Abstract
The stent geometrical design (e.g., inter-strut gap, length, and strut cross-section) is responsible for stent–vessel contact problems and changes in the blood flow. These changes are crucial for causing some intravascular abnormalities such as vessel wall injury and restenosis. Therefore, structural optimization of stent design is necessary to find the optimal stent geometry design. In this study, we performed a multiobjective stent optimization for minimization of average stress and low wall shear stress ratio while considering the wall deformation in 3D flow simulations of triangular and rectangular struts. Surrogate-based optimization with Kriging method and expected hypervolume improvement (EHVI) are performed to construct the surrogate model map and find the best configuration of inter-strut gap (G) and side length (SL). In light of the results, G-SL configurations of 2.81–0.39 and 3.00–0.43 mm are suggested as the best configuration for rectangular and triangular struts, respectively. Moreover, considering the surrogate model and flow pattern conditions, we concluded that triangular struts work better to improve the intravascular hemodynamics.

ᅟ
Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Restenosis is the reoccurrence of stenosis or blockage on post-stented blood vessels [1]. Restenosis has been reported in one in ten patients with drug-eluted stents (DES) [2] and up to one-third of the patients with bare metal stents (BMSs) [1]. To the best of our knowledge, restenosis is induced by the mechanical and hemodynamic interactions between the stent and blood vessel. From a mechanical perspective, the stent–vessel contact may lead to cell inflammation owing to vessel injury problems [3, 4]. Moreover, the stent expansion deforms the vessel walls and may alter the flow around the strut [5, 6]. Then, changes in the flow pattern and orientation occur owing to flow obstruction because of the stent implantation [7,8,9,10]. When undesirable hemodynamic conditions occur (i.e., big recirculation zone), atherogenesis develops owing to low wall shear stress (WSS) causing restenosis. The stent geometrical design (e.g., porosity, length, and strut cross section) is responsible for stent–vessel contact problems and changes in the flow orientation [11, 12]. Consequently, many studies have concentrated on minimizing the undesirable effects of stent implantation.
Stent structural optimization studies have been performed to find the ideal stent configuration based on mechanical or hemodynamic objectives [13,14,15,16,17,18,19]. Srinivas et al. first optimized stents with different strut configurations to minimize vorticity and maximize WSS [13]. In contrast, studies on the strut cross sections suggest that alternative streamlined strut shapes offer improved stent performance [11, 12, 20]. Mejia et al. showed that streamlined shape stent struts are likely to reduce the occurrence of flow disturbance [11]. The experimental study by Chen et al. suggested that cells behave differently when different strut shapes are used; thus, the optimization of the cross-sectional shape of stent wires is necessary [12].
Recent optimization of the stent design for intravascular treatment targets mostly single objective or physical phenomena (i.e., flow dynamics or structural mechanics) [15, 17, 18, 21]. The review by Bressloff et al. covered the optimization of coronary stent systems and proposed future optimization targets, such as the WSS that strongly affects the endothelial cell activity [16]. Therefore, further optimization studies of fluid and structural mechanics using clinically or biologically focused objective functions are needed. To date, we performed single objective optimization based on the surrogate method and 2D axis-symmetrical stent models to minimized the low WSS area [22, 23]. We found that the surrogate model offers improvement on computational efficiency and design.
This study examines multiobjective stent optimization by minimizing mechanical and biological flow dynamics parameters and considering the wall deformation in 3D flow simulations of simple ring-shaped stents. The simulation was based on the finite-element method with high computational cost. Therefore, the surrogate-based optimization framework has been selected because the direct optimization of complex physical systems is time-consuming [24]. Basically, surrogate-based optimization predicts the objective function and then uses it as the metamodel of the physical system in the simulations [25]. By using the surrogate model, predictions of the objectives and research processes of the optimized design are efficiently performed. Besides, we also compare the optimized performance for different cross-sectional shapes on minimizing the objectives.
