Abstract
Metastatic tumor antigen 2 (MTA2) is a member of the MTA family that is closely associated with tumor progression and metastasis. In this study, the expression profile of MTA2 in 223 cases of non-small cell lung cancer (NSCLC) tissues and two lung cancer cell lines was investigated. Interestingly, we found MTA2, which was believed to have nuclear distribution only, was distributed in both nucleus and cytoplasm in normal and cancer cells. Nuclear MTA2 expression was detected in 148 cases of NSCLC (66.4 %), and was correlated with advanced TNM stages (p = 0.023), tumor size (p = 0.036), and lymph node metastasis (p = 0.004). Besides, the Ki-67 proliferation index was significantly higher in nuclear MTA2-positive tumors than in nuclear MTA2-negative tumors (r = 0.538, p = 0.006). However, there was no significant difference in cytoplasmic MTA2 status by age, gender, tumor stage, histology, grade, lymph node metastasis, and Ki-67 proliferation index. Univariate analysis revealed nuclear MTA2 expression was correlated with poor overall survival (p = 0.035), whereas there was a nonsignificant trend in the same direction for cytoplasmic MTA2 (p = 0.134). Multivariate Cox regression analysis revealed the overexpression of nuclear and cytoplasmic MTA2 not to be independent factors predictive of poor disease outcome. Our data suggested that MTA2 might play roles in both the nucleus and cytoplasm in the progression of NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-small cell lung cancer (NSCLC) is the primary classification of lung cancer and the prognosis of patients with NSCLC is principally correlated with tumor metastasis. In NSCLC, lymph node metastasis and invasion to neighboring organs are the most important indicators of poor prognosis. Metastasis is the major cause of morbidity and mortality among patients with lung cancer [1]. Metastasis is a complicated progression that involves various genes and can be divided into several stages, namely detachment of cancer cells from primary sites, penetration into the lymphatic channels and vessels, and growth at metastatic sites [2]. Despite the fact that numerous genes influence the biological behavior of NSCLC, the intrinsic mechanism of gene dysregulation that leads to metastasis still needs to be clarified further [3].
Metastasis-associated protein (MTA) genes are a novel gene family that includes three different genes at separate loci (MTA1 at 14q, MTA2 at 11q, and MTA3 at 2q) with several alternative splice forms (MTA1s, MTA1-ZG29p, and MTA3L) [4]. Both MTA1 and MTA2 are components of NuRD adenosine-5'-triphosphate (ATP)-dependent chromatin remodeling and histone deacetylase complexes [5, 6]. The transcription factor complex that contains MTA1 is highly expressed in metastatic cells, such as breast, colorectal, gastric, esophageal, hepatocellular carcinoma, and NSCLC [7–11].
There is more and more evidence showing that the MTA family is closely linked to tumor progression and metastasis [12–14]. However, clinical study regarding MTA2 expression in NSCLC is sparse [15]. In our study, the expression profile of MTA2 in NSCLC was measured and the relationship between MTA2 expression and patients’ pathological features or clinical outcomes was analyzed. Furthermore, we examined MTA2 distribution in two lung cancer cell lines and found that MTA2 was located not only in nucleus but also in cytoplasm. To further clarify the functional role of nuclear and cytoplasmic MTA2 in NSCLCs, we performed a retrospective clinical study on MTA2 expression in relation to clinical characteristics and biological markers, such as Ki-67 proliferation index. Additionally, the effect of both nuclear and cytoplasmic MTA2 on NSCLC prognosis was analyzed by looking into follow-up data.
