progression of 55 % in patients treated with combination, a profile toxicity acceptable although about 20 % of patients in the experimental arm presented significant toxicity such as stomatitis, pneumonitis, hyperglycemia, or fatigue. The overall survival results so far are favorable, although these will be final in late 2013. This study led to the approval of everolimus and is currently the standard treatment for postmenopausal women with ER+ HER2− MBC, progressing to IA. Moreover, TAMRAD, a phase 2 study that evaluated the combination of tamoxifen with everolimus compared to tamoxifen alone in the same patient population, demonstrated significant differences in favor of the combination, mainly in the subgroup of patients with IA secondary resistance [23, 24].
Studies are being conducted in this population with different inhibitors that interact in the PI3K pathway such as buparlisib (BKM-120), a pan-class 1 PI3K inhibitor, or in other signaling pathways such as dovitinib (TKI-258), a fibroblast growth factor receptor inhibitor, which leads us to a new era of treatment of this disease.
The rationale of this trial was developed because in HR-positive breast carcinomas that are initially HER2-negative, EGFR and HER2 pathways may become upregulated and explain the development of endocrine resistance. A combined inhibition of the kinase and endocrine pathways could delay acquired resistance. An example was the anti-epidermal growth factor receptor agent gefitinib. In vitro, this drug improves antihormone response and prevents development of resistance in breast cancer [25].
This phase II trial was designed to evaluate the efficacy and the safety of lapatinib in combination to letrozole in MBC pretreated with aromatase inhibitor after a primary (cohort 1) or secondary (cohort 2) clinical resistance. Only patients from the second group, defined by secondary resistance to AI, were included.
We included all resistant patients independently of HER2 status, with a total population of 28. Nevertheless, based on the results of HER2-positive populations treated with letrozole and lapatinib, published by Johnston, three patients with this overexpression were excluded, and the present analyses were performed in an HER2-negative population.
This trial was prematurely closed in November 2009 due to low recruitment, and an interim analysis from the advice of an independent committee review board based on ORR was performed.
This present study showed that lapatinib plus letrozole failed to achieve response in the majority of patients with endocrine-sensitive, HER2-negative disease with a secondary resistance to aromatase inhibitors. Nevertheless, these preliminary results demonstrate that this combination seems to be able to control tumor progression over AI resistance in which 29 % of patients achieved a clinical benefit. Moreover, 8 % of complete response demonstrated the combination is able, in a limited number of cases, to overcome the resistance to AI that provides an encouraging signal of activity. This point is suggesting a need to search for the identification of tumor biological characteristics related to this activity of the association letrozole–lapatinib.
Lapatinib is an oral receptor tyrosine kinase inhibitor that targets HER-2 and has not been associated with significant symptomatic cardiotoxicity [26]. This study reported no significant clinical cardiac toxicity. Otherwise, severe adverse events were infrequent. The most frequent all grade non-hematological toxicity was diarrhea. It was reversible after reduction or interruption of lapatinib. This toxicity explained a dose adaptation or interruption in around 10 % of patients, which is consistent with other publications [27]. Surprisingly, a case of asymptomatic grade 1 hypertriglyceridemia probably related to lapatinib was observed. We have no rational explanation for the only event although there might be a relation between this alteration and liver injury. A reversible alteration in the metabolism of lipids has been recently described in animal models [28], especially associated with hepatotoxicity. Rare incidence of serious hepatic adverse effects led to routine monitoring of liver function and this preclinical finding and our observation could also motivate a routine triglyceridemia monitoring.
The addition of targeted therapies to standard anticancer agent has been enhancing the activities of numerous standard treatments. A need to found targeted therapies able to emphasize the activity of HT is warranted. A better knowledge of biological interaction mechanisms with ER/PgR pathway could be of interest and it will allow an effective combination. The addition of lapatinib seems to be reversing the resistance to IA in a small proportion of patients. These results would however require a better understanding of biological mechanisms to confirm that a subpopulation of patients with ER+, HER2− tumors benefits from such therapeutic strategy.