2 Methods
2.1 Design variables and sampling strategy
Finding the best configuration of inter-strut gap length (G) and side length (SL) configuration is the main purpose of this work with the general framework is shown in Fig. 1. For accurate observation, a sufficient sample set is necessary. Twenty-six samples of G-SL combinations were generated by the Halton sampling method for each strut shape. As previously explained on our work [23], the Halton sampling points were obtained by pairing two sets of mutually prime-based Halton sequences in a unit square [26]. These sequences can be obtained for any m-th Halton number firstly within 0 to 1 intervals by
where m is any integer, which is written in the base-b notation of
For this sample generation, we used pairs of Halton sequences based on 2 and 3. Afterwards, normalization was performed to fit to our G-SL configurations that were generated for G between 1 and 3 mm and SL between 0.05 and 0.45 mm. This sampling strategy has been used to obtain the “space-filling” distribution along the sampling range. In addition to these 26 samples (Fig. 2), more points were added to the dataset when necessary.
2.2 Geometry construction
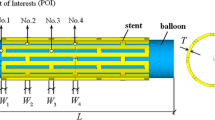
The wall deformation in post-stented blood vessels was observed in in vivo studies [5, 27]. Moreover, studies show the parent artery expansion and straightening due to stent deployment based on the in vivo vessel reconstructions. These works also suggest that the inflow and hemodynamic properties change owing to the alteration of post-stented blood vessel geometry [10, 28,29,30]. Therefore, it is necessary to include the wall deformation in the simulation. To obtain the deformed geometry and investigate the effect of this deformation to the flow condition, the following two consecutive simulations were performed: structural mechanics and solid-flow coupled simulation (Fig. 3). The 4-mm blood vessel diameter geometry settings are shown in Fig. 4 along with the design variables for the different strut cross-sectional shapes, G, and SL. Rectangular struts are typical laser-cut stents, while triangular struts are proposed as streamlined strut shapes.
Geometry creation was begun with obtaining the desired blood vessel deformation by applying 5.5% displacement for all stent geometries [5]. Once the deformed geometry was obtained, it was taken as the initial wall geometry in the fluid flow simulation. Afterwards, the wall geometry is rotated to obtain the symmetrical 3D geometry of the deformed blood vessel. The new cylindrical geometry was added followed by Boolean operations to add the flow domain on the central part of the vessel body. Finally, the geometry was divided into four symmetrical parts to reduce the computational cost.
2.3 Boundary and simulation settings
The stent struts are defined as NiTi material with Young’s modulus and Poisson’s ratio of 80 GPa and 0.33, respectively [31]. The Young’s modulus and Poisson’s ratio of the blood vessel layer and muscle tissues are 0.7 GPa and 0.45 for the no-slip wall vessel layer and 1.16 MPa and 0.45 for the muscle tissue [32, 33]. In the fluid domain, the blood was assumed to be an incompressible Newtonian fluid with a viscosity of 3.5 mPa·s and density of 1050 kg m−3 [34].
The flow boundary condition was that of stationary laminar inflow with a mean velocity of 0.33 ms−1 and 0 Pa laminar outflow boundary condition. The flow boundary condition corresponds to Reynolds number of 396 [13]. In addition, along the flow–vessel boundary, static pressure of 100 mmHg from the average normal human blood pressure and dynamic pressure from flow phenomena were set as fluid–solid coupled boundary loads to test if any wall deformation occurs. All simulation steps and boundary condition settings were the same as well as both strut cross-sectional shapes. Triangular and hexagonal meshes were used in the mechanical and fluid simulations. A mesh dependency test was performed for each representative case of both mechanical stress and fluid–solid coupled simulation with the solution converged within 1% (Fig. 5). Similar mesh quality criteria were maintained for all simulations during the optimization process.
The simulations were performed using COMSOL Multiphysics 5.0 (Comsol Inc., Stockholm, Sweden) with structural mechanics and laminar flow coupled systems. This simulation is performed in two consecutive steps, as shown in Fig. 3. The solid domain parts were defined as linear elastic materials that work based on the equation of motion with strain displacement equation based on Young’s modulus and Poisson’s ratio. The fluid domain parts were considered to belong to laminar flow by solving the Navier–Stokes equation. Interaction between deformation and stress normal work based on Cauchy stress which formulated as the pressure and viscous terms in Navier–Stokes, read as
where n is the normal vector, P is pressure, μ is the viscosity, V is blood velocity, ρ is the blood density, and g is the body acceleration or gravity. The convergence criteria were set to a maximum residual of 10−5.