Materials and methods
Tissue samples and tissue microarrays development
Tumor specimens including NSCLC tissues and paired non-tumor portion (with >5 cm distance from the primary tumor’s edge) from 223 patients with NSCLC were obtained between 2000 and 2005 following surgical resection at the Hunan Province People’s Hospital (Changsha, Hunan Province, China). None of the patients had received radiotherapy, chemotherapy, or immunotherapy prior to tumor excision. Patients’ ages at the time of surgery ranged from 35 to 77, with an average age of 54.38 years old. Based on the TNM stage revised by the International Union Against Cancer in 2007 [16] and the classification of the World Health Organization [17], we classified the patients with primary lung cancer into the following groups: (1) 104 squamous cell carcinomas, with 25 cases at stage I, 40 cases at stage II, 34 cases at stage III, and five at stage IV; 15 cases were well differentiated, 24 cases were moderately differentiated, and 65 cases were poorly differentiated; and 42 cases with lymph node metastasis. (2) One hundred nineteen adenocarcinomas, with 43 cases at stage I, 13 cases at stage II, 62 cases at stage III, and 1 cases at stage IV; 30 cases were highly differentiated, 52 cases were moderately differentiated, and 37 cases were poorly differentiated; and 44 cases with lymph node metastasis. Among the 223 cases, 70 cases had complete follow-up records and the lymph node metastases of 35 patients were available.
Tissue microarrays (TMA) were constructed by selecting viable and representative region enriched for tumor cells from formalin fixed and paraffin-embedded tumor tissue. Core samples were removed (1.6 mm diameter) from each tumor and rearranged on empty paraffin-blocks using a manual tissue microarray device (BEECHER MTA II; Beecher Instruments, Inc., Sun Prairie, WI, USA).
Cell culture
Human lung carcinoma cell lines A549 and H460 were cultured in RPMI 1640 tissue culture medium (Invitrogen, Carlsbad, CA, USA), containing 10 % fetal calf serum (Invitrogen), 100 IU/mL penicillin (Sigma, St. Louis, MO, USA), and 100 μg/mL streptomycin (Sigma) at 37°C in a humidified atmosphere (5 % CO2, 95 % air).
Immunohistochemical staining and evaluation
As described previously [18, 19], immunohistochemistry was done by using the ultrasensitive avidin–biotin–peroxidase complex method (Maixin Biotechnology, Fuzhou, Fujian, China) according to the manufacturer’s instructions. The antibodies used were MTA2 mouse monoclonal antibody (ab8106, 1:400, Abcam, Cambridge, UK) against a peptide mapping at the C-terminus of MTA2 of human origin, MTA2 goat polyclonal antibody (SC-9447, 1:100; Santa Cruz Biotechnology, CA, USA) against amino acids 652–668 of human MTA 2, and Ki-67 mouse monoclonal antibody (MIB-1, ready for use; Maxin Biotechnolog, Fuzhou, Fujian, China). Normal rabbit immunoglobulin-G was substituted for the primary antibody as a negative control. Staining with omission of the primary antibody was also performed as a negative control.
All of the immunostained sections were reviewed by the two authors (SDD and EHW) who had no knowledge of the patients’ clinical status. Cases with discrepancies were jointly re-evaluated by the investigators. The sections were evaluated at low magnification (×100) to identify “hot spots”. We counted 500 tumor cells and calculated the percentage of positively staining cells. Both cytoplasmic and nuclear stainings were regarded as MTA2 expression, but not only nuclear staining. To assess the roles of MTA2 both in nucleus and cytoplasm, they were judged separately. Thus, the proportion of cells exhibiting nuclear MTA2 expression was categorized as follows: score 0, no visible nuclear staining; score 1, <1/3; score 2, 1/3–2/3; and score 3, >2/3. The intensity of staining was not considered. To obtain final statistical results, score less than 2 was considered as low expression, while scores of 2 or more were considered as “nuclear MTA2 overexpression”.