References
Curado MP (2011) Breast cancer in the world: incidence and mortality. Salud Publ Mex 53:372–384
Parkin DM (2001) Global cancer statistics in the year 2000. Lancet Oncol 2:533–543
Hill C, Doyon F (2008) The frequency of cancer in France: mortality trends since 1950 and summary of the report on the causes of cancer. Bull Cancer 95:5–10
Ring A, Dowsett M (2003) Human epidermal growth factor receptor-2 and hormonal therapies: clinical implications. Clin Breast Cancer 4(Suppl 1):S34–S41
Nabholtz JM, Bonneterre J, Buzdar A et al (2003) Anastrozole (Arimidex™) versus tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: survival analysis and updated safety results. Eur J Cancer 39:1684–1689
Bonneterre J, Thurlimann B, Robertson JF et al (2000) Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol 18:3748–3757
Mouridsen H, Gershanovich M, Sun Y et al (2001) Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol 19:2596–2606
Dombernowsky P, Smith I, Falkson G et al (1998) Letrozole, a new oral aromatase inhibitor for advanced breast cancer: double-blind randomized trial showing a dose effect and improved efficacy and tolerability compared with megestrol acetate. J Clin Oncol 16:453–461
Buzdar AU, Jonat W, Howell A et al (1998) Anastrozole versus megestrol acetate in the treatment of postmenopausal women with advanced breast carcinoma: results of a survival update based on a combined analysis of data from two mature phase III trials. Arimidex Study Group. Cancer 83:1142–1152
Paridaens RJ, Dirix LY, Beex LV et al (2008) Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol 26:4883–4890
Dowsett M, Harper-Wynne C, Boeddinghaus I et al (2001) HER-2 amplification impedes the antiproliferative effects of hormone therapy in estrogen receptor-positive primary breast cancer. Cancer Res 61:8452–8458
Lipton A, Ali SM, Leitzel K et al (2002) Elevated serum Her-2/neu level predicts decreased response to hormone therapy in metastatic breast cancer. J Clin Oncol 20:1467–1472
Nicholson RI, Hutcheson IR, Harper ME et al (2001) Modulation of epidermal growth factor receptor in endocrine-resistant, oestrogen receptor-positive breast cancer. Endocr Relat Cancer 8:175–182
Grandis JR, Chakraborty A, Zeng Q et al (1998) Downmodulation of TGF-alpha protein expression with antisense oligonucleotides inhibits proliferation of head and neck squamous carcinoma but not normal mucosal epithelial cells. J Cell Biochem 69:55–62
Albanell J, Codony-Servat J, Rojo F et al (2001) Activated extracellular signal-regulated kinases: association with epidermal growth factor receptor/transforming growth factor alpha expression in head and neck squamous carcinoma and inhibition by anti-epidermal growth factor receptor treatments. Cancer Res 61:6500–6510
Earp HS, Dawson TL, Li X, Yu H (1995) Heterodimerization and functional interaction between EGF receptor family members: a new signaling paradigm with implications for breast cancer research. Breast Cancer Res Treat 35:115–132
Yarden RI, Wilson MA, Chrysogelos SA (2001) Estrogen suppression of EGFR expression in breast cancer cells: a possible mechanism to modulate growth. J Cell Biochem Suppl 36:232–246
Dowsett M, Houghton J, Iden C et al (2006) Benefit from adjuvant tamoxifen therapy in primary breast cancer patients according oestrogen receptor, progesterone receptor, EGF receptor and HER2 status. Ann Oncol 17:818–826
Higgins MJ, Stearns V (2009) Understanding resistance to tamoxifen in hormone receptor-positive breast cancer. Clin Chem 55:1453–1455
Shou J, Massarweh S, Osborne CK et al (2004) Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst 96:926–935
Chu I, Blackwell K, Chen S, Slingerland J (2005) The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer. Cancer Res 65:18–25
Leary AF, Drury S, Detre S et al (2010) Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin Cancer Res 16(5):1486–1497
Baselga J, Campone M, Piccart M et al (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Eng J Med 366(6):520–529
Bachelot T, Bougier C, Cropet C et al (2012) Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol 30(22):2718–2724
Gee JM, Harper ME, Hutcheson IR et al (2003) The antiepidermal growth factor receptor agent gefitinib (ZD1839/Iressa) improves antihormone response and prevents development of resistance in breast cancer in vitro. Endocrinology 144:5105–5117
Perez EA, Koehler M, Byrne J et al (2008) Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc 83:679–686
Johnston S, Pippen J Jr, Pivot X et al (2009) Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 27:5538–5546
Demirci U, Buyukberber S, Yilmaz G et al (2012) Hepatotoxicity associated with lapatinib in an experimental rat model. Eur J Cancer 48:279–285
Acknowledgments
This trial was sponsored by GlaxoSmithKline France.
Conflict of interest
The authors do not have any conflicts of interest for this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Villanueva, C., Romieu, G., Salvat, J. et al. Phase II study assessing lapatinib added to letrozole in patients with progressive disease under aromatase inhibitor in metastatic breast cancer—Study BES 06. Targ Oncol 8, 137–143 (2013). https://doi.org/10.1007/s11523-013-0279-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-013-0279-4