2.4 Design variables and objective functions
The endothelial cells on the blood vessel surface are greatly affected by the WSS conditions [7,8,9, 13, 35]. Low WSS (WSS < 0.5 Pa [7, 8]) is possibly the major reason for restenosis owing to cell migration and platelet activations. Therefore, we used the parameter of low WSS ratio (γ) (< 0.5 Pa), which appears on the blood vessel surface. The detail calculation of γ has been discussed previously [23]. Besides WSS, mechanical stress owing to stent deployment is also critical to the vessel wall conditions because of inflammation and cell traumas.
The average stress in the post fluid–solid coupled simulation is obtained at infinitesimal values compared to the initial deformation. Therefore, this parameter is neglected in the analysis which is also in line with previous work [36]. The minimization of γ and the initial average stress due to to the wall deformation are chosen as the objective functions.
2.5 Surrogate model construction and accuracy improvement method
The simulated system has been treated as a black box and finding the system’s objective function f(x) analytically is difficult; therefore, we chose the surrogate optimization method based on Kriging to estimate the objective function \( \left(\widehat{f}(x)\right) \) by generating random Gaussian functions and interpolating the responses [25, 37]. Next, the estimated objective function is used in the optimization steps. Moreover, surrogate model maps of the objective function are constructed. The surrogate model map is used to observe the effect of the stent design configuration and improve the stent design. The surrogate model and optimization code used in this study were developed by the Aerospace Fluid Engineering Laboratory, Institute of Fluid Science of Tohoku University.
Error-based exploration was performed to further refine the surrogate model. This method basically tries to minimize the Kriging error by suggesting new sample points with the highest predictor error values [25]. New simulations are then performed for the new sample point and the surrogate model is updated.
2.6 Multiobjective optimization and the expected hypervolume improvement method
Realistic optimization frequently features a trade-off between two and more objectives. Multiobjective optimization approach is necessary to handle problems with conflicting objectives. A Pareto optimal set is the collection of solutions in which an improvement in one objective cannot be optimized without degrading the other objectives [38]. Therefore, the proposed optimization aims to find the Pareto optimal solutions with γ and initial average stress as the objectives.
The EHVI method [39] was adopted as the multiobjective optimization algorithm. EHVI is based on the difference of the hypervolume between two sample sets of the Kriging model (Fig. 6). The EHVI is defined as
where fi is the Gaussian random variable, ϕi(Fi) is the probability function, and firef the hypervolume reference value [38]. The samples are enriched so that subsequent samples move closer to the Pareto front. Hypervolume is defined as the volume enclosed by the current nondominated solutions and the defined reference point for the hypervolume calculation. For a given reference point, the solutions that yield the highest hypervolume belong to the Pareto optimal set (Fig. 6). Each update of EHVI tries to find a solution that improves the hypervolume of the current nondominated set, hence the concept of hypervolume improvement.
3 Results
From the simulation, we obtain the response data for each γ and the average mechanical stress along the deployment area. Fifty-four different design configurations were simulated for each strut shape. The average computational time for each rectangular and triangular shape is 39 min and 16 s and 26 min and 30 s, respectively, excluding meshing and pre- and post-processing.
3.1 Rectangular stent: Kriging surrogate models
Two surrogate models were obtained for each objective function: γ (Fig. 7) and average stress (Fig. 8). As shown in Fig. 7, the system response based on each G and SL (G-SL) configuration shows the global increment in γ with increasing SL. The lowest rate of γ (%) is predicted to occur in the G range of 2.1–3 mm and SL less than 0.07 mm. Several interesting areas of low γ are obtained for G greater than 2 mm and different SL. Further investigations of the flow conditions under these particular configurations will be discussed in the Section 4.
In the average stress minimization case, the SL increase causes the average stress to decrease. Such behavior occurs for struts with SL greater than 0.2 mm and rapid increments are noticed under this size.