As for the cytoplasmic staining of MTA2, a proportion score was assigned initially, which represented the estimated proportion of positive tumor cells (0, none; 1, <1/3; 2, 1/3–2/3, and 3, >2/3). Next, an intensity score was assigned, which represented the average intensity of the positive tumor cells (0, none; 1+, weak; 2+, intermediate; and 3+, strong). The proportion and intensity scores were then multiplied to obtain a total score. To obtain consistent judging result, a final score less than 3 was considered as low expression, while scores of 3 or more were considered as “cytoplasmic MTA2 overexpression”. Although two antibodies were used to detect the expression of MTA2 in our study, the results obtained by the monoclonal antibody were adopted to further analysis.
The immunohistochemical staining for Ki-67 was evaluated as the percentage of cancer cells with nuclear immunoreactivity counting at least 500 tumor cells per slide. The median value of this series (41 % of positive cells) was used as the cutoff value to distinguish tumors with low (0, <41 %) from tumors with high (1, ≥41 %) index of cell proliferation.
Immunofluorescent staining
Cells grown on glass coverslips were fixed with ice-cold 100 % methanol for 15 min at −20°C, followed by permeabilization with 0.2 % Triton X-100. MTA2 was detected by two kinds of antibodies used in immunohistochemistry (IHC) detection. Primary antibodies were applied overnight at 4°C followed by incubation with secondary antibody conjugated to fluorescein isothiocyanate-labeled. The nuclei were counterstained with propidium iodide. The cells were examined with a laser scanning confocal microscopy (MTC-600, BIO-RAD, USA).
Study design
This is a retrospective study based on archival material. The cases analyzed represent a sequential series of patients according to the archival number given at the Hunan Province People’s Hospital, Changsha, Hunan Province, China, upon receipt of the surgical specimen. The selection of this specific material was performed to include patients who underwent surgery alone without chemotherapy of radiotherapy, thus simulating at a time when, in the lack of clinical evidence from properly designed randomized trials, these adjunctive therapies were not the golden standard. In this way, the analysis of data will reflect the actual impact of the tumor biology on the clinical outcome, without introducing into our model unpredictable differences in terms of chemo- or radiosensitivity of individual carcinomas. Patients with perioperative death and those with missing demographic or histopathological data were excluded from this study. The endpoints of analysis were the association of MTA2 expression with histopathological and immunohistochemical variables and its impact on the overall disease-specific survival. Informed consent was obtained from all enrolled patients prior to surgery. This study was conducted under the regulations of the Institutional Review Board of China Medical University.
Statistical analysis
Pearson’s chi-square test was used to analyze the relationship between expression of MTA2 and clinicopathological factors. McNemar’s test was used to compare the MTA2 expression in normal lung tissues and lung cancer tissues. The probabilities of overall survival were calculated using the Kaplan–Meier method and were compared using the log-rank test. For determining factors related to overall survival, a Cox proportional hazard model was utilized (Backward Stepwise (Conditional LR)). All statistical analyses were performed using SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA). P values less than 0.05 were considered statistically significant.
Results
MTA2 expression in normal, NSCLC tissues, and cancer cell lines
Normal lung tissue cells (including bronchial epithelial, serous gland cells, and alveolar epithelial cells) showed nuclear and cytoplasmic staining of MTA2 by IHC staining (Fig. 1). The total positive rate of MTA2 expression in normal lung tissues was 55.2 % (53/223), with a positive rate of 43.5 % (97/223) in bronchial epithelial cells, and 39.5 % (88/223) in submucosal gland cells. Overall, immunostaining was mainly seen in cytoplasm with a positive rate of 46.2 % (103/223), which was higher than in nuclei (35.9 %, 80/223).
MTA2 expression in adjacent noncancerous lung tissues using immunohistochemical staining. a Weak to moderate staining of MTA2 was expressed in the cytoplasm and nucleus of normal adult bronchial epithelial cells without hematoxylin staining. b The ciliated cells in terminal bronchiole showing MTA2 immunoreactivity localized in their nucleus. c The ciliated cells in terminal bronchiole showing MTA2 immunoreactivity localized in their nucleus and cytoplasm. d The goblet cells in the small bronchus showing MTA2 immunoreactivity localized in their cytoplasm. e The serous gland, but not mucos gland, in tracheal gland of submucosa showing MTA2 immunoreactivity localized in their cytoplasm. f Macrophage showing MTA2 immunoreactivity localized in their cytoplasm. Weak staining of MTA2 detected in alveolar epithelial cells. Red cells are negative in this section, indicating that the staining is specific. Scale bar 20 μm
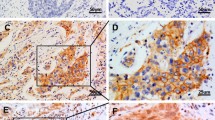
We next examined the subcellular locations of MTA2 in paraffin sections of lung tumor sections from patients. Once again, we observed MTA2 in the cytoplasm and nucleus (Fig. 2). Figure 2a shows mainly cytoplasmic MTA2 staining in a lung cancer specimen, Fig. 2b shows mainly nuclear MTA2 staining in a lung cancer specimen. The staining specificity of MTA2 was confirmed with another polyclonal antibody (SC-9446). The staining patterns by these two antibodies were obtained high correlation (Tables 1 and 2; Fig. 3). Some score variations were presented when we judged the cytoplasmic staining between these two antibodies, but consistent statistical results could be conducted since we conduct the criteriation. Furthermore, several controls were also used in the staining, which included eliminating the primary antibody from the procedure to ensure that the secondary antibody did not give any background staining and replacing the primary antibody by normal rabbit immunoglobulin-G before use in the assay(data not shown). Both of these control slides showed no background staining, suggesting that the staining is specific for MTA2. Furthermore, MTA2 distributions in two lung cancer cell lines were observed by laser confocal scanning microscopy. The results also demonstrated that MTA2 was distributed in both nucleus and cytoplasm (Fig. 4). In both these two cell lines, MTA2 expression was detected mainly in the nucleus with some visible staining in the cytoplasm. We also noticed that the staining in nucleus was mostly diffuse but was also associated with small punctate dot structures.
MTA2 expression in primary non-small cell lung cancer tissues using IHC without hematoxylin staining. a Lung cancer cells showing MTA2 immunoreactivity mainly localized in their cytoplasm. Scale bar 15 μm. b Lung cancer cells showing MTA2 immunoreactivity mainly localized in their nucleus. Scale bar 15 μm
Immunohistochemical staining of lung cancer specimens expressing MTA2 by two antibodies. Scale bar 20 μm. a Squamous cell carcinoma cells showing MTA2 immunoreactivity in their nuclear and cytoplasm by monoclonal antibody (ab8106). b Squamous cell carcinoma cells showing MTA2 immunoreactivity in their nuclear and cytoplasm by polyclonal antibody (SC-9447). c Adenocarcinoma cells showing MTA2 immunoreactivity in their nuclear and cytoplasm by monoclonal antibody (ab8106). d Adenocarcinoma cells showing MTA2 immunoreactivity in their nuclear and cytoplasm by polyclonal antibody (SC-9447)
Nuclear and cytoplasmic distribution of MTA2 in non-small cell lung cancer cell lines. In both H460 (a–c) and A549 (d–f) cell lines, MTA2 expression was detected mainly in the nucleus with some visible staining in the cytoplasm. The staining in nucleus was mostly diffuse but was also associated with small punctate dot structures (white arrows). Scale bar 14 μm
Relationship between MTA2 expression and clinicopathological factors in NSCLC
The relationships between MTA2 expression and the different clinicopathological factors are shown in Table 3. Nuclear MTA2 immunoreactivity was higher in NSCLCs with lymph node metastasis than node-negative cases. IHC staining showed a positive correlation between protein expression and node metastasis (p = 0.004). Increased nuclear MTA2 expression in NSCLC was also found to be correlated with larger tumor size (≥3 cm) and advanced TNM stages (III + IV; p < 0.05). No other clinicopathological factors were found to be correlated with nuclear MTA2 expression (p > 0.05). On the other hand, subject characteristics by cytoplasmic MTA2 status are also shown in Table 3. There was no significant difference in cytoplasmic MTA2 status by age, gender, tumor stage, histology, grade, and lymph node metastasis (Table 3).