3.2 Triangular stent: Kriging surrogate models
The surrogate model in Fig. 7b suggests that generally lower γ values are obtained by the triangular strut. High γ values are associated with small G and large SL. On the other hand, low γ areas are observed under several design configurations, i.e., along small SL, long G and medium SL, and long G and large SL. The lowest γ values are concentrated and are associated with small to medium G.
With respect to the second objective (Fig. 8b), general behavior is similar to that of the rectangular strut is observed. These results suggest that large struts minimize the average stress along the deployment area. Generally, we observed that there is a trade off between the two objectives. Therefore, multiobjective optimization is necessary.
3.3 Multiobjective solutions: nondominated point set
The set of multiobjective solutions is given in Table 1 and shown in Fig. 9. Seven nondominated points were obtained for rectangular and triangular struts after 14 and 12 EHVI iterations, respectively. The final optimal design is chosen after considering each point of interest in the analysis. The nondominated points in both cross sections are concentrated on the extreme left side of the γ and average stress curve in Fig. 9. From the nondominated sets, the corner pointyields the best performance in minimization of γ and average stress. However, further investigation of the flow and mechanical physical conditions is needed.
4 Discussion
Based on the obtained surrogate model, we observe that for both cross-sectional cases, the configurations of small G and big SL strut is not recommended. Focusing on the γ-minimization surrogate model, broader areas with γ less than 40% are produced by the triangular strut. In addition, due to the different γ values suggested by each cross section in particular on G-SL 1.5–0.32 mm and G-SL 1.19–0.17 mm, thus we give special focus on these configurations. The flow patterns for both cross-sectional shapes are shown in Fig. 10 with each recirculation zone (RCZ) length listed in Table 2. Shorter RCZs are observed with the triangular strut, which produces smaller γ (%) near the strut [30]; thanks to the smaller flow disturbance generated by triangular design owing to its streamlined shape.
For the second objective, both surrogate models produce similar general trend, in which the average stress increases with decreasing SL. Apparently, there is a trade-off between G-SL configurations and the objectives. Past single objective optimization works [22, 23] has shown that small struts minimize γ (%) because of the smaller flow disturbance owing to RCZ at the narrow strut–vessel gap and the wall deformation, whereas the rest of the blood vessel wall surface is exposed directly to the main flow. However, small struts are responsible for the high average stresses on the vessel surface because of the narrower contact surface area.
Regarding the nondominated sets for both cross sections, a wide average stress gap is observed in the nondominated solution of low γ between points d and e, and f and g for rectangular and triangular shapes, respectively. After checking the G-SL configurations between these points (Table 1), the wide average stress gap is likely the result of the significant difference strut SL between nondominated points; points a, b, c, and d in Fig. 9a are distributed in the narrow region of low average stress with significantly different γ values. Based on the flow pattern of points a and d (Fig. 11), small RCZ is observed in point d which causes small γ values. In contrast, the narrow distribution of nondominated points corresponds to the triangular cross-sectional shape, in particular, points c, d, e, and f. The combination of similar SL (Table 1) and RCZ–G ratios (Table 2) is the reason for this. For analysis based on nondeformed wall conditions, either the flow pattern or γ will differ [30]. From Fig. 11, we can also observe that the general tendency of the RCZ shape of rectangular struts is similar, as previously obtained by study of Jimenez, et al. in [40].
Previous studies have shown that the WSS condition is transferred through the endothelial cells to the smooth muscle cells (SMC). Therefore, the low WSS could trigger a phenotype change of SMC, which could cause the atherosclerotic plaque to grow [35]. In addition, excess mechanical stresses on the vessel surface may also promote cell inflammation owing to injury [9]. Hence, it is important to balance the post deployment γ area and average stress on the blood vessel surface. Combinations of relatively big SL struts with long G are the best to satisfy both objective functions for rectangular and triangular struts. Further evaluation of cell behavior toward this optimized design is interesting to be investigated. Although strut with an exact parameter criteria are sometimes hard to be fabricated, the process of design exploration from the utilization of surrogate model contour map can be beneficial to solve the problem of fabrication constraint.