We also investigated the relation between MTA2 expression and NSCLC proliferation by examining the expression of Ki-67/MIB-1. Ki-67 proliferation index varied greatly among 147 NSCLC cases studied (40.4 ± 29.3 %). Ki-67 proliferation index was higher in nuclear MTA2-positive tumors (57.0 ± 38.1 %) than in nuclear MTA2-negative tumors (33.2 ± 24.5 %). The correlation between nuclear MTA2 and Ki-67 expression is significant (r = 0.538, p = 0.000, Table 3). Furthermore, Ki-67 staining had identical distribution to nuclear MTA2 staining for most of the tumors studied (Fig. 3). On the other hand, there was no significant correlation between Ki-67 staining and cytoplasmic MTA2 expression (r = 0.057, p = 0.397).
Clinical outcome and multivariate analysis of overall survival rate
The overall Kaplan–Meier survival curves for nuclear and cytoplasmic MTA2 expression are shown in Fig. 5. Univariate analysis revealed nuclear MTA2 expression was correlated with poor overall survival (p = 0.035), whereas there was a nonsignificant trend in the same direction for cytoplasmic MTA2 (p = 0.134). To further evaluate prognostic utility of MTA2 expression, a multivariate Cox regression analysis was carried out. The effects of MTA2, both nuclear and cytoplasmic MTA2, on overall survival in a Cox proportional hazards model are summarized Table 4.
After enrolling sex, age at diagnosis, stage at diagnosis, histology, differentiation, lymph node metastasis, and Ki-67 index, we found that lymph node metastasis (p = 0.021) and TNM staging (p = 0.034) and Ki67 index (p = 0.044) were independent prognostic factors. Both nuclear and cytoplasmic MTA2 expression was not independent prognostic factors (p > 0.01).
Discussion
There is little documentation about MTA2 expression profile in lung cancer. As to our knowledge, only one report examined MTA2 expression in lung cancer tissues. The study showed that MTA2 was only located in nucleus [15]. However, we found that MTA2 was located not only in nucleus but also in cytoplasmic compartments in NSCLC tissues. This observation was further confirmed in two lung cancer cell lines, A549 and H460. This observation was confirmed by two MTA2 antibodies, against the COOH terminus of the MTA2 and amino acids 652–668 of human MTA 2, separately. Using statistical analysis, we also found that MTA2 expression was correlated with advanced TNM staging, tumor size, and lymph node metastasis (Fig. 6).
Overall survival of NSCLC patients in relation to the MTA2 status. a. The overall survival of patients who had NSCLC with low nuclear MTA2 expression versus high nuclear MTA2 expression. Overexpression of nuclear MTA2 was significantly correlated with poor prognosis (p = 0.035). b The overall survival of patients who had NSCLC with low cytoplasmic MTA2 expression versus high cytoplasmic MTA2 expression. There was a nonsignificant trend for cytoplasmic MTA2 overexpression (p = 0.134)
MTA2 has been identified as a component of NuRD [5, 20], a protein complex that has both ATP-dependent chromatin remodeling activity and histone deacetylase activity. NuRD was found to be located in nucleus of human cells. The regulation of histone and chromatin acetylation by NuRD complex plays an important role in tumor progression. It has been reported that MTA-regulated chromatin remodeling pathways interact with transcriptional signaling pathways that govern events in tumor progression and metastasis [21]. The dynamics of the histone acetylation pathway is also very important for tumor progression. Acetylation of histone opens up the chromatin structure and leads to transcriptional activation. Deacetylation by MTA2 may trigger NSCLC progression by inhibiting the transcription of tumor suppressors. Nuclear MTA2 might also exert its function by interacting with p53. It has been reported that MTA2 specifically interacts with p53 and reduces the steady-state level of acetylated p53. In addition, it has been reported that MTA2 overexpression resulted to suppression of p53-dependent transcriptional activation, and inhibits p53-mediated cell growth arrest and apoptosis [22]. MTA2 might modulate p53-mediated cell growth and apoptosis by suppressing p53-dependent transcriptional activity.