Several assumptions were applied to this work that put the simulation under some limitations. Although the real blood flow is pulsatile, we used steady flow assumption since it is efficient in terms of the computational cost. Moreover, our previous work shows that the difference between the surrogate model construction, which was derived from pulsatile transient simulation, was insignificant [30]. We also treat the blood fluid as a Newtonian fluid, which may lead to some flaw simulation results, particularly, on the near-wall area where the non-Newtonian properties of blood are likely to appear. However, based on the Fahraeus–Lindqvis effect, this non-Newtonian effect begins to play a role on the vessel size with a diameter less than 1 mm [9]. For the solid mechanics domain, an assumption of simple linear mechanical model for blood vessels was applied. This assumption cannot appropriately represent the nonlinear and anisotropic behavior of the blood vessel, which comprises many layers of complex fibers [32, 41] Therefore, more complex models of the intravascular environment and stent geometry with high-order design variables need to be considered. Although this simulation has shown the results with certain level of convergence from both meshes dependency and solver convergence, it is also important to increase the simulation accuracy by improving the computational resources to handle more mesh elements, especially for small struts. As the validation of this optimization study, future work on in vitro investigations of how the optimized solution will affect the cellular activities is also necessary, as is necessary to find the exact threshold of the best γ (%) and average stress for healthy vascular conditions.
5 Conclusions
In this study, 3D computational multiobjective optimization by minimizing low WSS ratio (γ) and the average stress along the deployment area under deformed wall conditions for triangular and rectangular struts was performed. To balance both objectives, we suggest design configurations for G-SL of 2.81–0.39 mm (rectangular nondominated point d) and 3.00–0.43 mm (triangular nondominated point f) for rectangular and triangular struts, respectively. The triangular strut is predicted to produce better intravascular hemodynamics; however, regarding the average stress, both strut types show no significant differences.
References
Elmore JB, Mehanna E, Parikh SA, Zidar DA (2016) Restenosis of the coronary arteries: past, present, future directions. Interv Cardiol Clin 5:281–293. https://doi.org/10.1016/j.iccl.2016.03.002
Giacoppo D, Gargiulo G, Aruta P, Capranzano P, Tamburino C, Capodanno D (2015) Treatment strategies for coronary in-stent restenosis: systematic review and hierarchical Bayesian network meta-analysis of 24 randomised trials and 4880 patients. BMJ:h5392. https://doi.org/10.1136/bmj.h5392
Chen HY, Hermiller J, Sinha AK, Sturek M, Zhu L, Kassab GS (2009) Effects of stent sizing on endothelial and vessel wall stress: potential mechanisms for in-stent restenosis. J Appl Physiol 106:1686–1691. https://doi.org/10.1152/japplphysiol.91519.2008
Patel SM, Li J, Parikh SA (2016) Design and comparison of large vessel stents. Interv Cardiol Clin 5:365–380. https://doi.org/10.1016/j.iccl.2016.03.005
Freeman JW, Snowhill PB, Nosher JL (2010) A link between stent radial forces and vascular wall remodeling: the discovery of an optimal stent radial force for minimal vessel restenosis. Connect Tissue Res 51:314–326. https://doi.org/10.3109/03008200903329771
Otsuka F, Nakano M, Ladich E, Kolodgie FD, Virmani R (2012) Pathologic etiologies of late and very late stent thrombosis following first-generation drug-eluting stent placement. Thrombosis 2012:1–16. https://doi.org/10.1155/2012/608593
Lewis G (2008) Materials, fluid dynamics, and solid mechanics aspects of coronary artery stents: a state-of-the-art review. J Biomed Mater Res Part B Appl Biomater 86B:569–590. https://doi.org/10.1002/jbm.b.31028