It is interesting to discuss the role of cytoplasmic MTA2 in cancer cells. Recently, MTA2 has been reported to be a repressor of estrogen receptor α (ERα), a protein predominantly located in cytoplasm for both normal and cancer lung cells. The predominant form of ERα in lung cancer is believed to be one that lacks exon 4, the exon encoding the nuclear localization signal near amino-terminus of ERα [23]. Since MTA2 participates in the deacetylation of ERα in human breast cancer cells, we suspect that cytoplasmic MTA2 might also interact with ERα in lung cancer cells. In fact, we have detected the interaction between MTA2 and ERα in some lung cancer tissues (data not shown). Of course, further experiments are still needed to resolve this issue.
It has been reported that the expression level of nuclear MTA2 was especially high in rapidly dividing cells [24]. Our study demonstrated that nuclear MTA2 expression also correlates with lung cancer cell proliferation. The Ki-67 proliferation index was significantly higher in nuclear MTA2-positive tumors than in nuclear MTA2-negative tumors. Furthermore, by using serial sections, the immunostaining pattern of Ki-67 was found to be identical to that of nuclear MTA2 in most tumor samples. These findings indicated that nuclear MTA2 expression might play a role in regulating tumor proliferation in NSCLC. In fact, Xia et al. [25] found that elevated level of nuclear MTA2 correlates with cell proliferation.
By analyzing the relationship between MTA2 expression in NSCLC tissues and clinicopathological features of patients, it was found that nuclear MTA2 expression was correlated with larger tumor size, advanced TNM stage, and lymph node metastasis. This indicated that nuclear MTA2 might play certain role in invasion and metastasis of lung cancer and nuclear MTA2 might be taken as a reference index in molecular staging. In order to clarify the prognostic utility of MTA2 expression for NSCLS patients, NSCLC tissues with follow-up data were tested by MTA2 staining. The results showed that postoperative survival period of nuclear MTA2-positive group was notably shorter than that of nuclear MTA2-negative group, indicating that higher nuclear MTA2 expression is a negative prognostic predictor and MTA2 might be involved in malignant behaviors of tumor. There was a nonsignificant trend in the same direction for cytoplasmic MTA2. As to our knowledge, the present study is the first in identifying a correlation between MTA2 expression and NSCLC prognosis.
Reference
Ganti AK, Huang CH, Klein MA, Keefe S, Kelley MJ (2011) Lung cancer management in 2010. Oncology (Williston Park, NY) 25:64–73
Liotta LA, Steeg PS, Stetler-Stevenson WG (1991) Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 64:327–336
Sleeman J, Steeg PS (2010) Cancer metastasis as a therapeutic target. Eur J Cancer 46:1177–1180
Yaguchi M, Wada Y, Toh Y, Iguchi H, Kono A, Matsusue K et al (2005) Identification and characterization of the variants of metastasis-associated protein 1 generated following alternative splicing. Biochim Biophys Acta 1732:8–14
Bowen NJ, Fujita N, Kajita M, Wade PA (2004) Mi-2/NuRD: multiple complexes for many purposes. Biochim Biophys Acta 1677:52–57
Kumar R, Wang RA, Bagheri-Yarmand R (2003) Emerging roles of MTA family members in human cancers. Semin Oncol 30:30–37
Toh Y, Kuninaka S, Endo K, Oshiro T, Ikeda Y, Nakashima H et al (2000) Molecular analysis of a candidate metastasis-associated gene, MTA1: possible interaction with histone deacetylase 1. J Exp Clin Cancer Res 19:105–111
Moon WS, Chang K, Tarnawski AS (2004) Overexpression of metastatic tumor antigen 1 in hepatocellular carcinoma: relationship to vascular invasion and estrogen receptor-alpha. Hum Pathol 35:424–429
Hamatsu T, Rikimaru T, Yamashita Y, Aishima S, Tanaka S, Shirabe K et al (2003) The role of MTA1 gene expression in human hepatocellular carcinoma. Oncol Rep 10:599–604
Sasaki H, Moriyama S, Nakashima Y, Kobayashi Y, Yukiue H, Kaji M et al (2002) Expression of the MTA1 mRNA in advanced lung cancer. Lung Cancer 35:149–154
Toh Y, Kuwano H, Mori M, Nicolson GL, Sugimachi K (1999) Overexpression of metastasis-associated MTA1 mRNA in invasive oesophageal carcinomas. Br J Cancer 79:1723–1726
Hofer MD, Kuefer R, Varambally S, Li H, Ma J, Shapiro GI et al (2004) The role of metastasis-associated protein 1 in prostate cancer progression. Cancer Res 64:825–829
Iguchi H, Imura G, Toh Y, Ogata Y (2000) Expression of MTA1, a metastasis-associated gene with histone deacetylase activity in pancreatic cancer. Int J Oncol 16:1211–1214
Jang KS, Paik SS, Chung H, Oh YH, Kong G (2006) MTA1 overexpression correlates significantly with tumor grade and angiogenesis in human breast cancers. Cancer Sci 97:374–379
Wang S, Qi Y, Zhang J, Zhang Q, Li H, Qiu X (2010) Expression and significance of MTA2 in non-small cell lung cancer. Zhongguo fei ai za zhi Chinese J Lung Cancer 13:777–780
Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R et al (2007) The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2:706–714
Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC (2004) World health organization classification of tumours: pathology and genetics of tumours of the lung, pleura, thymus and heart. IARC Press, Lyon
Dai SD, Wang Y, Jiang GY, Zhang PX, Dong XJ, Wei Q et al (2010) Kaiso is expressed in lung cancer: its expression and localization is affected by p120ctn. Lung Cancer 67:205–215
Dai SD, Wang Y, Miao Y, Zhao Y, Zhang Y, Jiang GY et al (2009) Cytoplasmic Kaiso is associated with poor prognosis in non-small cell lung cancer. BMC Cancer 9:178
Cui Y, Niu A, Pestell R, Kumar R, Curran EM, Liu Y et al (2006) Metastasis-associated protein 2 is a repressor of estrogen receptor alpha whose overexpression leads to estrogen-independent growth of human breast cancer cells. Molecular Endocrinol 20:2020–2035
Manavathi B, Singh K, Kumar R (2007) MTA family of coregulators in nuclear receptor biology and pathology. Nucl Recept Signal 5:e010
Luo J, Su F, Chen D, Shiloh A, Gu W (2000) Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature 408:377–381
Chakraborty S, Ganti AK, Marr A, Batra SK (2010) Lung cancer in women: role of estrogens. Expert Rev Respir Med 4:509–518
Zhang Y, Ng HH, Erdjument-Bromage H, Tempst P, Bird A, Reinberg D (1999) Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev 13:1924–1935
Xia L, Zhang Y (2001) Sp1 and ETS family transcription factors regulate the mouse Mta2 gene expression. Gene 268:77–85
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grants 81071717 to S-D Dai, 81071905 to E-H Wang, 81000995 to Y Han and 81000942 to Y Miao) and Doctoral Foundation of Ministry of Education of China (no. 20102104120027 to S-D Dai)
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, SL., Han, Y., Zhang, Y. et al. Expression of metastasis-associated protein 2 (MTA2) might predict proliferation in non-small cell lung cancer. Targ Oncol 7, 135–143 (2012). https://doi.org/10.1007/s11523-012-0215-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-012-0215-z