Beier S, Ormiston J, Webster M, Cater J, Norris S, Medrano-Gracia P, Young A, Cowan B (2015) Hemodynamics in idealized stented coronary arteries: important stent design considerations. Ann Biomed Eng 44:315–329. https://doi.org/10.1007/s10439-015-1387-3
Westerhof N, Stergiopulos N, Noble MIM (2010) Snapshots of hemodynamics
Putra NK, Anzai H, Ohta M (2016) Hemodynamic behaviours under blood vessel deformation by stent struts: two dimensional study. In: Thirteenth International Conference on Flow Dynamics pp 294–295
Mejia J, Ruzzeh B, Mongrain R, Leask R, Bertrand OF (2009) Evaluation of the effect of stent strut profile on shear stress distribution using statistical moments. Biomed Eng Online 8:8. https://doi.org/10.1186/1475-925X-8-8
Chen Z, Zhan F, Ding J, Zhang X, Deng X (2016) A new stent with streamlined cross-section can suppress monocyte cell adhesion in the flow disturbance zones of the endovascular stent. Comput Methods Biomech Biomed Eng 19:60–66. https://doi.org/10.1080/10255842.2014.984701
Srinivas K, Nakayama T, Ohta M, Obayashi S, Yamaguchi T (2008) Studies on design optimization of coronary stents. J Med Device 2:11004-1–11004-7
Srinivas K, Townsend S, Lee C-J, Nakayama T, Ohta M, Obayashi S, Yamaguchi T (2010) Two-dimensional optimization of a stent for an aneurysm. J Med Device 4:21003-1–21003-7
Anzai H, Falcone JL, Chopard B, Hayase T, Ohta M (2014) Optimization of strut placement in flow diverter stents for four different aneurysm configurations. J Biomech Eng 136:61006-1–61006-7
Bressloff NW, Ragkousis G, Curzen N (2015) Design optimisation of coronary artery stent systems. Ann Biomed Eng 44:1–11. https://doi.org/10.1007/s10439-015-1373-9
Zhang M, Anzai H, Chopard B, Ohta M (2016) Towards the patient-specific design of flow diverters made from helix-like wires: an optimization study. Biomed Eng Online 15(Suppl):371–382
Li H, Gu J, Wang M, Zhao D, Li Z, Qiao A, Zhu B (2016) Multi-objective optimization of coronary stent using kriging surrogate model. Biomed Eng Online 15:148. https://doi.org/10.1186/s12938-016-0268-9
Janiga G, Daróczy L, Berg P, Thévenin D, Skalej M, Beuing O (2015) An automatic CFD-based flow diverter optimization principle for patient-specific intracranial aneurysms. J Biomech 48:3846–3852. https://doi.org/10.1016/j.jbiomech.2015.09.039
Kim YH, Xu X, Lee JS (2010) The effect of stent porosity and strut shape on saccular aneurysm and its numerical analysis with lattice Boltzmann method. Ann Biomed Eng 38:2274–2292. https://doi.org/10.1007/s10439-010-9994-5
Li H, Liu T, Wang M, Zhao D, Qiao A, Wang X, Gu J, Li Z, Zhu B (2017) Design optimization of stent and its dilatation balloon using kriging surrogate model. Biomed Eng Online 16(13):13. https://doi.org/10.1186/s12938-016-0307-6
Putra NK, Palar PS, Anzai H, et al (2017) Variation of strut parameter effects with wall deformation on stent deployment via surrogate model. In: 5th International Conference on Computational and Mathematical Biomed Eng pp 1007–1010
Putra NK, Palar PS, Anzai H, et al (2018) Comparative Study Between Different Strut’s Cross Section Shape on Minimizing Low Wall Shear Stress Along Stent Vicinity via Surrogate-Based Optimization. In: Schumacher A, Vietor T, Fiebig S, et al (eds) Advances in Structural and Multidisciplinary Optimization: Proceedings of the 12th World Congress of Structural and Multidisciplinary Optimization (WCSMO12). Springer International Publishing, Cham, pp 2097–2109
Yang XS, Koziel S, Leifsson L (2012) Computational optimization, modelling and simulation: smart algorithms and better models. In: Procedia Computer Science. Elsevier Masson SAS, pp 852–856
AIJ F, Sobester A, Keane AJ (2008) Engineering design via surrogate modelling. Wiley., West-Sussex
Kolar M, OS F (1993) Fast, portable and reliable algorithm for the calculation of Halton numbers. Comput Math Appl 25:3–13
Otsuka F, Finn AV, Yazdani SK, Nakano M, Kolodgie FD, Virmani R (2012) The importance of the endothelium in atherothrombosis and coronary stenting. Nat Rev Cardiol 9:439–453. https://doi.org/10.1038/nrcardio.2012.64
Mori F, Ohta M, Matsuzawa T (2015) Changes in blood flow due to stented parent artery expansion in an intracranial aneurysm. Technol Health Care 23:9–21. https://doi.org/10.3233/THC-140871
Kono K, Shintani A, Terada T (2014) Hemodynamic effects of stent struts versus straightening of vessels in stent-assisted coil embolization for sidewall cerebral aneurysms. PLoS One 9:e108033. https://doi.org/10.1371/journal.pone.0108033
Putra NK, Palar PS, Anzai H, et al (2017) Stent design optimization based on Kriging surrogate model under deformed vessel wall: pulsatile inlet flow. In: ICA 2017 Proceedings. IEEE
Components JMM (2015) Nitinol technical properties. http://jmmedical.com/resources/221/Nitinol-Technical-Properties.html. Accessed 9 Sept 2015
Fung YC (1996) Blood flow in arteries. In: Biomechanics: circulation, second. Springer-Verlag, New York, pp 108–205
COMSOL Multiphysics (2014) Fluid structure interaction in a network of blood vessels. In: Structural mechanics module model library manual, vol 1, p 20
Li Y, Anzai H, Nakayama T et al (2014) Simulation of hemodynamics in artery with aneurysm and stenosis with different geometric configuration. J Biomech Sci Eng 9:1–11. https://doi.org/10.1299/jbse.2014jbse0003
Han X, Sakamoto N, Tomita N et al (2017) Influence of shear stress on phenotype and MMP production of smooth muscle cells in a co-culture model. J Biorheol 31:50–56. https://doi.org/10.17106/jbr.31.50
Chiastra C, Migliavacca F, Martínez MÁ, Malvè M (2014) On the necessity of modelling fluid-structure interaction for stented coronary arteries. J Mech Behav Biomed Mater 34:217–230. https://doi.org/10.1016/j.jmbbm.2014.02.009
Shimoyama K, Yoshimizu S, Jeong S et al (2011) Multi-objective design optimization for a steam turbine stator blade using LES and GA. J Comput Sci Technol 5:134–147. https://doi.org/10.1299/jcst.5.134
Luo C, Shimoyama K, Obayashi S (2015) A study on many-objective optimization using the Kriging-surrogate-based evolutionary algorithm maximizing expected hypervolume improvement. Math Probl Eng 2015:1–15. https://doi.org/10.1155/2015/162712
Emmerich MTM, Deutz AH, Klinkenberg JW (2011) Hypervolume-based expected improvement: monotonicity properties and exact computation. In: 2011 IEEE Congress of Evolutionary Computation, CEC 2011. pp 2147–2154
Jimenez JM, Prasad V, Yu MD, Kampmeyer CP, Kaakour AH, Wang PJ, Maloney SF, Wright N, Johnston I, Jiang YZ, Davies PF (2014) Macro- and microscale variables regulate stent haemodynamics, fibrin deposition and thrombomodulin expression. J R Soc Interface 11:20131079–20131079. https://doi.org/10.1098/rsif.2013.1079
Yeh HH, Rabkin SW, Grecov D (2017) Hemodynamic assessments of the ascending thoracic aortic aneurysm using fluid-structure interaction approach. Med Biol Eng Comput 56:1–17. https://doi.org/10.1007/s11517-017-1693-z
Acknowledgments
We would like to thank Dr. Yasutomo Shimizu for his help and suggestions during the preparation of this manuscript.
Funding
This research is supported by Indonesia Endowment for Education Fund (LPDP), Ministry of Finance, Republic of Indonesia through Beasiswa Pendidikan Indonesia Scholarship Program for Doctorate Students and the ImPACT program of Council for Science, Technology and Innovation (Cabinet Office, Government of Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Putra, N.K., Palar, P.S., Anzai, H. et al. Multiobjective design optimization of stent geometry with wall deformation for triangular and rectangular struts. Med Biol Eng Comput 57, 15–26 (2019). https://doi.org/10.1007/s11517-018-1864-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-018-1864-6